Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fiber Meshes Preparation
2.3. Hydrogel Preparation
2.4. Characterizations of the Hydrogels
2.4.1. Structural Properties by XRD
2.4.2. Chemical Analysis by FTIR
2.4.3. Thermal Stability by TGA
2.4.4. Morphological Analysis by SEM
2.4.5. Surface Roughness
2.4.6. Mechanical Properties
2.4.7. Contact Angle Measurements
2.4.8. Water Retention Analysis
2.4.9. Cell Growth Tests
3. Results and Discussions
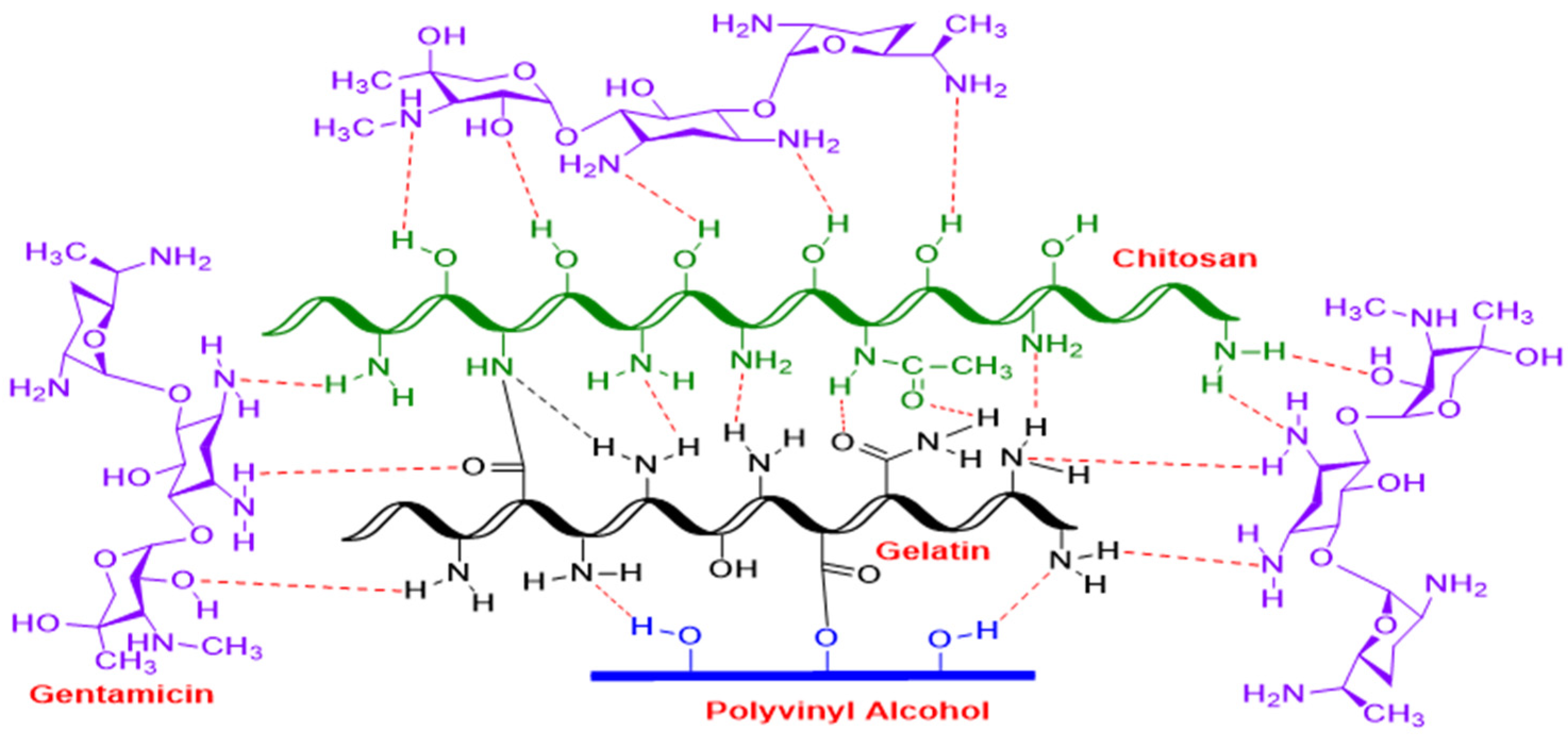
3.1. Hypothetical Reaction Mechanism of PVA/GL/CH Hydrogel with Gentamicin Sulfate as Crosslinker
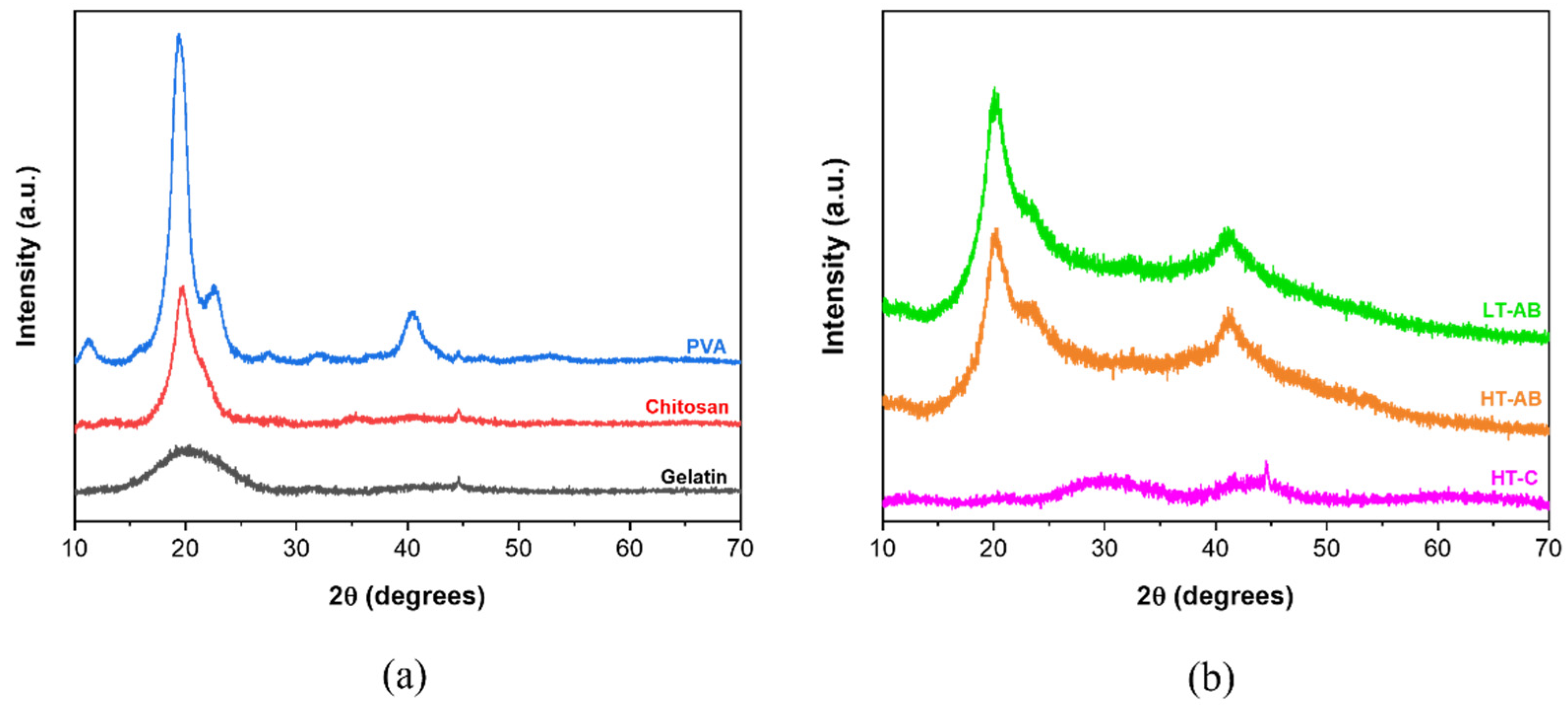
3.2. Crystallographic Analysis by X-Ray-Diffraction (XRD)
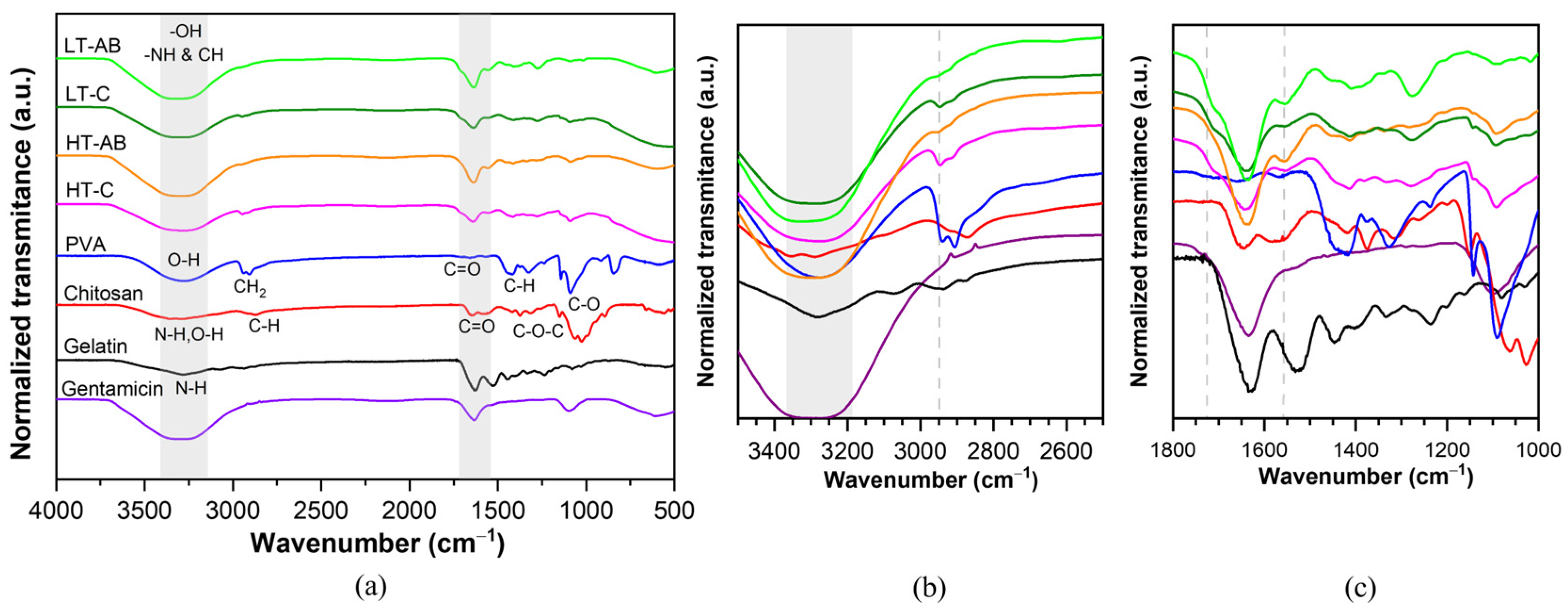
3.3. Fourier-Transform Infrared Spectroscopy Measurements
3.4. Thermal Stability Analysis
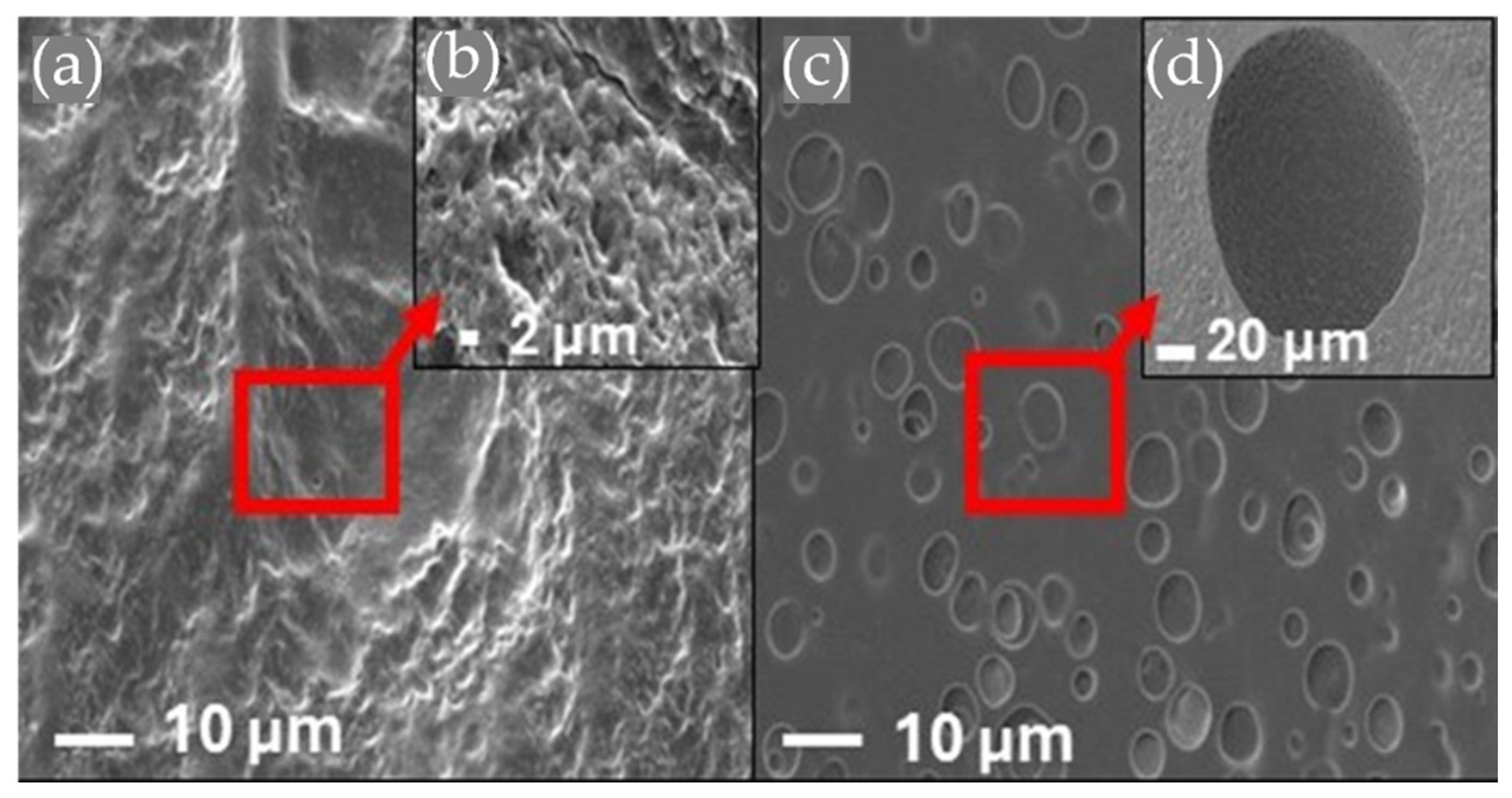
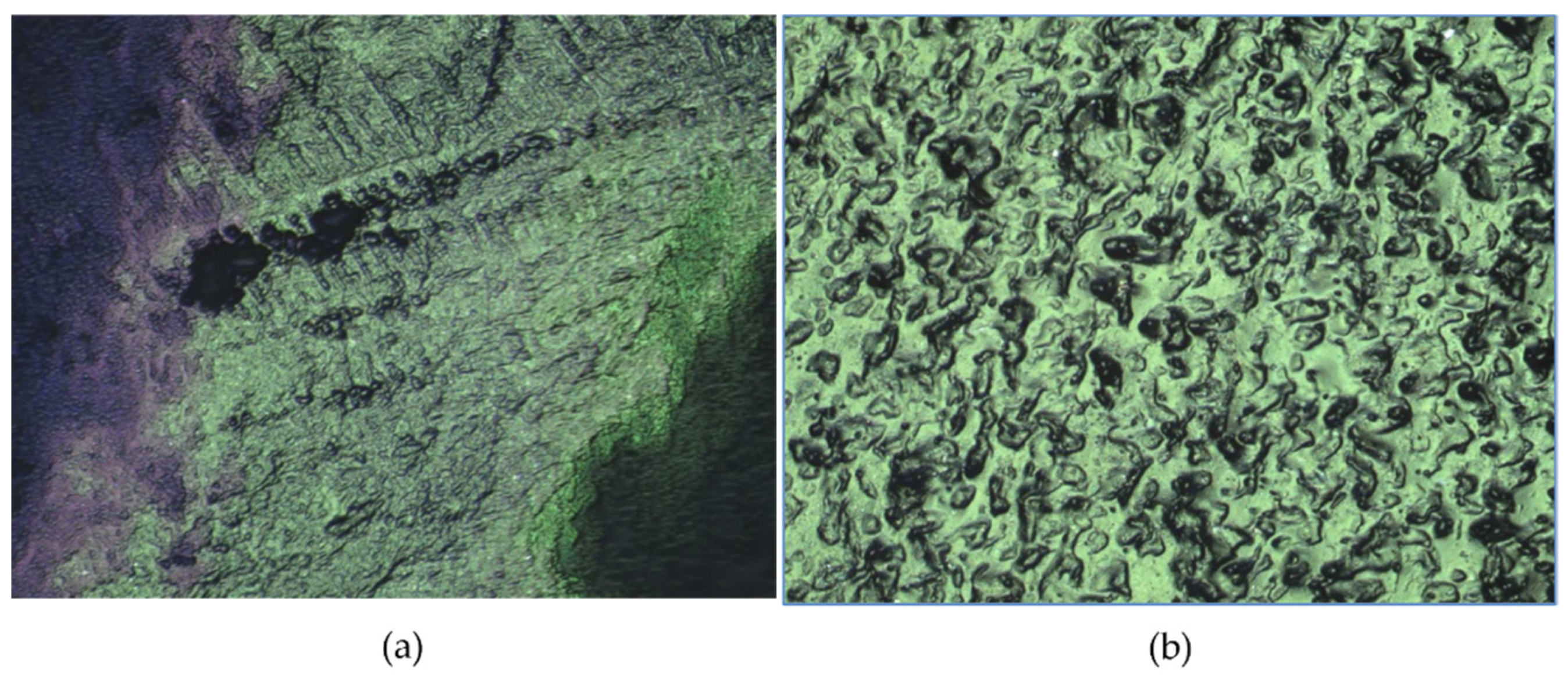
3.5. Morphology by Scanning Electron Microscopy
3.6. Hydrogels’ Surface Roughness
3.7. Mechanical Tests
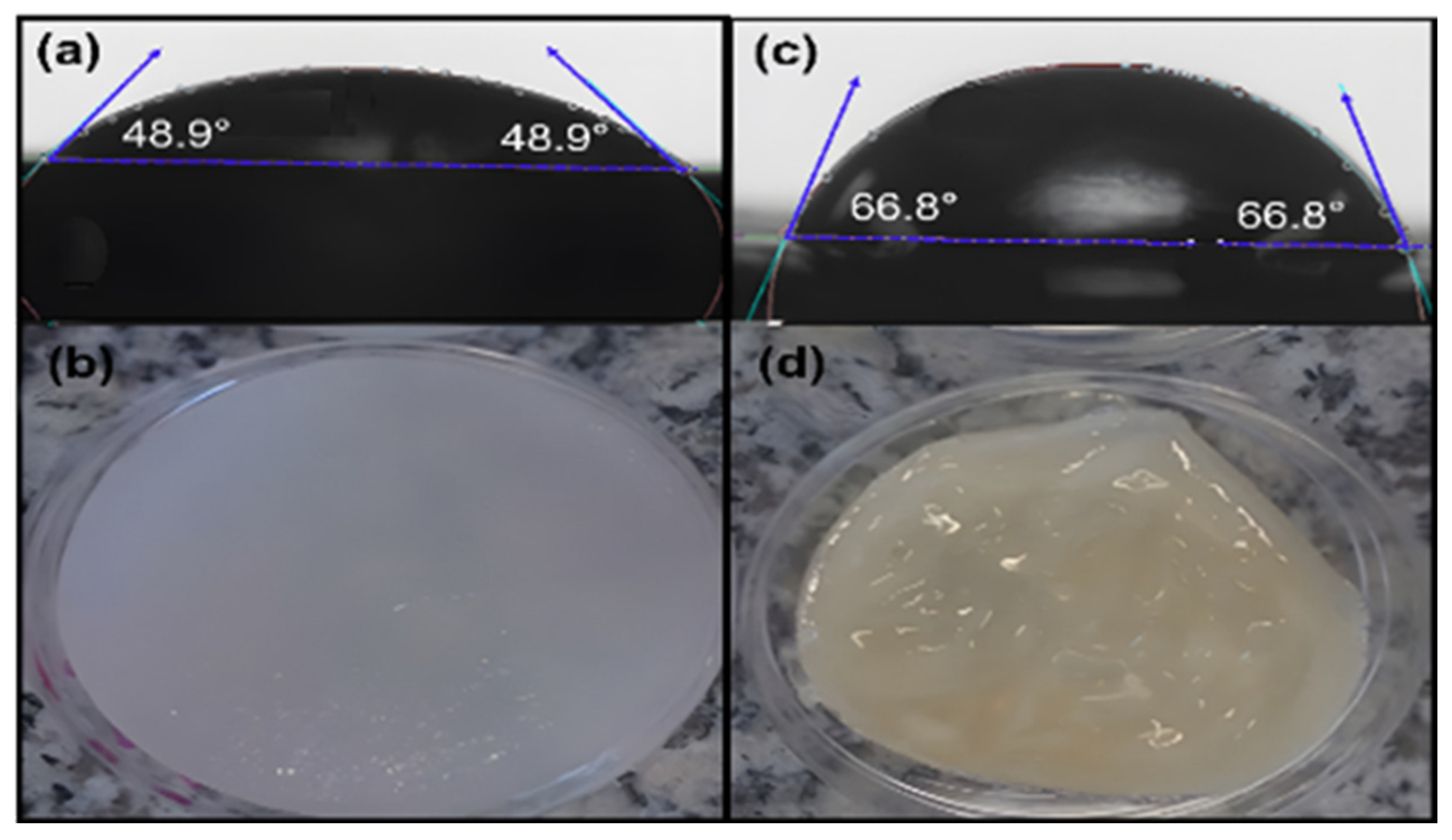
3.8. Contact Angle Tests
