Development of a Water-Sensitive Self-Thickening Emulsion Temporary Plugging Diverting Agent for High-Temperature and High-Salinity Reservoirs
Abstract
1. Introduction
2. System Preparation and Analysis Methods
2.1. System Development
2.2. Properties Characterization
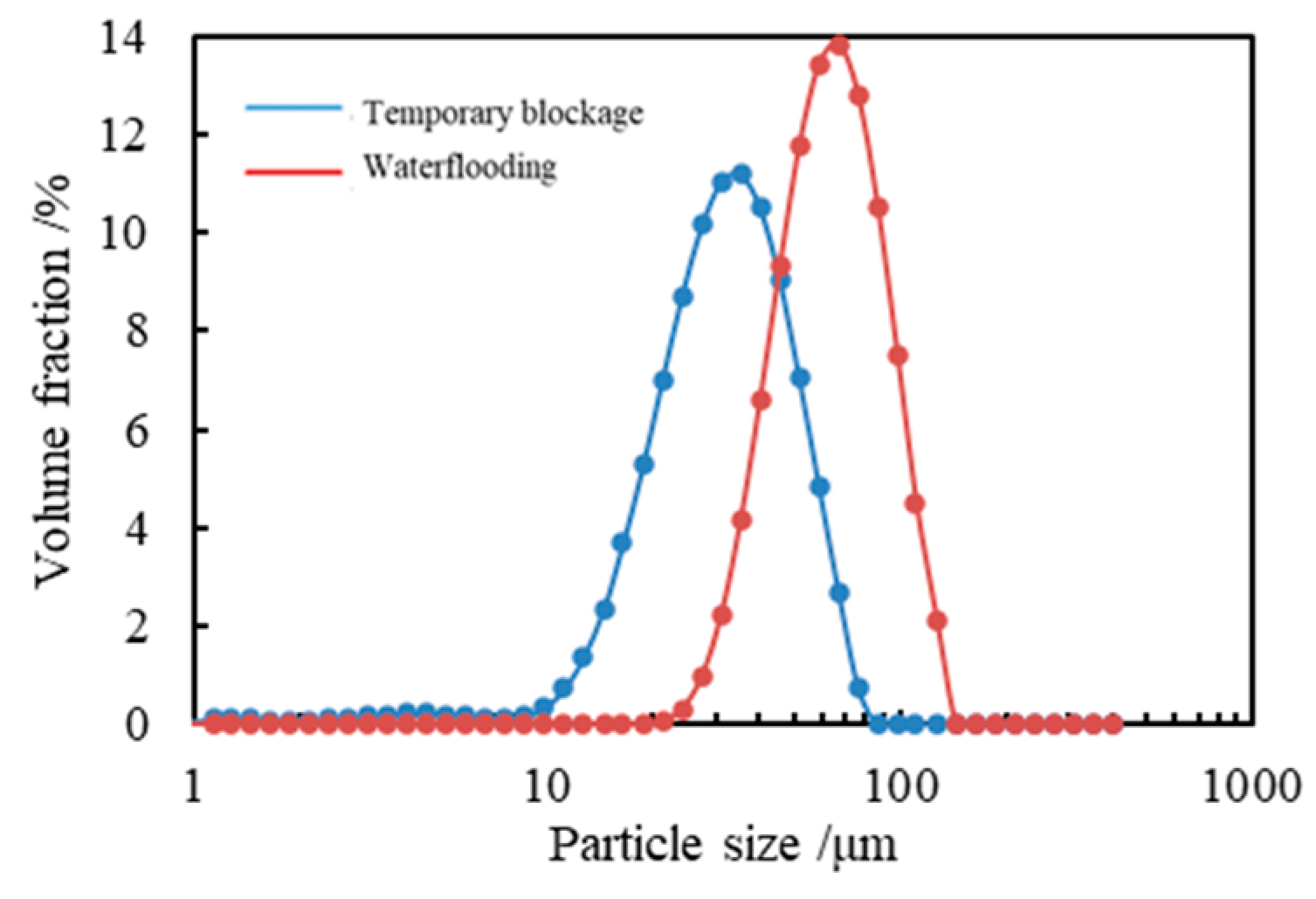
2.2.1. Particle Size Distribution Test
