Urea–Formaldehyde Strengthened by Polyvinyl Alcohol: Impact on Mulch Film Properties and Cucumber Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PUF
2.3. Determination of Basic Performance of PUF
2.4. Sample Characterization
2.5. Preparation of Biodegradable Film
2.6. Biodegradable Mulch Performance
2.7. Spraying of the Biodegradable Materials
2.8. Bioassay Under a Greenhouse
2.9. Statistical Analysis
3. Results and Discussion
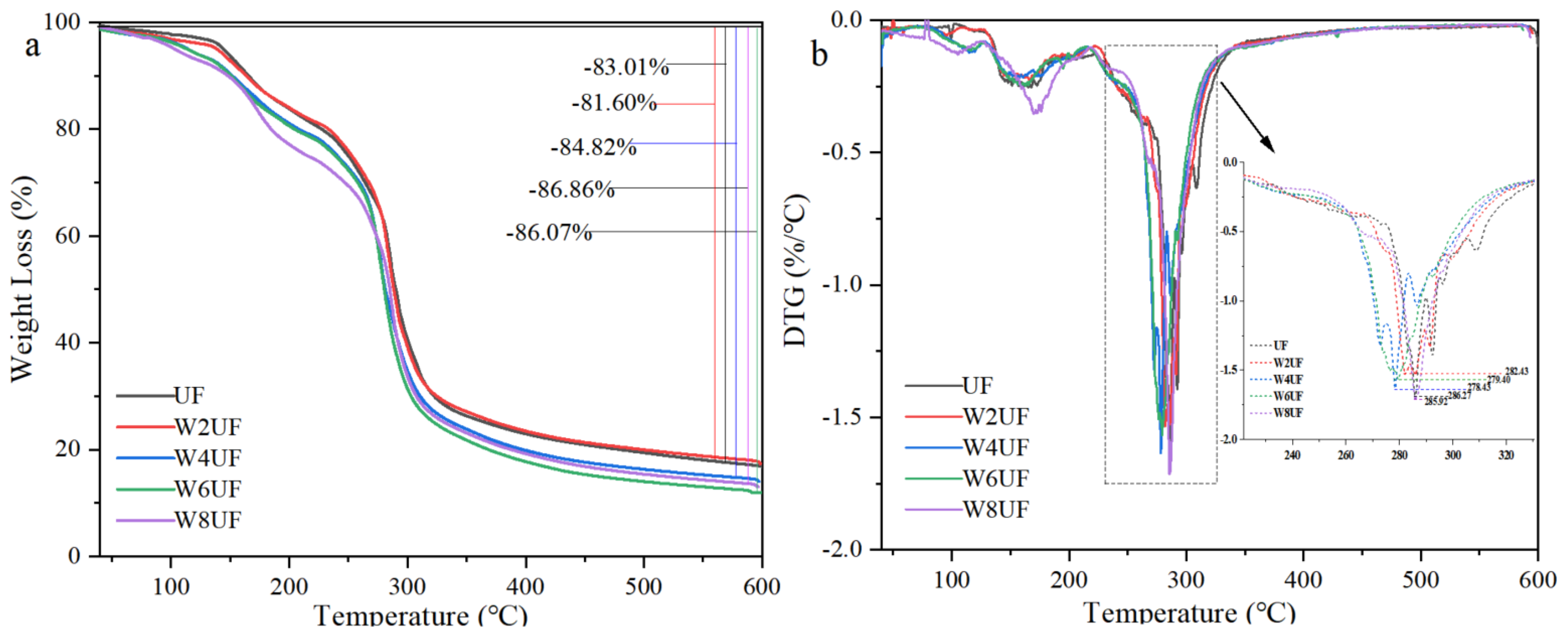
3.1. Basic Characteristics of PUF
3.2. Micro-Structural Characterization of PUF
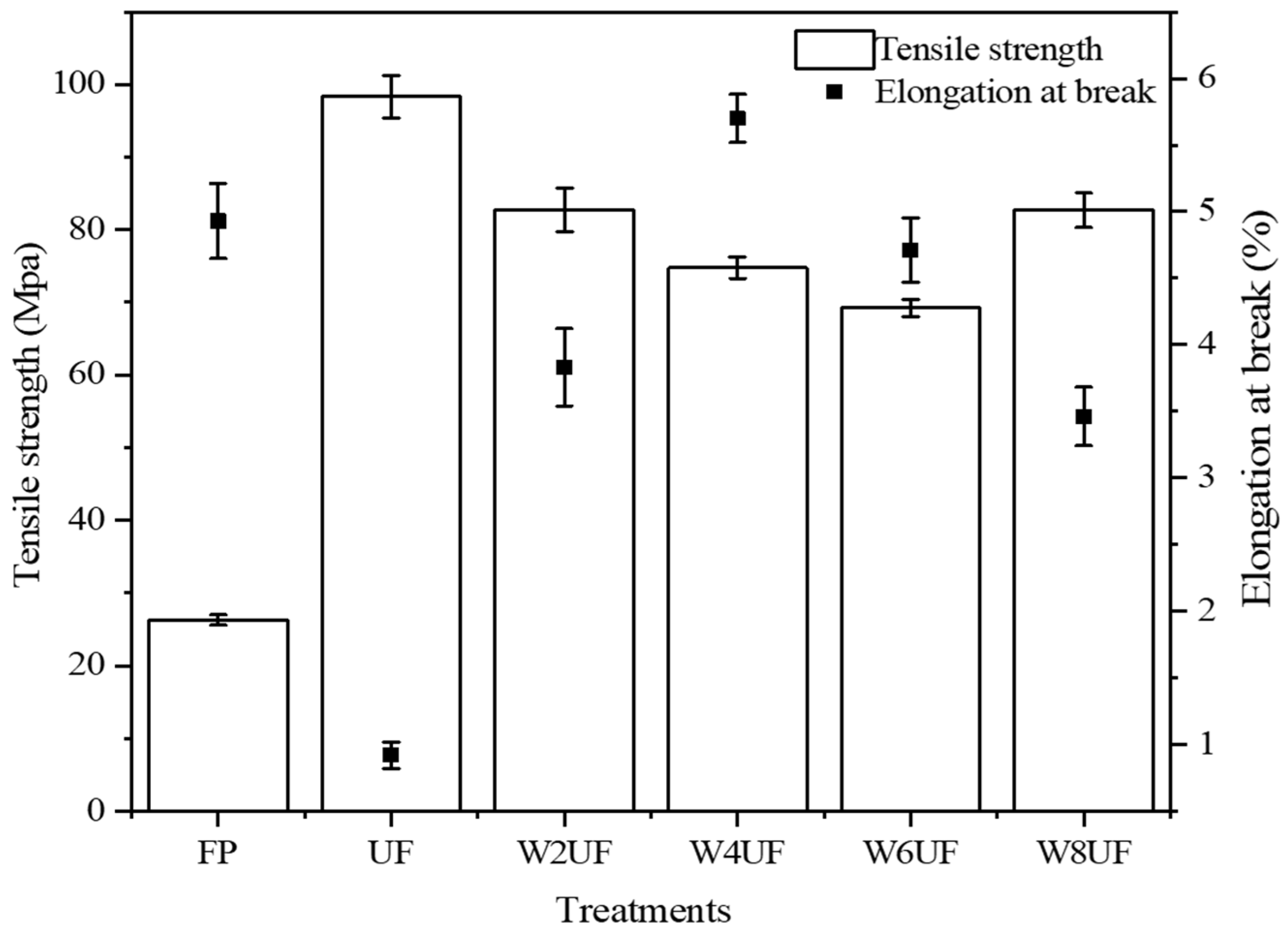
3.3. Mechanical Properties of PUF
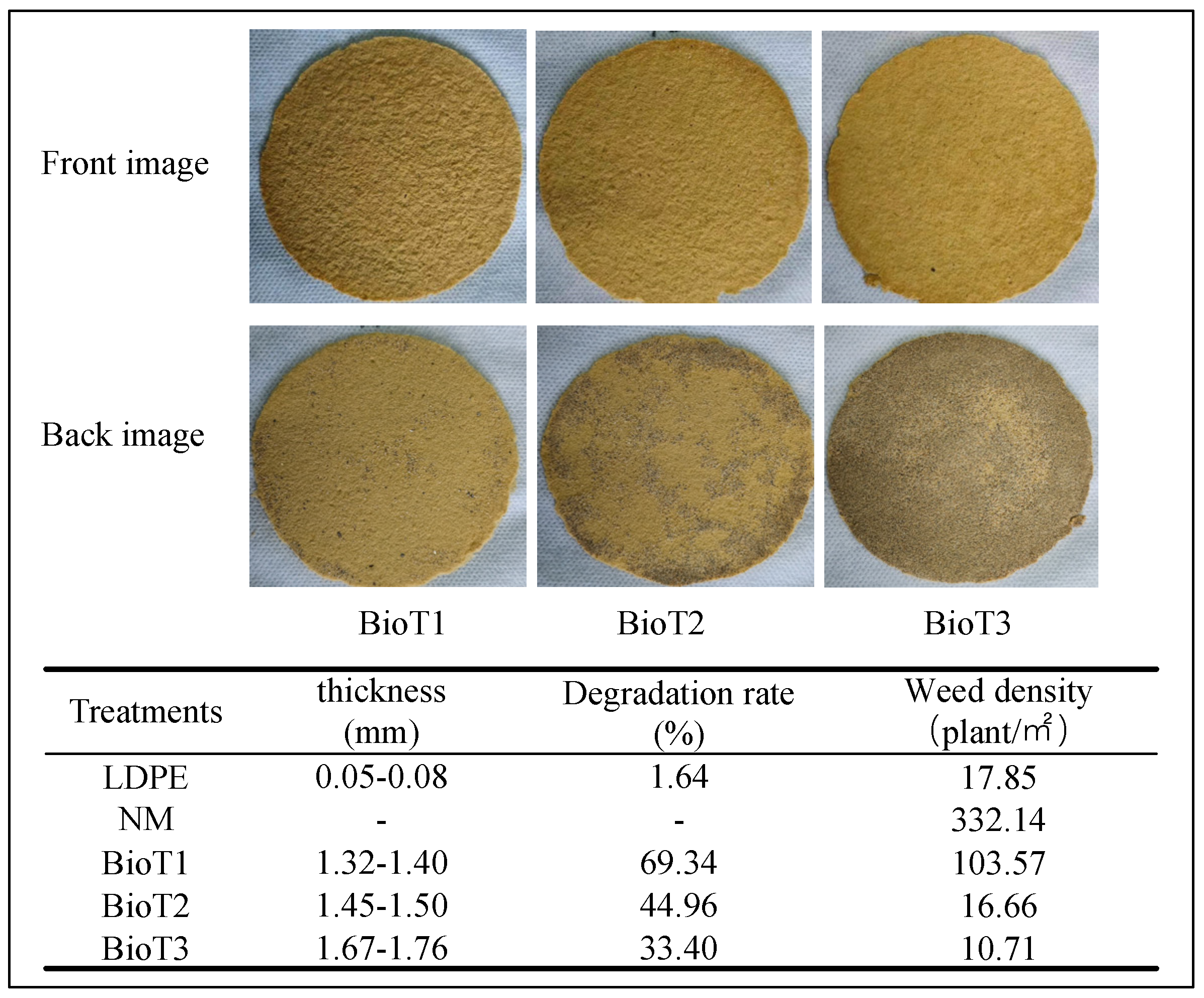
3.4. Biodegradable Mulch Characteristics