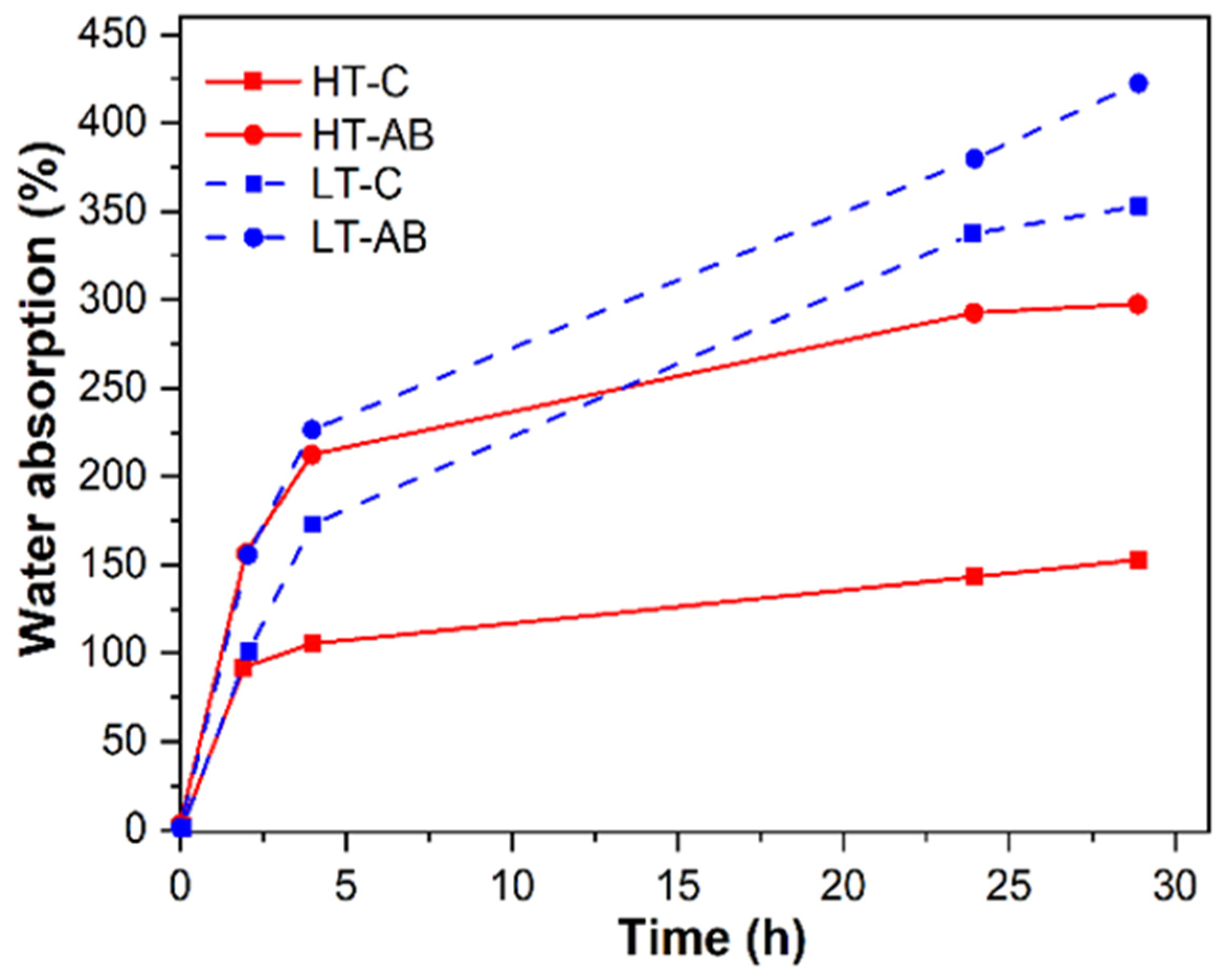
3.9. Hydrogels Water Retention Tests
3.10. Cell Growth of Hydrogels
4. Constitutive Material Model
4.1. Material Model Formulation
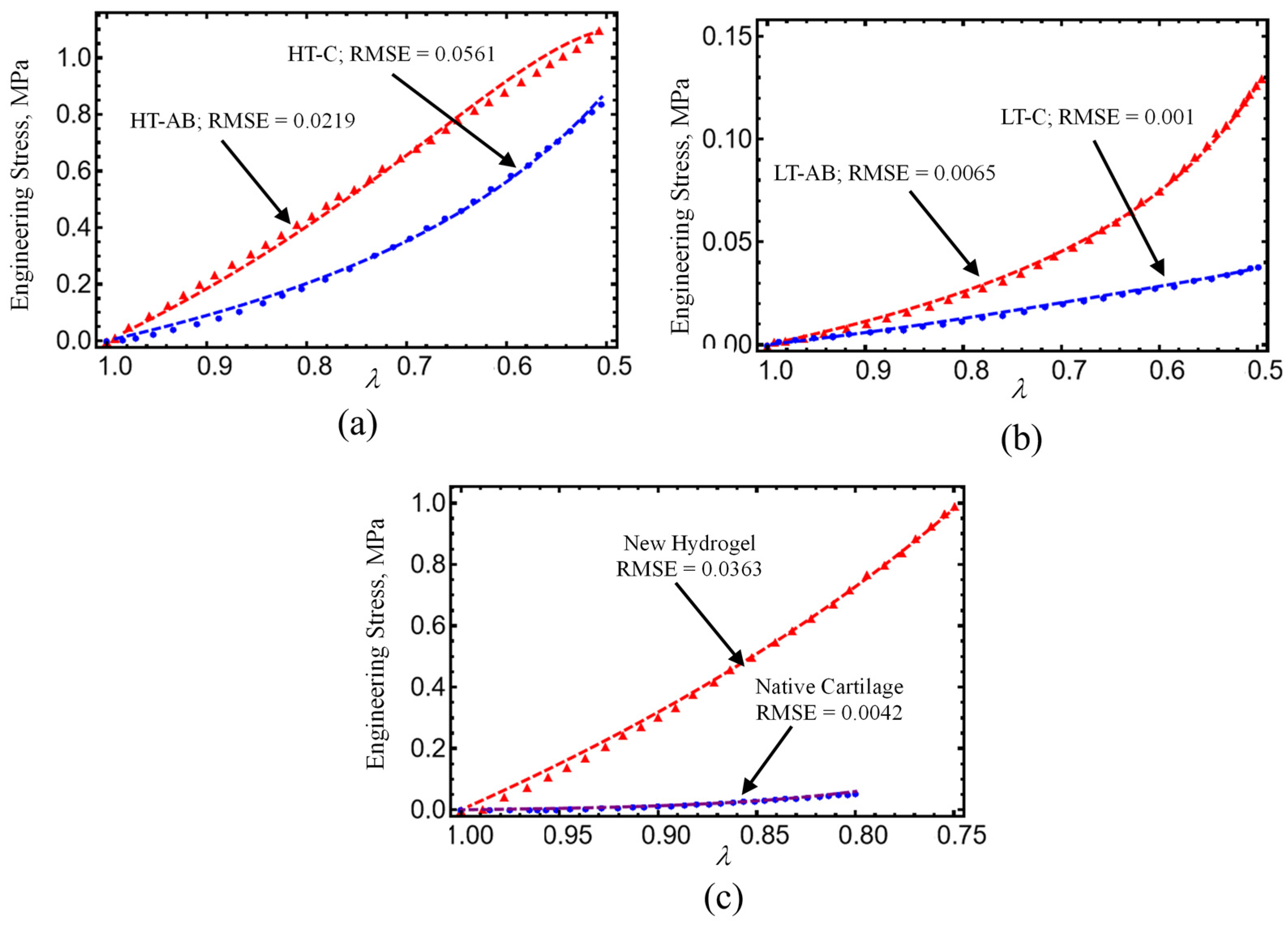
4.2. Comparison with Experimental Data
5. Conclusions
- Hydrogels crosslinked with GS exhibited improved tensile and compressive performance. The HT-AB samples achieved an ultimate tensile strength of 1.768 MPa and a compressive strength of 1.196 MPa, compared with 1.389 MPa and 0.921 MPa in non-GS (HT-C) samples. The LT-AB hydrogels showed a 49.2% increase in tensile strength and a 244.75% increase in compressive strength relative to their LT-C counterparts.
- FTIR spectroscopy revealed changes near 3000 cm−1, indicating hydrogen bonding between GS and polymer components. An additional absorption band around 3325 cm−1 further supported interactions with hydroxyl and amine groups. Thermal analysis showed enhanced stability in GS-crosslinked hydrogels, with maximum decomposition temperatures increasing to 380.2 °C and 445.3 °C in HT-AB samples, compared with 312.7 °C in HT-C samples.