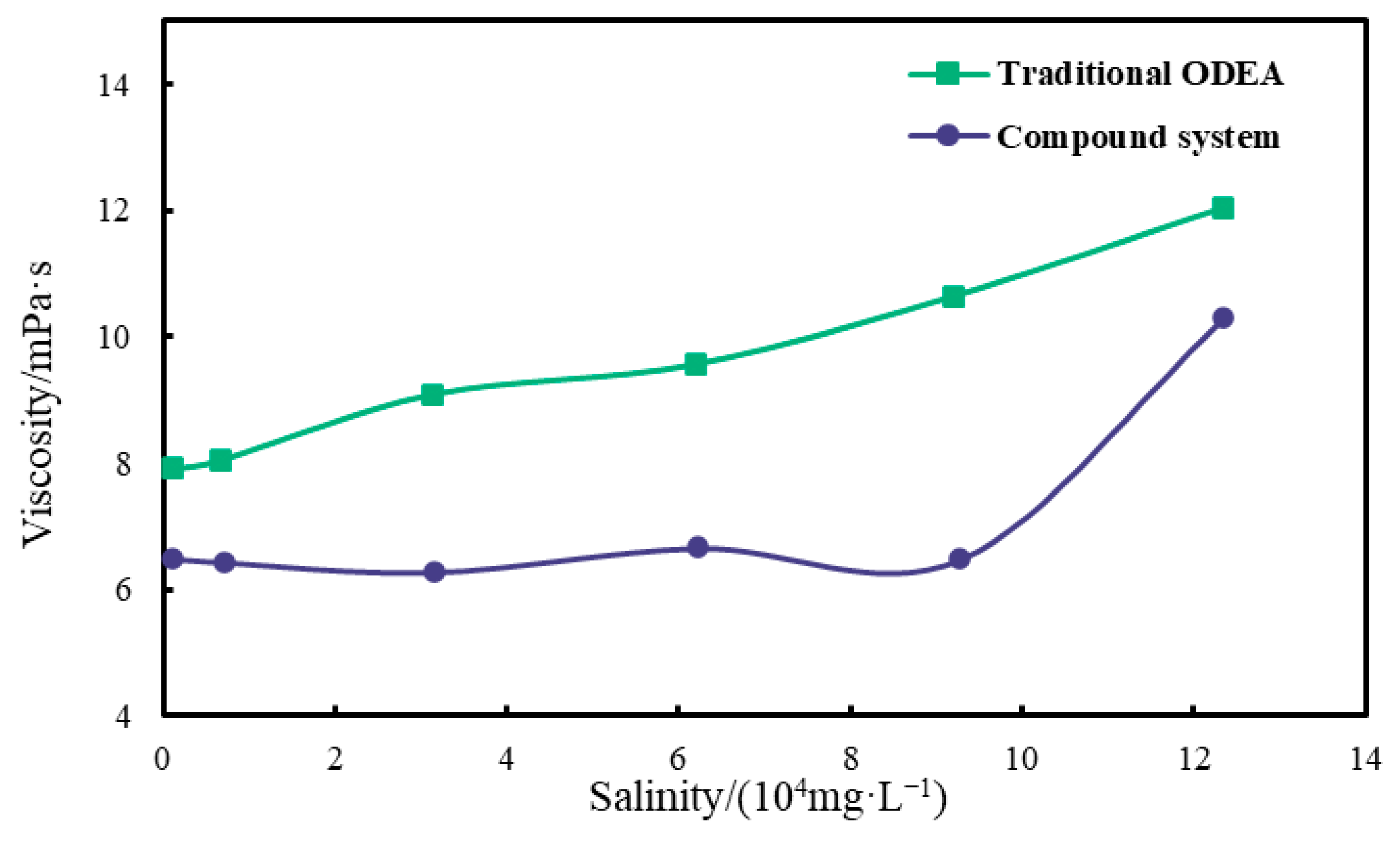
2.2.2. Salt Resistance Test
2.2.3. Oil–Water Selectivity Test
2.2.4. Temperature Resistance Evaluation
2.2.5. System Compatibility Test
2.3. Evaluation of the Temporary Plugging and Diverting Ability
3. Results
3.1. System Optimization
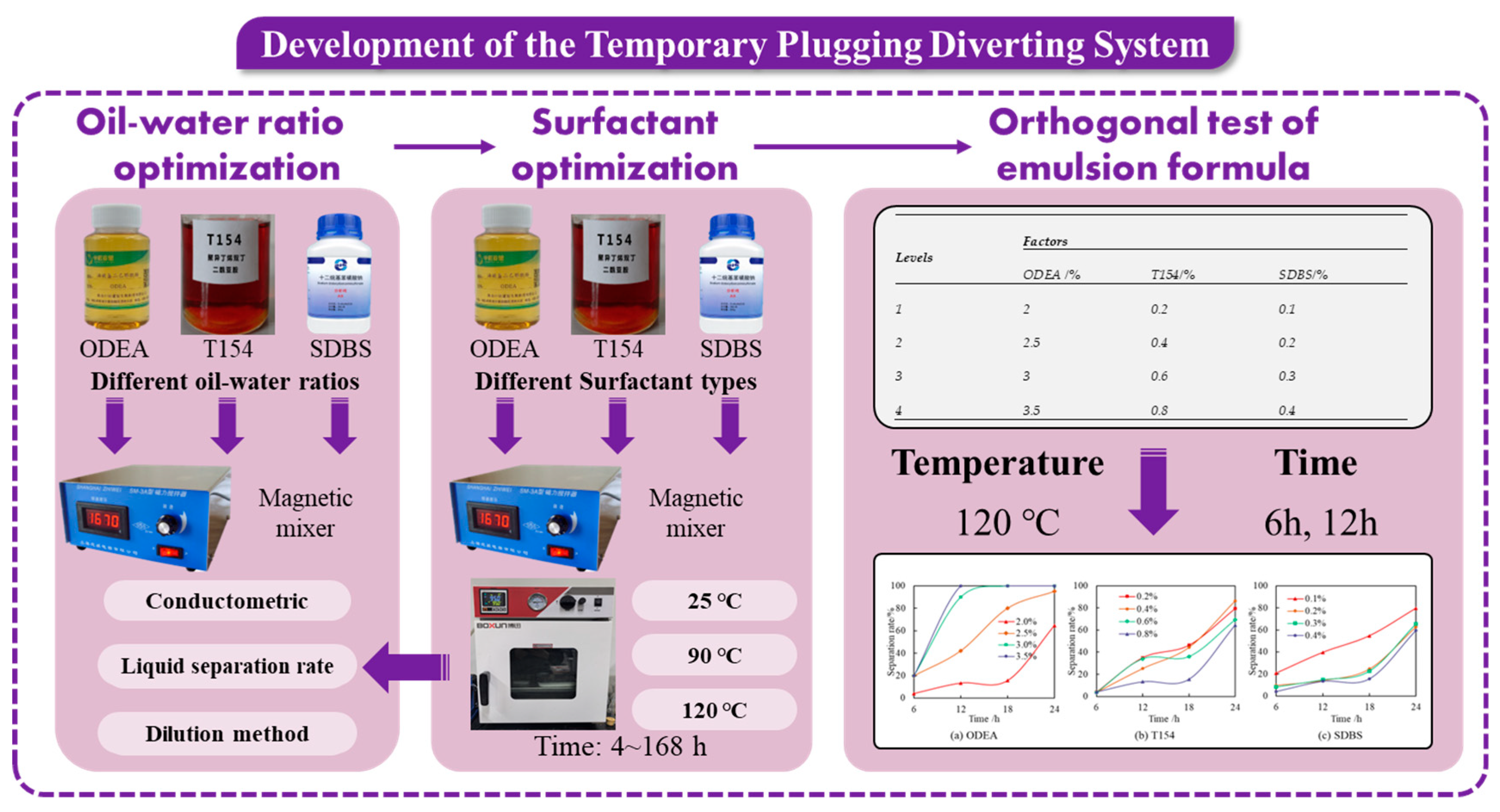