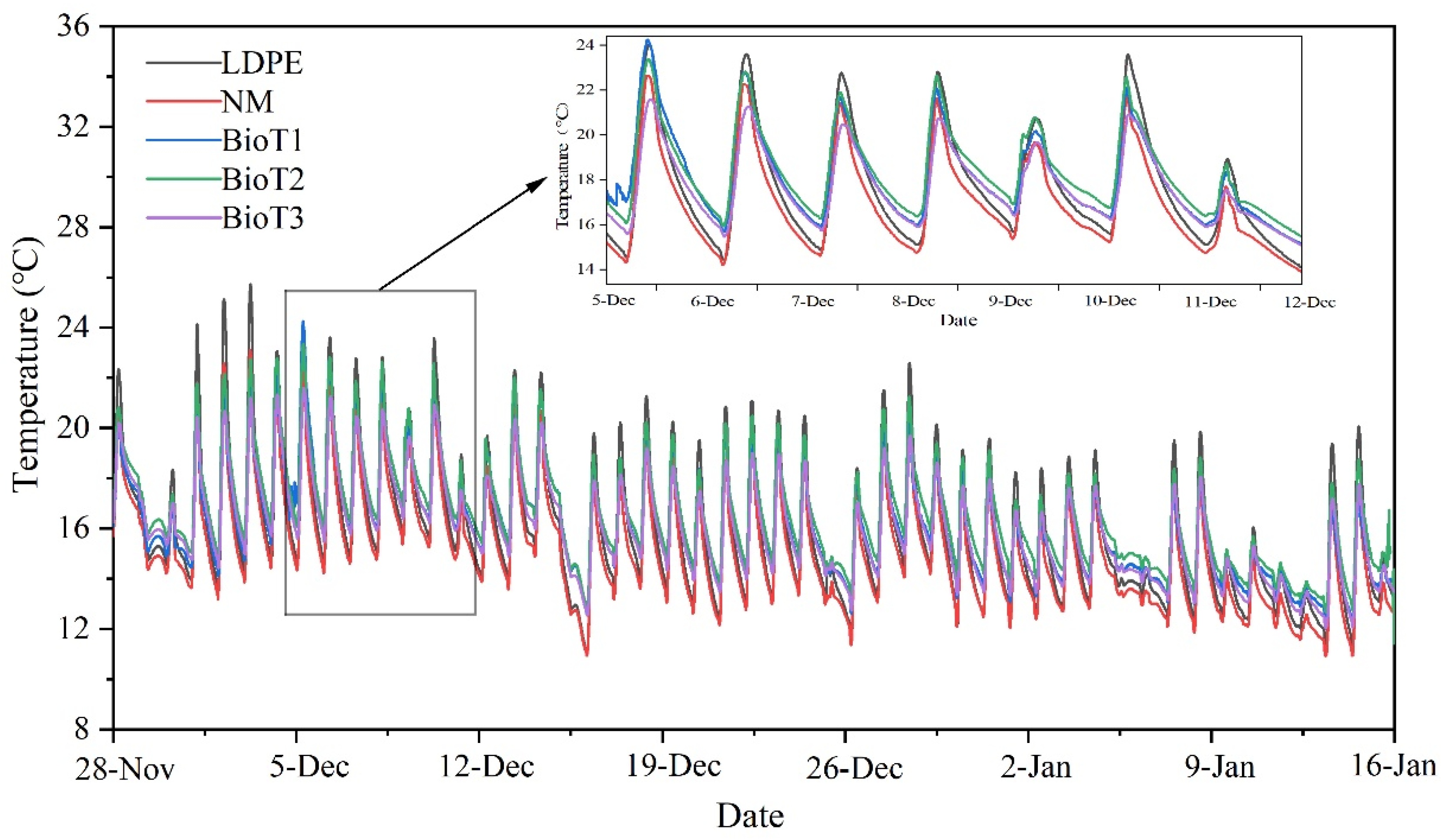
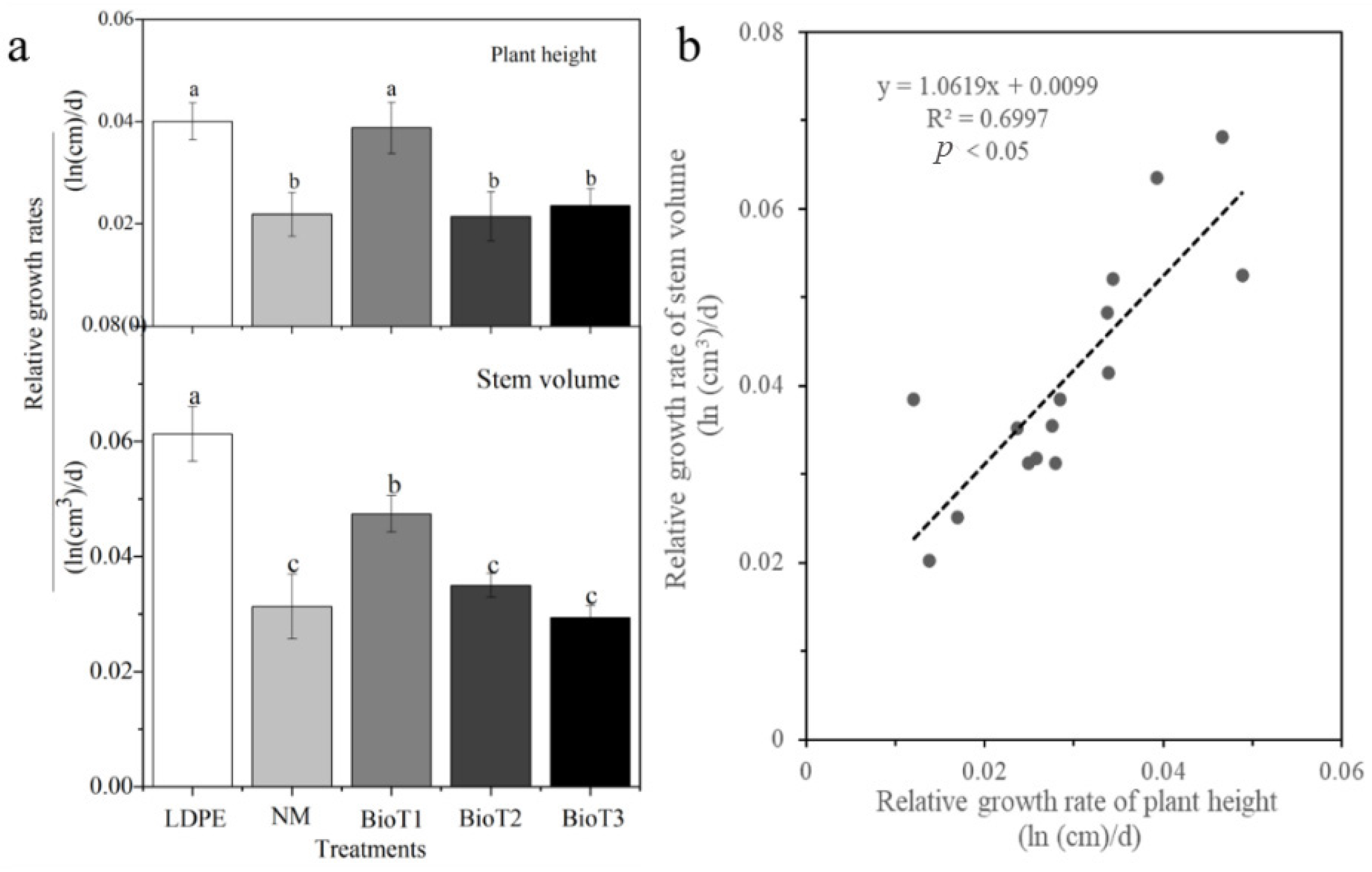
3.5. Plant Growth Trial
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem. Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of Plastic Film, Biodegradable Paper and Bio-Based Film Mulching for Summer Tomato Production: Soil Properties, Plant Growth, Fruit Yield and Fruit Quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- Dun, M.; Fu, H.; Hao, J.; Shan, W.; Wang, W. Tailoring Flexible Interphases in Bamboo Fiber-Reinforced Linear Low-Density Polyethylene Composites. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106606. [Google Scholar] [CrossRef]