- SEM imaging confirmed uniform PCL fiber distribution and integration into the hydrogel matrix. Surface roughness values of 2.54 µm (HT-AB) and 2.74 µm (LT-AB) closely matched the 1–6 µm range of native articular cartilage. Fiber inclusion did not compromise water retention, and LT-AB samples retained water more effectively over 29 h than other formulations.
- XRD measurements showed that the gelation method does not influence the crystallographic properties of HT- and LT-hydrogels.
- A non-Gaussian strain energy density model accurately reproduced the hydrogel’s mechanical response under uniaxial loading/unloading conditions. The predicted hydrogel shear modulus values closely matched the human cartilage values previously reported in the literature.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Jiang, Z.; Zou, X.; You, X.; Cai, Z.; Huang, J. Advancements in Tissue Engineering for Articular Cartilage Regeneration. Heliyon 2024, 10, e25400. [Google Scholar] [CrossRef] [PubMed]
- Zylinska, B.; Sobczynska-Rak, A.; Lisiecka, U.; Stodolak-Zych, E.; Jarosz, L.; Szponder, T. Structure and Pathologies of Articular Cartilage. In Vivo 2021, 35, 1355–1363. [Google Scholar] [CrossRef]
- Cederlund, A.A.; Aspden, R.M. Walking on Water: Revisiting the Role of Water in Articular Cartilage Biomechanics in Relation to Tissue Engineering and Regenerative Medicine. J. R. Soc. Interface 2022, 19, 20220364. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yung, P.; Lu, G.; Liu, Y.; Ding, C.; Mao, C.; Li, Z.A.; Tuan, R.S. Musculoskeletal Organs-on-Chips: An Emerging Platform for Studying the Nanotechnology–Biology Interface. Adv. Mater. 2024, 37, 2401334. [Google Scholar] [CrossRef] [PubMed]
- Beckett, L.E.; Lewis, J.T.; Tonge, T.K.; Korley, L.S.T.J. Enhancement of the Mechanical Properties of Hydrogels with Continuous Fibrous Reinforcement. ACS Biomater. Sci. Eng. 2020, 6, 5453–5473. [Google Scholar] [CrossRef]
- Newsroom American Joint Replacement Registry Releases 2020 Annual Report. 2020. Available online: https://www.aaos.org/aaos-home/newsroom/press-releases/american-joint-replacement-registry-releases-2020-annual-report/ (accessed on 12 May 2025).