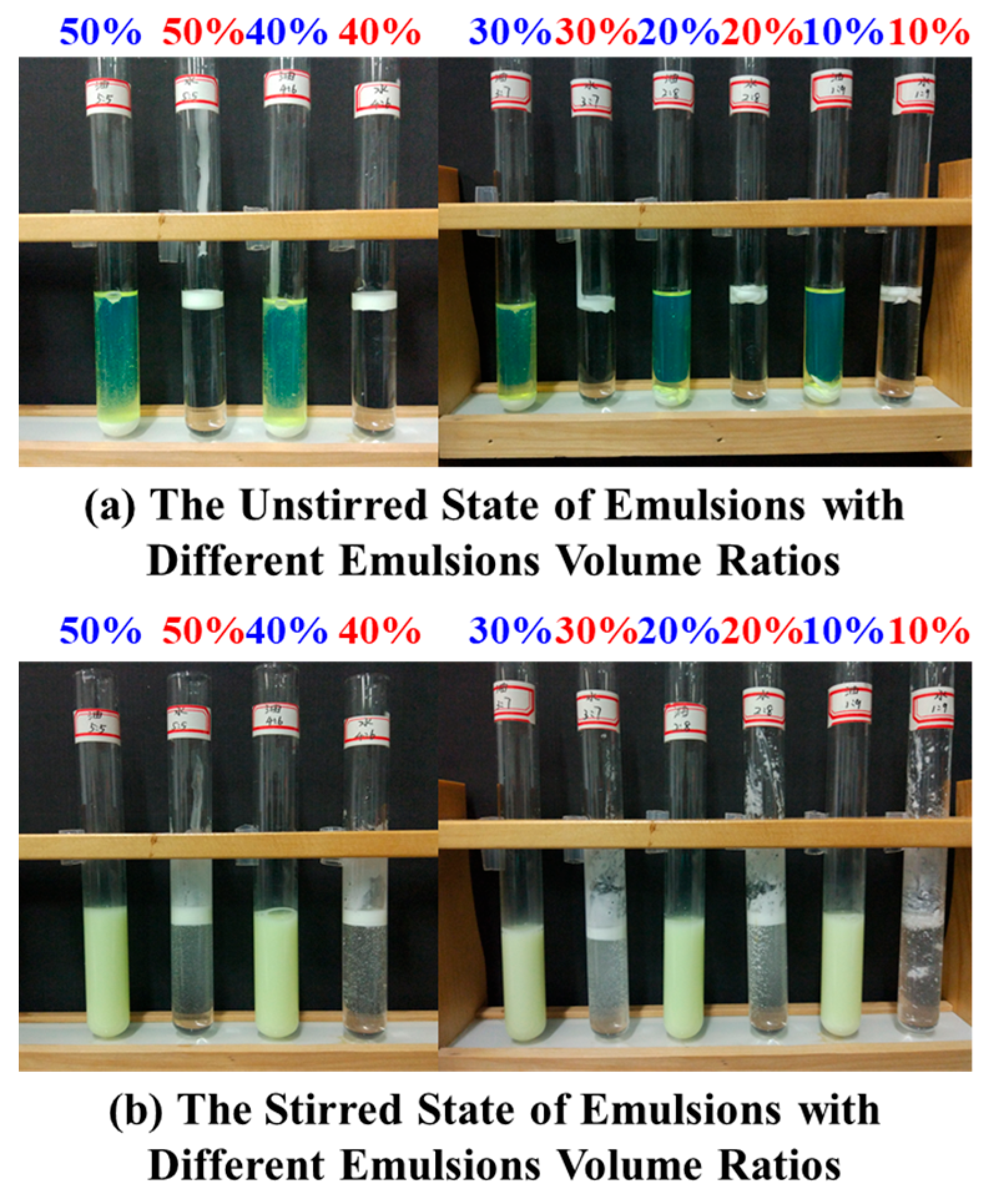
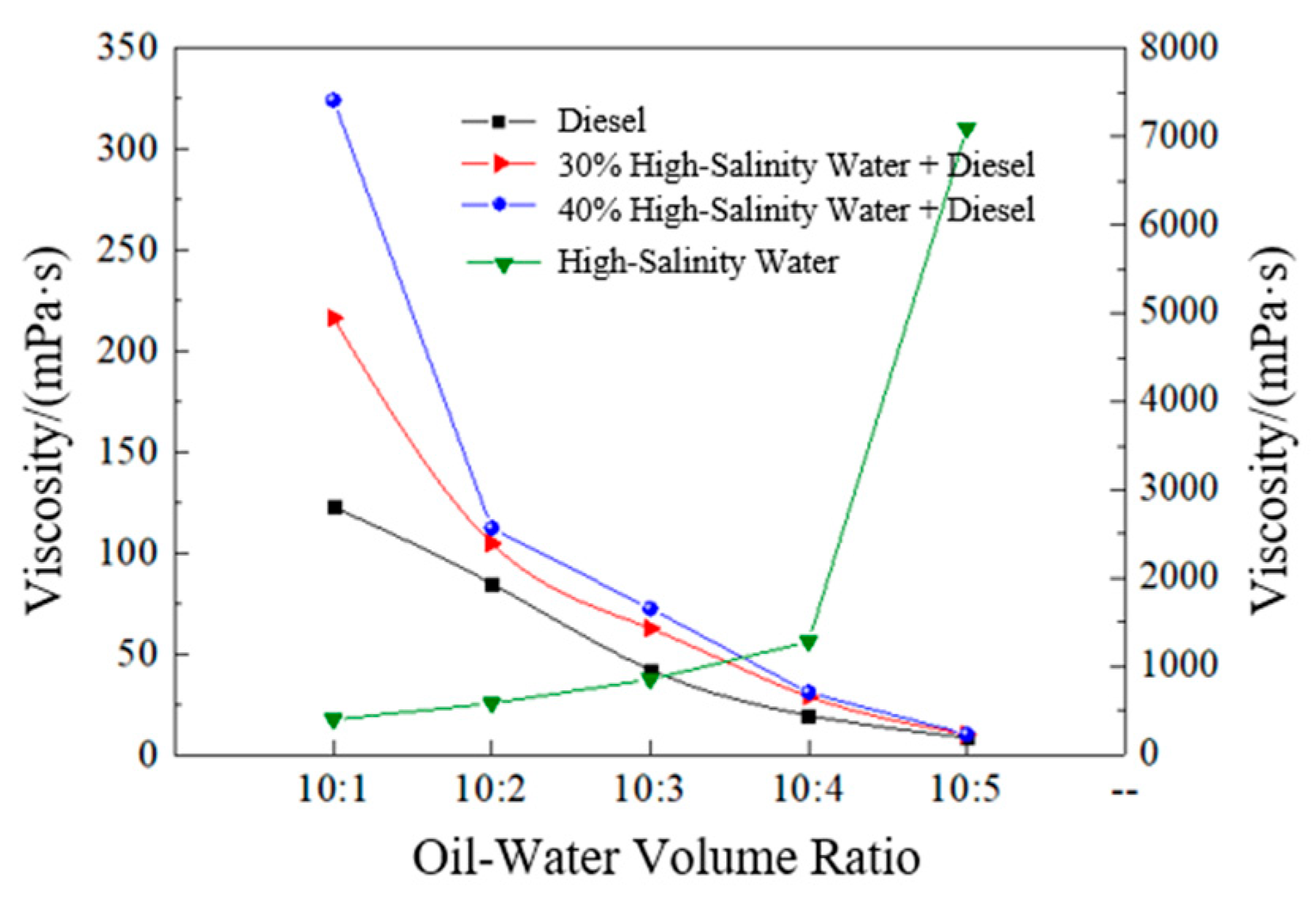
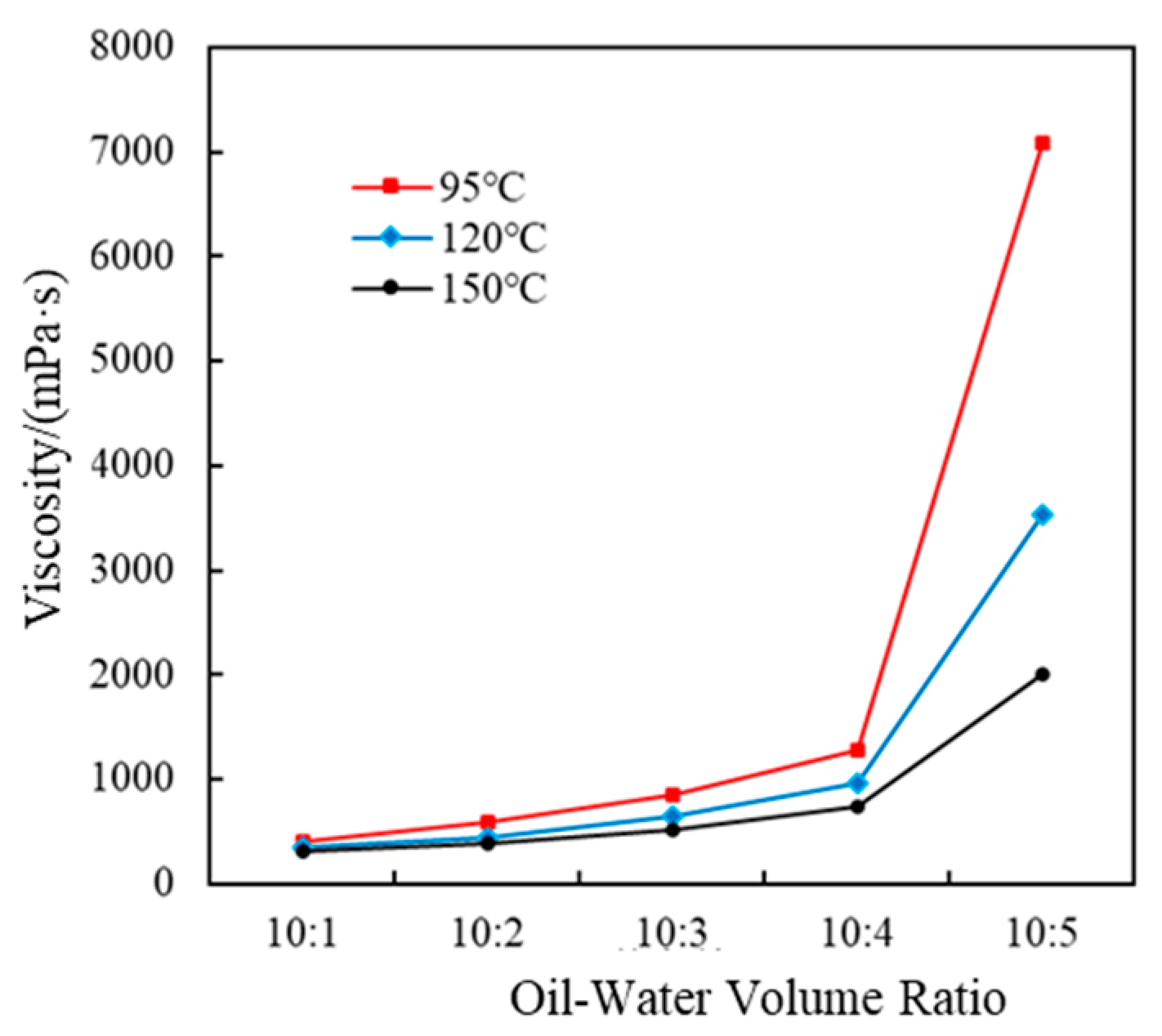
3.1.1. Oil–Water Ratio Optimization
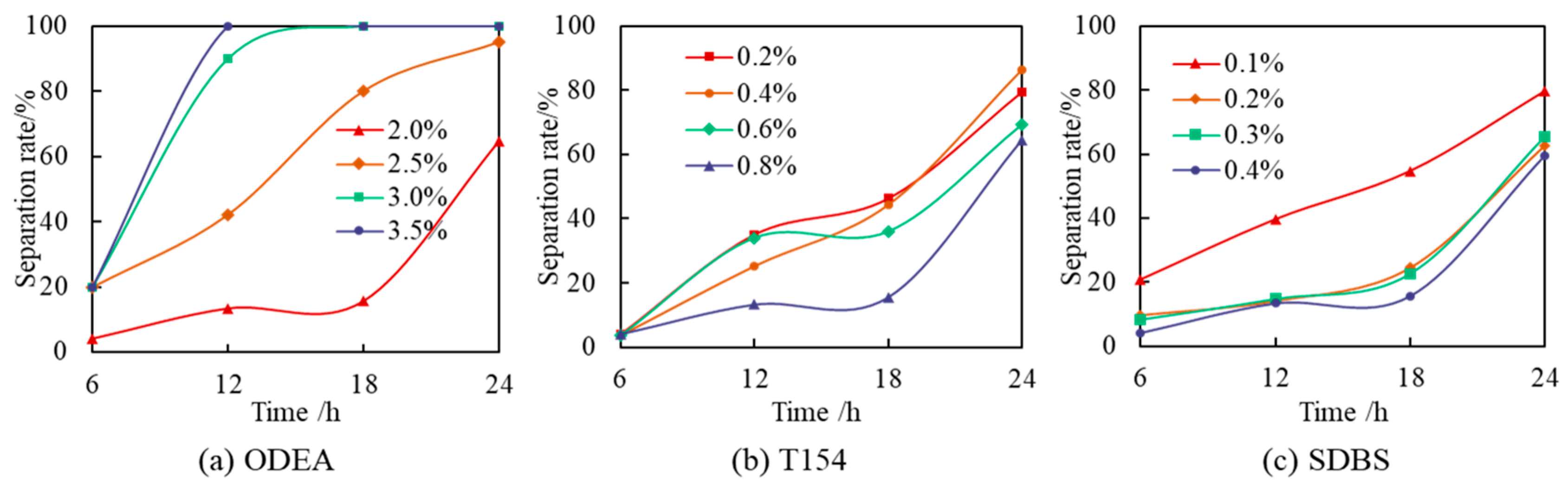
3.1.2. Surfactant Optimization
3.1.3. Orthogonal Test of Emulsion Formula
3.2. Characteristic Evaluation
3.2.1. Particle Size Distribution
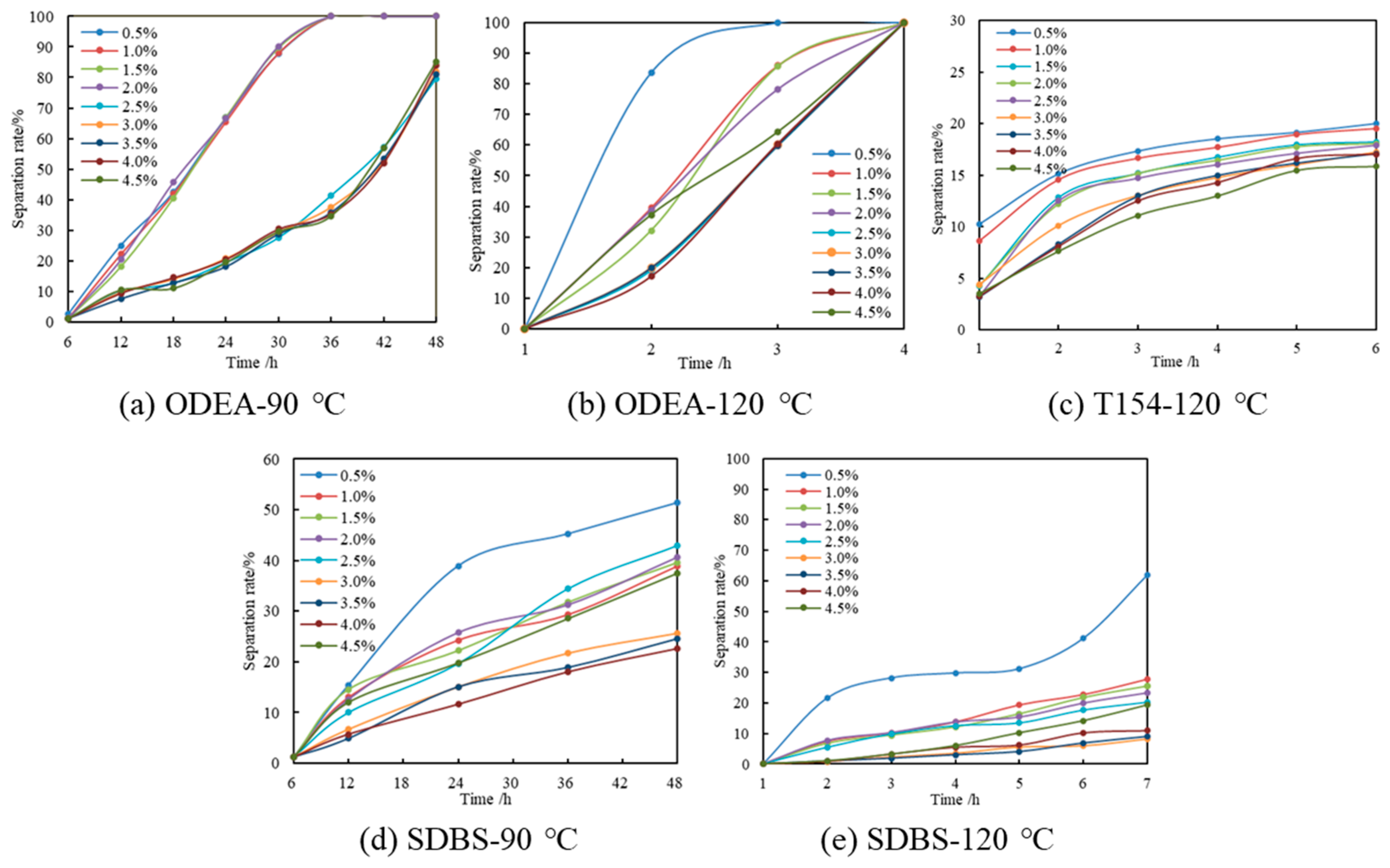