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, Regional, and National Burden of Osteoarthritis, 1990-2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A Review of Biomimetic Scaffolds for Bone Regeneration: Toward a Cell-Free Strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for Bone-Tissue Engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ye, H.; Wu, D. Recent Advances on Polymeric Hydrogels as Wound Dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int. J Mol. Sci. 2023, 24, 9841. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. Articular Cartilage Repair Biomaterials: Strategies and Applications. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Tsumaki, N. Regeneration of Joint Surface Defects by Transplantation of Allogeneic Cartilage: Application of IPS Cell-Derived Cartilage and Immunogenicity. Inflamm. Regen. 2023, 43, 56. [Google Scholar] [CrossRef]
- Koukos, C.; Giotis, D.; Kotsapas, M.; Kapetanakis, S.; Saseendar, S.; Kyriakidis, T. Osteochondral Allograft Transplantation for a Large Focal Osteochondral Defect of the Lateral Femoral Condyle: Technical Note and Case Report. J. Orthop. Case Rep. 2025, 15, 199–204. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D Smart Scaffolds Using Natural, Synthetic and Hybrid Derived Polymers for Skin Regenerative Applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Shabbir Ali, A.; Gul, R.; Usman, H.; Naseer, F. Applications of Natural Polymers in Regenerative Medicine and Tissue Engineering. Pharm. Commun. 2023, 2, 47–61. [Google Scholar] [CrossRef]
- Zhao, F.; Xiong, Y.; Ito, K.; van Rietbergen, B.; Hofmann, S. Porous Geometry Guided Micro-Mechanical Environment Within Scaffolds for Cell Mechanobiology Study in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 736489. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Rodriguez-Cabello, J.C.; Gonzalez De Torre, I.; González-Pérez, M.; González-Pérez, F.; Montequi, I. Fibrous Scaffolds From Elastin-Based Materials. Front. Bioeng. Biotechnol. 2021, 9, 652384. [Google Scholar] [CrossRef]
- Kołakowska, A.; Gadomska-Gajadhur, A.; Ruśkowski, P. Biomimetic Scaffolds Based on Chitosan in Bone Regeneration. A Review. Chem. Process Eng. 2022, 43, 305–330. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Cellulose-Based Composites as Scaffolds for Tissue Engineering: Recent Advances. Molecules 2022, 27, 8830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Su, W.T.; Liu, C.Y.; Huang, C.C. Highly Organized Porous Gelatin-Based Scaffold by Microfluidic 3D-Foaming Technology and Dynamic Culture for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 8449. [Google Scholar] [CrossRef] [PubMed]
- Humaira; Raza Bukhari, S.A.; Shakir, H.A.; Khan, M.; Saeed, S.; Ahmad, I.; Muzammil, K.; Franco, M.; Irfan, M.; Li, K. Hyaluronic Acid-Based Nanofibers: Electrospun Synthesis and Their Medical Applications; Recent Developments and Future Perspective. Front. Chem. 2022, 10, 1092123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, T.; Tao, M.; Wang, Y.; Yao, X.; Zhu, C.; Xin, F.; Jiang, M. Development of Recombinant Human Collagen-Based Porous Scaffolds for Skin Tissue Engineering: Enhanced Mechanical Strength and Biocompatibility. Polymers 2025, 17, 303. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Yang, Y. A Review of Sodium Alginate-Based Hydrogels: Structure, Mechanisms, Applications, and Perspectives. Int. J. Biol. Macromol. 2025, 292, 139151. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Yang, Y.; Zhang, D.; Hao, G. Multifunctional Natural Starch-Based Hydrogels: Critical Characteristics, Formation Mechanisms, Various Applications, Future Perspectives. Carbohydr. Polym. 2025, 357, 123458. [Google Scholar] [CrossRef]
- Kundak, H.; Bilisik, K. Development of Three-Dimensional (3D) Biodegradable Polyglycolic Acid Fiber (PGA) Preforms for Scaffold Applications: Experimental Patterning and Fiber Volume Fraction-Porosity Modeling Study. Polymers 2023, 15, 2083. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Z.; Liu, K.; Ma, C.; Zhang, G. Cartilage-Inspired Smart Anti-Impact Gel with Highly Stable and Tailored Properties. Cell Rep. Phys. Sci. 2023, 4, 101289. [Google Scholar] [CrossRef]
- Chen, Y.; Etxabide, A.; Seyfoddin, A.; Ramezani, M. Fabrication and Characterisation of Poly(Vinyl Alcohol)/Chitosan Scaffolds for Tissue Engineering Applications. Mater Today Proc. 2023. [Google Scholar] [CrossRef]
- Aitchison, A.H.; Allen, N.B.; Mitra, K.; Abar, B.; O’Neill, C.N.; Bagheri, K.; Anastasio, A.T.; Adams, S.B. Tunable Alginate-Polyvinyl Alcohol Bioinks for 3D Printing in Cartilage Tissue Engineering. Gels 2024, 10, 829. [Google Scholar] [CrossRef]
- Gonella, S.; Domingues, M.F.; Miguel, F.; Moura, C.S.; Rodrigues, C.A.V.; Ferreira, F.C.; Silva, J.C. Fabrication and Characterization of Porous PEGDA Hydrogels for Articular Cartilage Regeneration. Gels 2024, 10, 422. [Google Scholar] [CrossRef]
- Savin, G.; Sastourne-Array, O.; Caillol, S.; Bethry, A.; Assor, M.; David, G.; Nottelet, B. Evaluation of Porous (Poly(Lactide-Co-Glycolide)-Co-(ϵ-Caprolactone)) Polyurethane for Use in Orthopedic Scaffolds Molecules Evaluation of Porous (Poly(Lactide-Co-Glycolide)-Co-(ε-Caprolactone)) Polyurethane for Use in Orthopedic Scaffolds. Molecules 2024, 29, 776. [Google Scholar] [CrossRef]
- Ramírez-Ruiz, F.; Núñez-Tapia, I.; Piña-Barba, M.C.; Alvarez-Pérez, M.A.; Guarino, V.; Serrano-Bello, J. Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering 2025, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.; Song, W.; Yu, B.; Liu, H. Rational Design in Functional Hydrogels towards Biotherapeutics. Mater. Des. 2022, 223, 111086. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in Natural Polymer-Based Hydrogels: Synthesis, Applications, and Future Directions in Biomedical and Environmental Fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Petros, S.; Tesfaye, T.; Ayele, M. A Review on Gelatin Based Hydrogels for Medical Textile Applications. J. Eng. 2020, 2020, 866582. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Yom-Tov, O.; Frisman, I.; Seliktar, D.; Bianco-Peled, H. A Novel Method for Hydrogel Nanostructuring. Eur. Polym. J. 2014, 52, 137–145. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Bercea, M. Recent Advances in Poly(Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Cheng, H.; Zhu, J.; Li, X.; Ye, S.; Li, X. Hydrogel-Based Dressings Designed to Facilitate Wound Healing. Mater. Adv. 2023, 5, 1364–1394. [Google Scholar] [CrossRef]
- Kang, Y.; Guan, Y.; Li, S. Innovative Hydrogel Solutions for Articular Cartilage Regeneration: A Comprehensive Review. Int. J. Surg. 2024, 110, 7984–8001. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in Hydrogel Design for Articular Cartilage Regeneration: A Comprehensive Review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Nishat, N.; Jadoun, S.; Ansari, M.Z. Polysaccharide Based Superabsorbent Hydrogels and Their Methods of Synthesis: A Review. Carbohydr. Polym. Technol. Appl. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Wang, J.; Minev, I.R. Electro-Assisted Printing of Soft Hydrogels via Controlled Electrochemical Reactions. Nat. Commun. 2022, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, J.; Zhao, Z.; Wu, M.; Gong, L.; Sun, Y.; Huang, C.; Yan, B.; Zeng, H. Recent Advances in Fabricating Injectable Hydrogels via Tunable Molecular Interactions for Bio-Applications. J. Mater. Chem. B 2023, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Y.; Xiang, Y.; Zhang, S.; Guo, X. Application of Smart Hydrogel Materials in Cartilage Injury Repair: A Systematic Review and Meta-Analysis. J. Biomater. Appl. 2024, 39, 96–116. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, D.; Liu, Y.; Liu, X.; Zhao, H.; Zhou, G.; Liu, S.; Sun, K. Recent Research Progress of Hydrogel on Cartilage. Eur. Polym. J. 2024, 213, 113115. [Google Scholar] [CrossRef]
- Zhang, Z.; Bu, J.; Li, B.; Xuan, H.; Jin, Y.; Yuan, H. Dynamic Double Cross-Linked Self-Healing Polysaccharide Hydrogel Wound Dressing Based on Schiff Base and Thiol-Alkynone Reactions. Int. J. Mol. Sci. 2022, 23, 13817. [Google Scholar] [CrossRef]
- Pruksawan, S.; Lim, J.W.R.; Lee, Y.L.; Lin, Z.; Chee, H.L.; Chong, Y.T.; Chi, H.; Wang, F.K. Enhancing Hydrogel Toughness by Uniform Cross-Linking Using Modified Polyhedral Oligomeric Silsesquioxane. Commun. Mater. 2023, 4, 75. [Google Scholar] [CrossRef]
- Hassan, C.; Peppas, N. Structure and Properties of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable Polyvinyl Alcohol Hydrogels That Can Be Modified with Cell Adhesion Peptides for Use in Tissue Engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Liu, Y.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G.B. Thermal Behavior and Mechanical Properties of Physically Crosslinked PVA/Gelatin Hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of Hydrogels Using Three-Dimensionally Printed Microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- Curley, C.; Hayes, J.C.; Rowan, N.J.; Kennedy, J.E. An Evaluation of the Thermal and Mechanical Properties of a Salt-Modified Polyvinyl Alcohol Hydrogel for a Knee Meniscus Application. J. Mech. Behav. Biomed. Mater. 2014, 40, 13–22. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Królicka, E.; Malina, D. Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(Vinyl Alcohol) Hydrogels. Molecules 2021, 26, 2381. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Rahman Khan, M.M. Progress in Hydrogel Toughening: Addressing Structural and Crosslinking Challenges for Biomedical Applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Xu, Y.; Lin, W.; Peng, Z. A Ternary Heterogeneous Hydrogel with Strength Elements for Resilient, Self-Healing, and Recyclable Epidermal Electronics. npj Flex. Electron. 2022, 6, 51. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Li, P.; Fan, Y. Ternary Hydrogels with Tunable Mechanical and Self-Healing Properties Based on the Synergistic Effects of Multiple Dynamic Bonds. J. Mater. Chem. B 2020, 8, 4660–4671. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and Evaluation of PH-Sensitive Biodegradable Ternary Blended Hydrogel Films (Chitosan/Guar Gum/PVP) for Drug Delivery Application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Carvalho, S.M.; Brito, R.M.d.M.; Capanema, N.S.V.; Duval, I.d.B.; Cardozo, M.E.; Rihs, J.B.R.; Lemos, G.G.M.; Lima, L.C.D.; Reys, M.P.d.; et al. Arginine-Biofunctionalized Ternary Hydrogel Scaffolds of Carboxymethyl Cellulose–Chitosan–Polyvinyl Alcohol to Deliver Cell Therapy for Wound Healing. Gels 2024, 10, 679. [Google Scholar] [CrossRef]
- Kohoolat, G.; Alizadeh, P.; Motesadi Zarandi, F.; Rezaeipour, Y. A Ternary Composite Hydrogel Based on Sodium Alginate, Carboxymethyl Cellulose and Copper-Doped 58S Bioactive Glass Promotes Cutaneous Wound Healing in Vitro and in Vivo. Int. J. Biol. Macromol. 2024, 259, 129260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, X.; Chen, Y.; Huang, C.; Zhong, N.; Hu, Y. Preparation and Characterization of Ternary Polysaccharide Hydrogels Based on Carboxymethyl Cellulose, Carboxymethyl Chitosan, and Carboxymethyl β-Cyclodextrin. Int. J. Biol. Macromol. 2024, 271, 132604. [Google Scholar] [CrossRef]
- López-Velázquez, J.C.; Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Qui-Zapata, J.A.; García-Morales, S.; Navarro-López, D.E.; Luna-Bárcenas, G.; Vassallo-Brigneti, E.C.; García-Carvajal, Z.Y. Gelatin–Chitosan–PVA Hydrogels and Their Application in Agriculture. J. Chem. Technol. Biotechnol. 2019, 94, 3495–3504. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite Hydrogels Based on Gelatin, Chitosan and Polyvinyl Alcohol to Biomedical Applications: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Sánchez-Cardona, Y.; Echeverri-Cuartas, C.E.; López, M.E.L.; Moreno-Castellanos, N. Chitosan/Gelatin/Pva Scaffolds for Beta Pancreatic Cell Culture. Polymers 2021, 13, 2372. [Google Scholar] [CrossRef]
- Taborda, M.I.; Catalan, K.N.; Orellana, N.; Bezjak, D.; Enrione, J.; Acevedo, C.A.; Corrales, T.P. Micropatterned Nanofiber Scaffolds of Salmon Gelatin, Chitosan, and Poly(Vinyl Alcohol) for Muscle Tissue Engineering. ACS Omega 2023, 8, 47883–47896. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Liu, W.; Li, R.; Wang, D.; Zhang, N.; Chen, D.; Lee, S. Polyvinyl Alcohol/Chitosan-Fe3+/Gelatin/Tri-Network Composite Hydrogel Wound Dressing with Effective Hemostasis and Self-Healing Properties. Polymer 2024, 307, 127304. [Google Scholar] [CrossRef]
- Ortega-Sánchez, C.; Melgarejo-Ramírez, Y.; Rodríguez-Rodríguez, R.; Jiménez-Ávalos, J.A.; Giraldo-Gomez, D.M.; Gutiérrez-Gómez, C.; Rodriguez-Campos, J.; Luna-Bárcenas, G.; Velasquillo, C.; Martínez-López, V.; et al. Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture. Polymers 2024, 16, 479. [Google Scholar] [CrossRef]
- Hassan, S.N.; Ahmad, F. The Relevance of Antibiotic Supplements in Mammalian Cell Cultures: Towards a Paradigm Shift. Gulhane Med. J. 2020, 62, 224–230. [Google Scholar] [CrossRef]
- Kilinç, S.; Tunç, T.; Pazarci, Ö.; Sümer, Z. Research into Biocompatibility and Cytotoxicity of Daptomycin, Gentamicin, Vancomycin and Teicoplanin Antibiotics at Common Doses Added to Bone Cement. Jt. Dis. Relat. Surg. 2020, 31, 328–334. [Google Scholar] [CrossRef]
- Liang, F.; Li, X.; Li, X.; Liu, X.; Pan, R.; Su, W.; Li, C.; Liu, K. Preparation and Characterization of a Chitosan-Gentamicin Derivative and Its Effect on Tropical Fruit Preservation. Food Hydrocoll. 2025, 158, 110485. [Google Scholar] [CrossRef]
- Farioli, A.S.; Martinez, M.V.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Antimicrobial Activity of Gentamicin-Loaded Biocomposites Synthesized through Inverse Vulcanization from Soybean and Sunflower Oils. Sustain. Chem. 2024, 5, 229–243. [Google Scholar] [CrossRef]
- Vale de Macedo, G.H.R.; Chagas, V.L.; Cruz dos Santos, M.H.; Costa dos Santos, G.D.; Bazán, J.M.N.; Zagmignan, A.; Carvalho, E.M.; de Cássia Mendonça de Miranda, R.; Teixeira, C.S.; da Silva, L.C.N. Development and Characterization of Alginate-Derived Crosslinked Hydrogel Membranes Incorporated with ConA and Gentamicin for Wound Dressing Applications. Biochem. Eng. J. 2022, 187, 108664. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of Cross-Linked Chitosan Quaternary Ammonium Salt Hydrogel Films Loading Drug of Gentamicin Sulfate for Antibacterial Wound Dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef]
- Cao, B.; Wang, C.; Guo, P.; Zhang, Q.; Wang, C.; Sun, H.; Wen, H.; Chen, X.; Wang, Y.; Wang, Y.; et al. Photo-Crosslinked Enhanced Double-Network Hydrogels Based on Modified Gelatin and Oxidized Sodium Alginate for Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 245, 125528. [Google Scholar] [CrossRef]
- Arshad, J.; Barkat, K.; Ashraf, M.U.; Badshah, S.F.; Ahmad, Z.; Anjum, I.; Shabbir, M.; Mehmood, Y.; Khalid, I.; Malik, N.S.; et al. Preparation and Characterization of Polymeric Cross-Linked Hydrogel Patch for Topical Delivery of Gentamicin. e-Polymers 2023, 23, 20230045. [Google Scholar] [CrossRef]
- Alimin, D.F.; Chuysinuan, P.; Niyompanich, J.; Choipang, C.; Ngamplang, P.; Kornsuthisopon, C.; Supaphol, P. In-Situ-Forming Alginate Hydrogel Embedded with Gentamicin-Loaded Thermosensitive Poly(N-Vinylcaprolactam) Nanogel for Cavity Wound Dressing. J. Drug Deliv. Sci. Technol. 2025, 108, 106871. [Google Scholar] [CrossRef]