3.2.2. Salt Resistance Performance
3.2.3. Oil–Water Selectivity
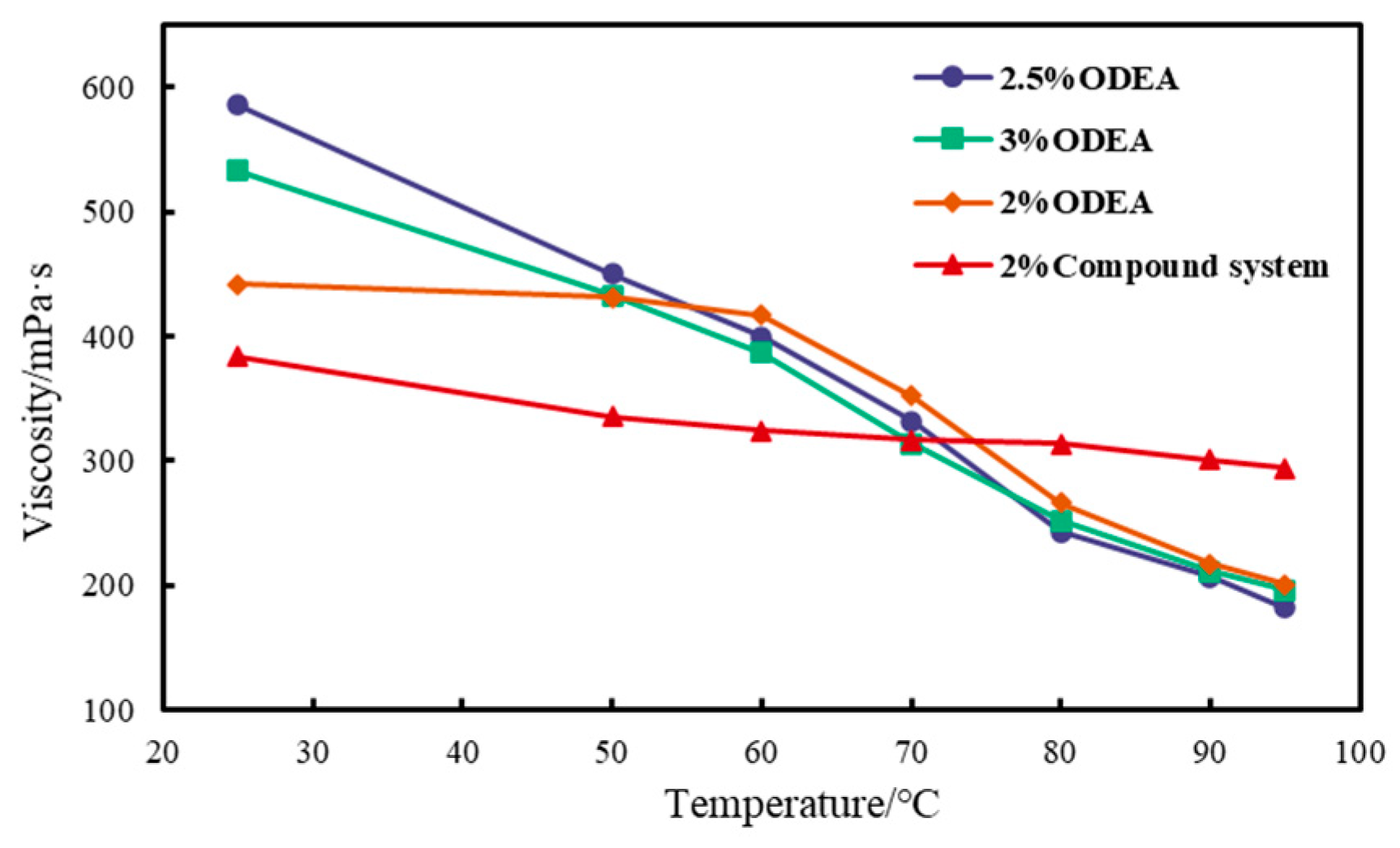
3.2.4. Temperature Resistance Performance
3.2.5. System Compatibility
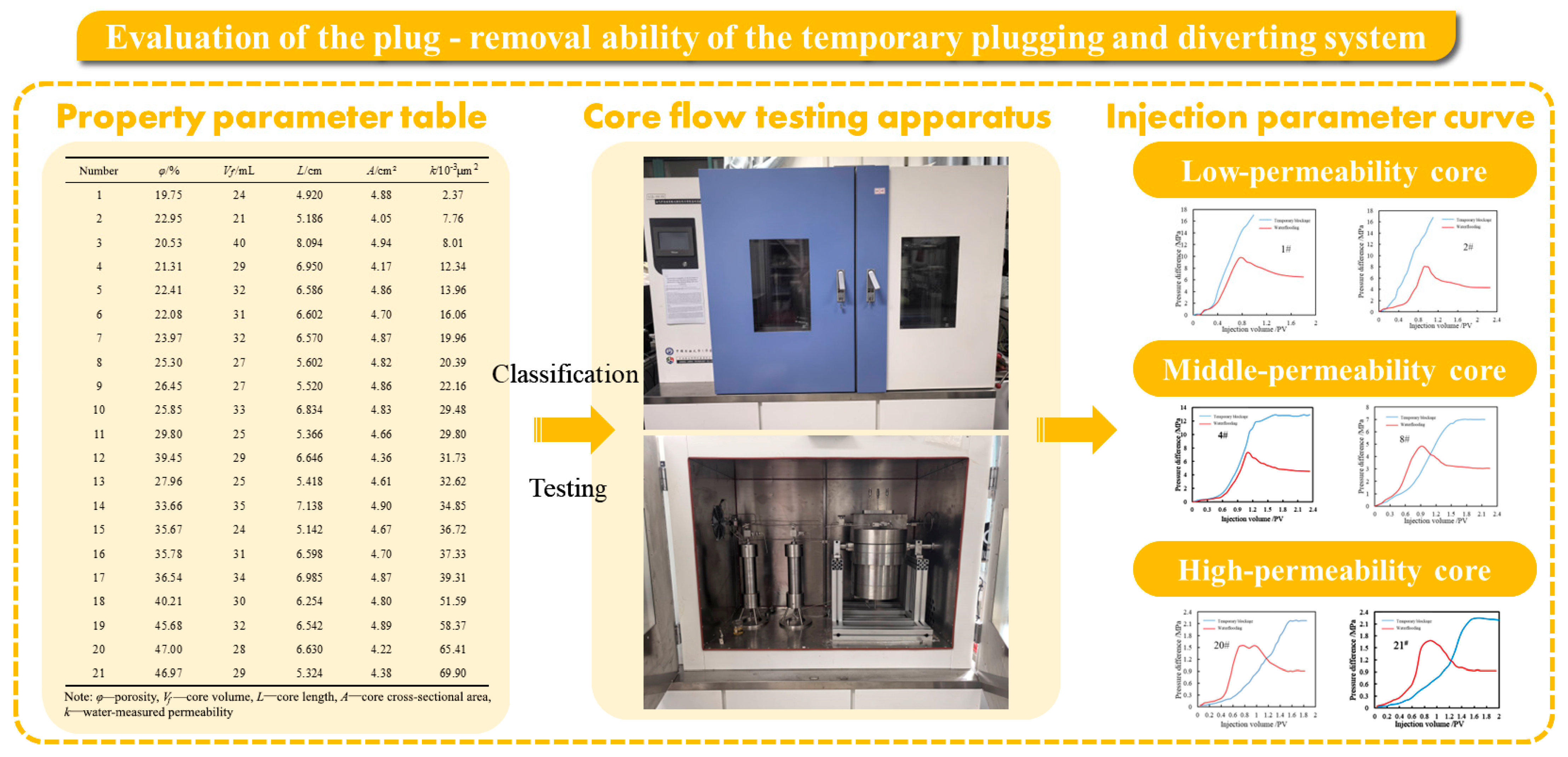
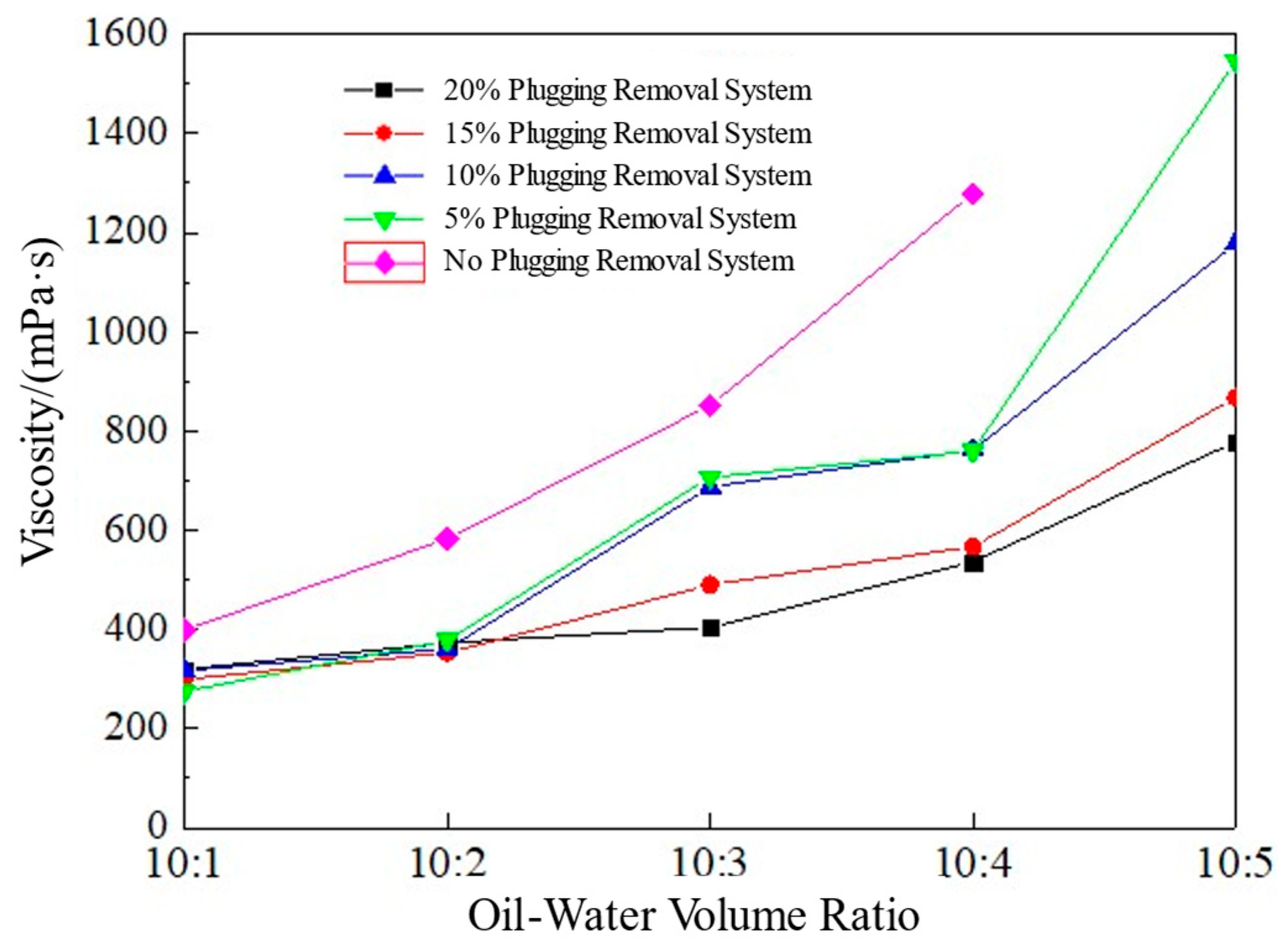
3.3. The Temporary Plugging and Diverting Ability
3.4. Analysis of Self-Thickening Mechanism
4. Conclusions and Prospects
4.1. Conclusions
4.2. Prospects
- This study is a material development and mechanism research at the laboratory scale. The determined formula resistant to high temperature and high salinity and the self-thickening mechanism provide a theoretical basis for field applications. In the follow-up, pilot trials will be carried out for the large-scale injection process to optimize the construction parameters, such as the dynamic ratio of emulsifiers and the matching relationship between the injection rate and the formation permeability, and they will further verify the temporary plugging effect under the actual reservoir conditions.