- Watson, A.L.; Eckhart, K.E.; Wolf, M.E.; Sydlik, S.A. Hyaluronic Acid-Based Antibacterial Hydrogels for Use as Wound Dressings. ACS Appl. Bio Mater. 2022, 5, 5608–5616. [Google Scholar] [CrossRef]
- Zaske, D.E.; Cipolle, R.J.; Rotschafer, J.C.; Solem, L.D.; Mosier, A.N.R.; Strate, R.G. Gentamicin Pharmacokinetics in 1,640 Patients: Method for Control of Serum Concentrations. Antimicrob. Agents Chemother. 1982, 21, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Sirousazar, M. Mechanism of Gentamicin Sulphate Release in Nanocomposite Hydrogel Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2013, 23, 619–621. [Google Scholar] [CrossRef]
- Khan, M.J.; Zhang, J.; Guo, Q. Covalent/Crystallite Cross-Linked Co-Network Hydrogels: An Efficient and Simple Strategy for Mechanically Strong and Tough Hydrogels. Chem. Eng. J. 2016, 301, 92–102. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M. Influence of Natural and Synthetic Crosslinking Reagents on the Structural and Mechanical Properties of Chitosan-Based Hybrid Hydrogels. Carbohydr. Polym. 2016, 151, 1073–1081. [Google Scholar] [CrossRef]
- Kooshe, M.; Aalipour, H.; Sarraf Shirazi, M.J.; Jebali, A.; Chi, H.; Hamedi, S.; Wang, N.; Li, T.; Moravvej, H. Physically Crosslinked Chitosan/PVA Hydrogels Containing Honey and Allantoin with Long-Term Biocompatibility for Skin Wound Repair: An In Vitro and In Vivo Study. J. Funct. Biomater. 2021, 12, 61. [Google Scholar] [CrossRef]
- Patricia, H.; Nilson, M.R.; Cristina, T.I. Gelatin Poly(Vinyl Alcohol) Based Hydrogel Film-A Potential Biomaterial for Wound Dressing: Experimental Design and Optimization Followed by Rotatable Central Composite Design. J. Biomater. Appl. 2021, 36, 682–700. [Google Scholar] [CrossRef]
- Wang, T.; Xu, B.; Yu, T.; Yu, Y.; Fu, J.; Wang, Y.; Gao, X.; Xue, Z.; Li, R.; Chang, G. PVA/Chitosan-Based Multifunctional Hydrogels Constructed through Multi-Bonding Synergies and Their Application in Flexible Sensors. Carbohydr. Polym. 2025, 350, 123034. [Google Scholar] [CrossRef]
- Utterström, J.; Naeimipour, S.; Selegård, R.; Aili, D. Coiled Coil-Based Therapeutics and Drug Delivery Systems. Adv. Drug Deliv. Rev. 2021, 170, 26–43. [Google Scholar] [CrossRef]
- Momtaz, M.; Momtaz, E.; Mehrgardi, M.A.; Momtaz, F.; Narimani, T.; Poursina, F. Preparation and Characterization of Gelatin/Chitosan Nanocomposite Reinforced by NiO Nanoparticles as an Active Food Packaging. Sci. Rep. 2024, 14, 519. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Yao, L.; Li, Y.; Weng, Y.; Qiu, D. Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold. Polymers 2022, 14, 1400. [Google Scholar] [CrossRef]
- Umadevi, S.; Musharaf, M.; Jenita, J.L. Design and Characterization of Timolol Maleate and Travoprost Hydrogel Drug Delivery System. J. Popul. Ther. Clin. Pharmacol. 2023, 30, e274–e290. [Google Scholar] [CrossRef]
- Cui, L.; Tong, W.; Zhou, H.; Yan, C.; Chen, J.; Xiong, D. PVA-BA/PEG Hydrogel with Bilayer Structure for Biomimetic Articular Cartilage and Investigation of Its Biotribological and Mechanical Properties. J. Mater. Sci. 2021, 56, 3935–3946. [Google Scholar] [CrossRef]
- Kandulna, R.; Choudhary, R.B. Concentration-Dependent Behaviors of ZnO-Reinforced PVA–ZnO Nanocomposites as Electron Transport Materials for OLED Application. Polym. Bull. 2018, 75, 3089–3107. [Google Scholar] [CrossRef]
- El Bahy, G.S.; El-Sayed, E.-S.M.; Mahmoud, A.A.; Gweily, N.M. Preparation and Characterization of Poly Vinyl Alcohol /Gelatin Blends. J. Appl. Sci. Res. 2012, 8, 3544–3551. [Google Scholar]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, Optical, Opto-Thermal and Thermal Properties of ZnS-PVA Nanofluids Synthesized through a Radiolytic Approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, N.; Khartini, W.; Khodir, W.A. In Vitro Evaluation of Crosslinked Polyvinyl Alcohol/Chitosan-Gentamicin Sulfate Electrospun Nanofibers. Malays. J. Chem. 2021, 23, 1–10. [Google Scholar]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Campaña Perilla, A.L.; Gomez-Bolivar, J.; Merroun, M.L.; Joudeh, N.; Saragliadis, A.; Røyne, A.; Linke, D.; Mikheenko, P. Characterization of Bimetallic Pd-Fe Nanoparticles Synthesized in Escherichia coli. ACS Appl. Bio Mater. 2024, 7, 8573–8589. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and Characterization of Chitosan/Gelatin/PVA Hydrogel for Wound Dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Kaviani, A.; Zebarjad, S.M.; Javadpour, S.; Ayatollahi, M.; Bazargan-Lari, R. Fabrication and Characterization of Low-Cost Freeze-Gelated Chitosan/Collagen/Hydroxyapatite Hydrogel Nanocomposite Scaffold. Int. J. Polym. Anal. Charact. 2019, 24, 191–203. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Johansen, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Valcarcel, J. Characterization of Gelatin and Hydrolysates from Valorization of Farmed Salmon Skin By-Products. Polymers 2021, 13, 2828. [Google Scholar] [CrossRef]
- Hermida-Merino, C.; Cabaleiro, D.; Lugo, L.; Valcarcel, J.; Vázquez, J.A.; Bravo, I.; Longo, A.; Salloum-Abou-jaoude, G.; Solano, E.; Gracia-Fernández, C.; et al. Characterization of Tuna Gelatin-Based Hydrogels as a Matrix for Drug Delivery. Gels 2022, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Elerian, A.F.; Mohamed, A.A.A.; Elnaggar, E.M.; Abu-Saied, M.A. Development of Novel Proton Exchange Membranes Based on Cross-Linked Polyvinyl Alcohol (PVA)/5-Sulfosalicylic Acid (SSCA) for Fuel Cell Applications. Discov. Appl. Sci. 2024, 6, 341. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Antor, R.I.; Bithi, A.M.; Nahin, A.M. Variation in Mechanical Properties of Polymer Composites with Reinforcements from Different Animal Origins—A Comprehensive Review. Int. J. Polym. Sci. 2025, 2025, 4184239. [Google Scholar] [CrossRef]
- Osman, M.A.; Alamoush, R.A.; Kushnerev, E.; Seymour, K.G.; Shawcross, S.; Yates, J.M. In-Vitro Phenotypic Response of Human Osteoblasts to Different Degrees of Titanium Surface Roughness. Dent. J. 2022, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yao, Y.; Yim, E.K.F. The Effects of Surface Topography Modification on Hydrogel Properties. APL Bioeng. 2021, 5, 031509. [Google Scholar] [CrossRef]
- Elshazly, T. Characterization of PVA Hydrogels with Regards to Vascular Graft Development; Georgia Institute of Technology: Atlanta, GA, USA, 2004. [Google Scholar]
- Clark, J.M.; Norman, A.G.; Kaab, M.J.; Notzli, H.P. The Surface Contour of Articular Cartilage in an Intact, Loaded Joint. J. Anat. 1999, 195, 45–56. [Google Scholar] [CrossRef]
- Van Der Heide, E.; Morales-Hurtado, M.; De Vries, E.G.; Zeng, L. Mimicking the Surface Properties of Human Skin: Tribo-Mechanical & Adhesive Properties of a Synthetic Stratum Corneum. J. Soc. Biomech. 2017, 43, 5. [Google Scholar]
- Petitjean, N.; Cañadas, P.; Royer, P.; Noël, D.; Le Floc, S. Cartilage Biomechanics: From the Basic Facts to the Challenges of Tissue Engineering. J. Biomed. Mater. Res. 2023, 111, 1067–1089. [Google Scholar] [CrossRef]
- Jurvelin, J.S.; Buschmann, M.D.; Hunziker, E.B. Mechanical Anisotropy of the Human Knee Articular Cartilage in Compression. Proc. Inst. Mech. Eng. H 2003, 217, 215–219. [Google Scholar] [CrossRef]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-Dependent Mechanical Properties of Human Articular Cartilage Obtained by Indentation Measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Villars, M.; Thibaud, S. Viscoelastic Modeling and Quantitative Experimental Characterization of Normal and Osteoarthritic Human Articular Cartilage Using Indentation. J. Mech. Behav. Biomed. Mater. 2013, 24, 41–52. [Google Scholar] [CrossRef]
- Kabir, W.; Di Bella, C.; Choong, P.F.M.; O’Connell, C.D. Assessment of Native Human Articular Cartilage: A Biomechanical Protocol. Cartilage 2021, 13, 427S–437S. [Google Scholar] [CrossRef] [PubMed]
- Nebelung, S.; Post, M.; Raith, S.; Fischer, H.; Knobe, M.; Braun, B.; Prescher, A.; Tingart, M.; Thüring, J.; Bruners, P.; et al. Functional in Situ Assessment of Human Articular Cartilage Using MRI: A Whole-Knee Joint Loading Device. Biomech. Model Mechanobiol. 2017, 16, 1971–1986. [Google Scholar] [CrossRef] [PubMed]
- Kos, P.; Varga, F.; Handl, M.; Kautzner, J.; Chudáček, V.; Držík, M.; Povýšil, C.; Trč, T.; Amler, E.; Hanus, M. Correlation of Dynamic Impact Testing, Histopathology and Visual Macroscopic Assessment in Human Osteoarthritic Cartilage. Int. Orthop. 2011, 35, 1733–1739. [Google Scholar] [CrossRef]
- Guilak, F.; Jones, W.R.; Ting-Beall, H.P.; Lee, G.M. The Deformation Behavior and Mechanical Properties of Chondrocytes in Articular Cartilage. J. Osteoarthr. Res. Soc. Int. 1999, 7, 59–70. [Google Scholar] [CrossRef]
- Jones, W.R.; Ting-Beall, H.P.; Lee, G.M.; Kelley, S.S.; Hochmuth, R.M.; Guilak, F. Alterations in the Young’s Modulus and Volumetric Properties of Chondrocytes Isolated from Normal and Osteoarthritic Human Cartilage. J. Biomech. 1999, 32, 119–127. [Google Scholar] [CrossRef]
- Elliott, D.M.; Kydd, S.R.; Perry, C.H.; Setton, L.A. Direct Measurement of the Poisson’s Ratio of Human Patella Cartilage in Tension. J. Biomech. Eng. 2002, 124, 223–228. [Google Scholar] [CrossRef]
- Grellmann, W.; Berghaus, A.; Haberland, E.J.; Jamali, Y.; Holweg, K.; Reincke, K.; Bierögel, C. Determination of Strength and Deformation Behavior of Human Cartilage for the Definition of Significant Parameters. J. Biomed. Mater. Res. A 2006, 78, 168–174. [Google Scholar] [CrossRef]
- Varga, F.; Držík, M.; Handl, M.; Chlpík, J.; Kos, P.; Filová, E.; Rampichová, M.; Nečas, A.; Trč, T.; Amler, E. Biomechanical Characterization of Cartilages by a Novel Approach of Blunt Impact Testing. Physiol. Res. 2007, 56, S61–S68. [Google Scholar] [CrossRef]
- Belluzzi, E.; Todros, S.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Berardo, A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes 2023, 11, 1014. [Google Scholar] [CrossRef]
- Malda, J.; Benders, K.E.M.; Klein, T.J.; de Grauw, J.C.; Kik, M.J.L.; Hutmacher, D.W.; Saris, D.B.F.; van Weeren, P.R.; Dhert, W.J.A. Comparative Study of Depth-Dependent Characteristics of Equine and Human Osteochondral Tissue from the Medial and Lateral Femoral Condyles. Osteoarthr. Cartil. 2012, 20, 1147–1151. [Google Scholar] [CrossRef]
- Gupta, S.; Webster, T.J.; Sinha, A. Evolution of PVA Gels Prepared without Crosslinking Agents as a Cell Adhesive Surface. J Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef]
- Lee, S.Y.; Pereira, B.P.; Yusof, N.; Selvaratnam, L.; Yu, Z.; Abbas, A.A.; Kamarul, T. Unconfined Compression Properties of a Porous Poly(Vinyl Alcohol)-Chitosan-Based Hydrogel after Hydration. Acta Biomater. 2009, 5, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, S.; Yu, Q.; Chen, T.; Liu, D. Application of Stem Cells in Regeneration Medicine. MedComm 2023, 4, e291. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Hashemibeni, B.; Masoud Ansar, M.; Hamid Zarkesh Esfahani, S.; Kazemi, M.; Goharian, V.; Esmaeili, N.; Esfandiary, E.; Esfahani, Z.S. Comparison between Chondrogenic Markers of Differentiated Chondrocytes from Adipose Derived Stem Cells and Articular Chondrocytes In Vitro. Iran. J. Basic Med. Sci. 2013, 16, 763–771. [Google Scholar]
- Elías-Zúñiga, A.; Baylón, K.; Ferrer, I.; Serenó, L.; Garcia-Romeu, M.L.; Bagudanch, I.; Grabalosa, J.; Pérez-Recio, T.; Martinez-Romero, O.; Ortega-Lara, W.; et al. On the Rule of Mixtures for Predicting Stress-Softening and Residual Strain Effects in Biological Tissues and Biocompatible Materials. Materials 2014, 7, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Elías-Zúñiga, A.; Beatty, M.F. Constitutive Equations for Amended Non-Gaussian Network Models of Rubber Elasticity. Int. J. Eng. Sci. 2002, 40, 2265–2294. [Google Scholar] [CrossRef]
- Elías-Zúñiga, A.; Montoya, B.; Ortega-Lara, W.; Flores-Villalba, E.; Rodríguez, C.A.; Siller, H.R.; Díaz-Elizondo, J.A.; Martínez-Romero, O. Stress-Softening and Residual Strain Effects in Suture Materials. Adv. Mater. Sci. Eng. 2013, 2013, 249512. [Google Scholar] [CrossRef]
- Bagudanch, I.; Martínez-Romero, O.; Elías-Zúñiga, A.; Garcia-Romeu, M.L. Identifying Polymeric Constitutive Equations for Incremental Sheet Forming Modeling. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 81, pp. 2292–2297. [Google Scholar]
- Palacios-Pineda, L.M.; Perales-Martínez, I.A.; Moreno-Guerra, M.R.; Elías-Zúñiga, A. An Optimum Specimen Geometry for Equibiaxial Experimental Tests of Reinforced Magnetorheological Elastomers with Iron Micro- and Nanoparticles. Nanomaterials 2017, 7, 254. [Google Scholar] [CrossRef] [PubMed]







| Sample | T10% (°C) | Tp1 (°C) | Tp2 (°C) | Rp1 (%/°C) | Rp2 (%/°C) | Residue (%) |
|---|---|---|---|---|---|---|
| Chitosan | 286.3 | 78.3 | 310.5 | 0.10 | 1.03 | 39.8 |
| Gelatin | 275.3 | 94.2 | 340.5 | 0.08 | 0.60 | 30.0 |
| PVA | 280.5 | 290.0 | 321.7 | 1.01 | 0.91 | 5.6 |
| HT-C | 63.1 | 70.6 | 312.7 | 0.91 | 0.43 | 7.0 |
| HT-AB | 266.4 | 380.2 | 445.3 | 0.66 | 0.46 | 7.7 |
| LT-C | 60.1 | 66.2 | 314.5 | 1.12 | 0.54 | 6.9 |
| Sample | Ra (μm) | Reference |
|---|---|---|
| Articular cartilage | 1.0–6.0 | [120] |
| PVA + Sodium Dodecyl Sulfate hydrogel | 21.04 ± 0.1 | [121] |
| HT-AB | 2.5 | This work |
| LT-AB | 2.7 | This work |
| Material | Crosslinker | Ultimate Stress (MPa) | Compressive Stress (MPA) | Reference |
|---|---|---|---|---|
| HT-C | Dried at 50 °C | 1.389 | 0.921 | This work |
| HT-AB | GS, dried at 50 °C | 1.768 | 1.196 | This work |
| LT-C | Freeze/Thaw Cycles | 0.203 | 0.038 | This work |
| LT-AB | GS, Freeze/Thaw Cycles | 0.303 | 0.131 | This work |
| PVA | Freeze/Thaw Cycles | 0.024 ± 0.012 | --- | [136] |
| PVA/Gelatin | Freeze/Thaw Cycles | 0.92 ± 0.018 | --- | [64] |
| Chitosan/PVA | Glutaraldehyde | Fracture stress: 196.82 | --- | [121] |
| GelMa reinforced 93% | APS/TEMED | 0.05 | [65] | |
| PVA/Chitosan | Irradiation | 0.072 | [137] | |
| Human knee cartilage | --- | 0.051 | [65,127] |
| Specimen | µ0 (MPa) | N (-) | A1 (MPa) | A2 (MPa) | b (-) | C (MPa) | f (-) |
|---|---|---|---|---|---|---|---|
| G1w | 0.23 | 20 | 0.45 | −0.05 | 0.5 | −120.25 | 0.04 |
| G1.5w | 0.25 | 25 | −4.175 | 0 | 0.65 | −40.25 | 0.06 |
| G2w | 0.25 | 20 | −3.31 | 0 | 0.475 | −60.25 | 0.0787 |
| G1r | 0.25 | 5.75 | −4.75 | 0 | 0.85 | −15.25 | 0.04 |
| G1.5r | 0.25 | 8.25 | −2.2 | 0 | 0.85 | −10.25 | 0.06 |
| G2r | 0.25 | 7.25 | −2.25 | 0 | 0.85 | −15.25 | 0.0787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Angel-Sánchez, K.; Treviño-Pacheco, A.V.; Perales-Martínez, I.A.; Martínez-Romero, O.; Olvera-Trejo, D.; Elías-Zúñiga, A. Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering. Polymers 2025, 17, 1520. https://doi.org/10.3390/polym17111520
Del Angel-Sánchez K, Treviño-Pacheco AV, Perales-Martínez IA, Martínez-Romero O, Olvera-Trejo D, Elías-Zúñiga A. Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering. Polymers. 2025; 17(11):1520. https://doi.org/10.3390/polym17111520
Chicago/Turabian StyleDel Angel-Sánchez, Karina, Ana Victoria Treviño-Pacheco, Imperio Anel Perales-Martínez, Oscar Martínez-Romero, Daniel Olvera-Trejo, and Alex Elías-Zúñiga. 2025. "Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering" Polymers 17, no. 11: 1520. https://doi.org/10.3390/polym17111520
APA StyleDel Angel-Sánchez, K., Treviño-Pacheco, A. V., Perales-Martínez, I. A., Martínez-Romero, O., Olvera-Trejo, D., & Elías-Zúñiga, A. (2025). Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering. Polymers, 17(11), 1520. https://doi.org/10.3390/polym17111520