- To initiate the process, during the pilot test stage, typical high-temperature and high-salinity blocks will be selected to conduct small-scale well group tests, aiming to verify the compatibility of the emulsion with formation fluids (e.g., crude oil compatibility and clay mineral reactivity) and optimize injection process parameters. Subsequently, in the process optimization stage, downhole pressure monitoring data will be integrated to establish a “permeability–droplet size–injection pressure” matching model and develop dynamic adjustment algorithms, enabling precise temporary plugging of different permeability intervals. Finally, in the large-scale application stage, the technology will be synergized with acidization, fracturing, and other techniques to form an integrated “emulsion temporary plugging and diverting + composite stimulation” process system. By leveraging the self-thickening property of the emulsion, deep interlayer temporary plugging will be achieved to enhance the stimulation efficiency of low-permeability layers.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Fan, Y.; Zhou, C.; Luo, Z.; Chen, W.; He, T.; Fang, H.; Fu, Y. Research and Application of Segmented Acid Fracturing by Temporary Plugging in Ultradeep Carbonate Reservoirs. ACS Omega 2021, 6, 28620–28629. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pu, C.; Wang, K.; Li, X.; Zhang, N.; Yan, D.; Huang, F. Investigation of the Oil-Soluble Particulate Temporary Plugging Agent-Assisted Water Huff ‘n’ Puff Enhanced Oil Recovery in Tight Oil Reservoirs. SPE J. 2023, 28, 2346–2364. [Google Scholar] [CrossRef]
- Chen, X.; Lu, X.; Liu, P.; Du, J.; Liang, C.; Huang, Q.; Zhu, D.; Liu, F. A critical review of key points in temporary plugging fracturing: Materials, injection, temporary plugging, and design. Geoenergy Sci. Eng. 2024, 240, 212981. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Peng, J.; Han, H.; Wang, Y.; Lv, Z. Temporary Plugging Agent Evaluation Technology and Its Applications in Shale Reservoirs in the Sichuan Basin. Processes 2023, 11, 2799. [Google Scholar] [CrossRef]
- Harrison, N.W. Diverting Agents-History and Application. J. Pet. Technol. 1972, 24, 593–598. [Google Scholar] [CrossRef]
- Obino, V.; Yadav, U. Application of Polymer Based Nanocomposites for Water Shutoff—A Review. Fuels 2021, 2, 304–322. [Google Scholar] [CrossRef]
- Hao, Y.; Zheng, X.; Fan, W.; Chen, S.; Ye, Z.; Tai, L. Research on Temperature-Resistant Polymer-Based Nano-SiO2 Composite Sealing Agent. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4729–4741. [Google Scholar] [CrossRef]
- Yao, L.; Quan, X.; Zhang, Y.; Huang, S.; Feng, Q.; Zhang, X. Preparation and Performance Evaluation of High-Temperature Polymer Nano-Plugging Agents for Water-Based Drilling Fluids Systems Applicable to Unconventional Reservoirs. Polymers 2025, 17, 588. [Google Scholar] [CrossRef]
- Navratil, M.; Sovak, M.; Mitchell, M.S. Formation Blocking Agents: Applicability in Water—And Steamflooding. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Francisco, CA, USA, 5–8 October 1983. [Google Scholar]
- Smith, C.L.; Anderson, J.L.; Roberts, P.G. New Diverting Techniques for Acidizing and Fracturing. In Proceedings of the SPE California Regional Meeting, San Francisco, CA, USA, 6 November 1969. [Google Scholar]
- Yan, Y.-L.; Xi, Q.; Una, C.-C.; He, B.-C.; Wu, C.-S.; Dou, L.-L. A novel acidizing technology in carbonate reservoir: In-Situ formation of CO2 foamed acid and its self-diversion. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123787. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Pang, Z.; Wu, C.; Gao, M. Pore-Scale Experiment on Blocking Characteristics and EOR Mechanisms of Nitrogen Foam for Heavy Oil: A 2D Visualized Study. Energy Fuels 2016, 30, 9106–9113. [Google Scholar] [CrossRef]
- Solbakken, J.S. Status of Foam as a Liquid Blocking Agent in Porous Media: A Review. Energies 2023, 16, 5063. [Google Scholar] [CrossRef]
- Church, D.C.; Quisenberry, J.L.; Fox, K.B. Field Evaluation of Gelled Acid for Carbonate Formations. J. Pet. Technol. 1981, 33, 2471–2474. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Samuel, M.; Card, R.J.; Nelson, E.B.; Brown, J.E.; Vinod, P.S.; Temple, H.L.; Qu, Q.; Fu, D.K. Polymer-Free Fluid for Hydraulic Fracturing. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Chang, F.; Qu, Q.; Frenier, W. A Novel Self-Diverting-Acid Developed for Matrix Stimulation of Carbonate Reservoirs. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar]
- van Santvoort, J.; Golombok, M. Viscoelastic surfactants for diversion control in oil recovery. J. Pet. Sci. Eng. 2015, 135, 671–677. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Mou, J.; Zhou, F.; Shi, Y. Diverting mechanism of viscoelastic surfactant-based self-diverting acid and its simulation. J. Pet. Sci. Eng. 2013, 105, 91–99. [Google Scholar] [CrossRef]
- Crowe, C.W.; Miller, B.D. New, Low Viscosity Acid in Oil Emulsions. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Houston, TX, USA, 6–9 October 1974. [Google Scholar]
- Buijse, M.A.; van Domelen, M.S. Novel Application of Emulsified Acids to Matrix Stimulation of Heterogeneous Formations. SPE Prod. Facil. 2000, 15, 208–213. [Google Scholar] [CrossRef]
- Sayed, M.A.; Nasr-El-Din, H.A. Acid Treatments in High Temperature Dolomitic Carbonate Reservoirs Using Emulsified Acids: A Coreflood Study. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 23–26 March 2013. [Google Scholar]
- Xu, H.; Zhang, L.; Wang, J.; Jiang, H. Evaluation of Self-Degradation and Plugging Performance of Temperature-Controlled Degradable Polymer Temporary Plugging Agent. Polymers 2023, 15, 3732. [Google Scholar] [CrossRef]
- Yang, F.; Liu, J.; Ji, R.; Yu, X.; Yang, H.; Su, G. Degradable Gel for Temporary Plugging in High Temperature Reservoir and Its Properties. Gels 2024, 10, 445. [Google Scholar] [CrossRef]
- Bera, B.; Khazal, R.; Schroën, K. Coalescence dynamics in oil-in-water emulsions at elevated temperatures. Sci. Rep. 2021, 11, 10990. [Google Scholar] [CrossRef]
- Mahdavi, M.S.; Tajikmansori, A.; Saeedi Dehaghani, A.H.; Seyed Mousavi, S.A.H. The Synergic Effect of Brine Salinity and Dispersed Clay Particles on Water-in-Heavy Oil Emulsion: Insight into Asphaltene Structure and Emulsion Stability. SPE J. 2024, 29, 7163–7179. [Google Scholar] [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Liu, P.; Li, W.; Wei, F.; Hu, F.; Zhu, X.; Jia, Z. Preparation of a fluid diversion agent for profile control in elevated temperature and high salinity reservoirs. J. Appl. Polym. Sci. 2021, 138, 50875. [Google Scholar] [CrossRef]
- Skauge, T.; Ormehaug, P.A.; Alsumaiti, A.; Masalmeh, S.; Skauge, A. Polymer Stability at Harsh Temperature and Salinity Conditions. In Proceedings of the SPE Conference at Oman Petroleum & Energy Show, Muscat, Oman, 21–23 March 2022. [Google Scholar]
- Dupuis, G.; Antignard, S.; Giovannetti, B.; Gaillard, N.; Jouenne, S.; Bourdarot, G.; Morel, D.; Zaitoun, A. A New Thermally Stable Synthetic Polymer for Harsh Conditions of Middle East Reservoirs. Part I. Thermal Stability and Injection in Carbonate Cores. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar]
- Muller, G.; Laine, J.P.; Fenyo, J.C. High-molecular-weight hydrolyzed polyacrylamides. I. Characterization. Effect of salts on the conformational properties. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 659–672. [Google Scholar] [CrossRef]
- Ge, J.; Sun, X.; Liu, R.; Wang, Z.; Wang, L. Emulsion Acid Diversion Agents for Oil Wells Containing Bottom Water with High Temperature and High Salinity. ACS Omega 2020, 5, 29609–29617. [Google Scholar] [CrossRef] [PubMed]
- Bist, N.; Nair, A.; Yadav, K.; Sircar, A. Diverting agents in the oil and gas industry: A comprehensive analysis of their origins, types, and applications. Pet. Res. 2024, 9, 72–84. [Google Scholar] [CrossRef]
- Zare, Y.; Park, S.P.; Rhee, K.Y. Analysis of complex viscosity and shear thinning behavior in poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes biosensor based on Carreau–Yasuda model. Results Phys. 2019, 13, 102245. [Google Scholar] [CrossRef]
- Fournier, C.O.; Fradette, L.; Tanguy, P.A. Effect of dispersed phase viscosity on solid-stabilized emulsions. Chem. Eng. Res. Des. 2009, 87, 499–506. [Google Scholar] [CrossRef]
- Wei, W.; Cai, J.; Xiao, J.; Meng, Q.; Xiao, B.; Han, Q. Kozeny-Carman constant of porous media: Insights from fractal-capillary imbibition theory. Fuel 2018, 234, 1373–1379. [Google Scholar] [CrossRef]



| Instrument Name | Manufacturer | Production Location |
|---|---|---|
| MY-P13-2S magnetic stirrer | Shanghai Yangyingpu Instrumentation Manufacturing Co., Ltd. | Shanghai, China |
| Digital constant temperature oil bath pot | Jiangsu Jintan Jincheng Guosheng Experimental Instrument Factory | Jintan, Jiangsu, China |
| BSA423 precision electronic balance | Sartorius (Beijing) Co., Ltd. | Beijing, China |
| 101-2A electric heating constant temperature oven | Shanghai Boxun Medical & Biological Instrument Co., Ltd. | Shanghai, China |
| Mastersizer 3000 laser particle size analyzer | Malvern Panalytical Co., Ltd. | Malvern, Worcestershire, United Kingdom |
| SNB-2 digital viscometer | Shanghai Precision Instrumentation Co., Ltd. | Shanghai, China |
| Instrument Name | Purity | Manufacturer | Production Location |
|---|---|---|---|
| Sodium dodecylbenzene sulfonate (SDBS) | AR | Tianjin Bodi Chemical Industry Co., Ltd. | Tianjin, China |
| Polyisobutylene succinimide (T154) | AR | Tianjin Bodi Chemical Industry Co., Ltd. | Tianjin, China |
| Oleic acid diethanolamide (ODEA) | AR | Yousuo Chemical Industry | Linyi, Shandong, China |
| NaCl | CP | Sinopharm Chemical Reagent Co., Ltd. | Shanghai, China |
| Diesel | Industrial goods | China Petroleum & Chemical Corporation (Sinopec) | Xi’an, Shaanxi, China |
| Deionized water | - | Self-prepared in laboratory | Qingdao, Shandong, China |
| Levels | Factors | ||
|---|---|---|---|
| ODEA/% | T154/% | SDBS/% | |
| 1 | 2 | 0.2 | 0.1 |
| 2 | 2.5 | 0.4 | 0.2 |
| 3 | 3 | 0.6 | 0.3 |
| 4 | 3.5 | 0.8 | 0.4 |
| Number | Porosity (φ)/% | Core Volume (Vf)/mL | Core Length (L)/cm | Core Cross-Sectional Area (A)/cm2 | Water-Measured Permeability (k)/10−3 μm2 |
|---|---|---|---|---|---|
| 1 | 19.75 | 24 | 4.920 | 4.88 | 2.37 |
| 2 | 22.95 | 21 | 5.186 | 4.05 | 7.76 |
| 3 | 20.53 | 40 | 8.094 | 4.94 | 8.01 |
| 4 | 21.31 | 29 | 6.950 | 4.17 | 12.34 |
| 5 | 22.41 | 32 | 6.586 | 4.86 | 13.96 |
| 6 | 22.08 | 31 | 6.602 | 4.70 | 16.06 |
| 7 | 23.97 | 32 | 6.570 | 4.87 | 19.96 |
| 8 | 25.30 | 27 | 5.602 | 4.82 | 20.39 |
| 9 | 26.45 | 27 | 5.520 | 4.86 | 22.16 |
| 10 | 25.85 | 33 | 6.834 | 4.83 | 29.48 |
| 11 | 29.80 | 25 | 5.366 | 4.66 | 29.80 |
| 12 | 39.45 | 29 | 6.646 | 4.36 | 31.73 |
| 13 | 27.96 | 25 | 5.418 | 4.61 | 32.62 |
| 14 | 33.66 | 35 | 7.138 | 4.90 | 34.85 |
| 15 | 35.67 | 24 | 5.142 | 4.67 | 36.72 |
| 16 | 35.78 | 31 | 6.598 | 4.70 | 37.33 |
| 17 | 36.54 | 34 | 6.985 | 4.87 | 39.31 |
| 18 | 40.21 | 30 | 6.254 | 4.80 | 51.59 |
| 19 | 45.68 | 32 | 6.542 | 4.89 | 58.37 |
| 20 | 47.00 | 28 | 6.630 | 4.22 | 65.41 |
| 21 | 46.97 | 29 | 5.324 | 4.38 | 69.90 |
| Content/% | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 |
|---|---|---|---|---|---|---|---|---|---|
| ODEA separation rate/% | 1.20 | 1.03 | 1.12 | 0.98 | 1.01 | 0.43 | 0.67 | 0.98 | 0.65 |
| T154 separation rate/% | 15.35 | 14.41 | 12.04 | 13.04 | 13.21 | 14.03 | 11.95 | 12.47 | 13.02 |
| SDBS separation rate/% | 11.03 | 10.45 | 10.06 | 9.87 | 10.01 | 8.78 | 9.05 | 9.12 | 8.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Qi, N.; Zhao, L.; Li, X.; Li, Z. Development of a Water-Sensitive Self-Thickening Emulsion Temporary Plugging Diverting Agent for High-Temperature and High-Salinity Reservoirs. Polymers 2025, 17, 1543. https://doi.org/10.3390/polym17111543
Liang C, Qi N, Zhao L, Li X, Li Z. Development of a Water-Sensitive Self-Thickening Emulsion Temporary Plugging Diverting Agent for High-Temperature and High-Salinity Reservoirs. Polymers. 2025; 17(11):1543. https://doi.org/10.3390/polym17111543
Chicago/Turabian StyleLiang, Chong, Ning Qi, Liqiang Zhao, Xuesong Li, and Zhenliang Li. 2025. "Development of a Water-Sensitive Self-Thickening Emulsion Temporary Plugging Diverting Agent for High-Temperature and High-Salinity Reservoirs" Polymers 17, no. 11: 1543. https://doi.org/10.3390/polym17111543
APA StyleLiang, C., Qi, N., Zhao, L., Li, X., & Li, Z. (2025). Development of a Water-Sensitive Self-Thickening Emulsion Temporary Plugging Diverting Agent for High-Temperature and High-Salinity Reservoirs. Polymers, 17(11), 1543. https://doi.org/10.3390/polym17111543