- Maurya, H.O.; Kumar, G.; Prasad, L.; Gupta, P. Development and characterization of microwave-processed linear low-density polyethylene based sisal/jute hybrid laminates. Polym. Compos. 2024, 45, 15. [Google Scholar] [CrossRef]
- Hachem, A.; Convertino, F.; Batista, F.T.; Baptista, D.; Briassoulis, D.L.V.; Martínez, M.; Ángeles Teruel, M.; Nizzetto, L.; Papardaki, N.G.; Ruggiero, G.; et al. Schettini Gis Mapping of Agricultural Plastic Waste in Southern Europe. Sci. Total Environ. 2024, 946, 174491. [Google Scholar] [CrossRef]
- Xiong, H.G.; Tang, S.W.; Tang, H.L.; Zou, P. The Structure and Properties of a Starch-Based Biodegradable Film. Carbohydr. Polym. 2008, 71, 263–268. [Google Scholar] [CrossRef]
- Araújo Veloso, M.C.R.; Scatolino, M.V.; Goncalves, M.M.B.P.; Valle, M.L.A.; De Paula Protásio, T.; Mendes, L.M.; Junior, J.B.G. Sustainable Valorization of Recycled Low-Density Polyethylene and Cocoa Biomass for Composite Production. Environ. Sci. Pollut. Res. 2021, 28, 32810–32822. [Google Scholar] [CrossRef]
- Jan, K.; Riar, C.S.; Saxena, D.C. Optimization of Pellet Production from Agro-Industrial by-Products: Effect of Plasticizers on Properties of Pellets and Composite Pots. J. Environ. Polym. 2017, 25, 56–73. [Google Scholar] [CrossRef]
- Santagata, G.; Malinconico, M.; Immirzi, B.; Schettini, E.; Scarascia Mugnozza, G.; Vox, G. An Overview of Biodegradable Films and Spray Coatings as Sustainable Alternative to Oil-Based Mulching Films. Acta Hortic. 2013, 1037, 921–928. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Zhang, A.; Chen, J.; Cheng, G.; Sun, B.; Pi, X.; Dyck, M.; Si, B.; Zhao, Y. Effects of Straw and Plastic Film Mulching on Greenhouse Gas Emissions in Loess Plateau, China: A Field Study of 2 Consecutive Wheat-Maize Rotation Cycles. Sci. Total Environ. 2016, 579, 814–824. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Gao, D.H.; Luo, W.H.; Xiao, N.Y.; Xiao, G.S.; Li, Z.Y.; Liu, C.F. Hemicelluloses-Based Sprayable and Biodegradable Pesticide Mulch Films for Chinese Cabbage Growth. Int. J. Biol. Macromol. 2023, 225, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Ls, A.; Es, B.; Ldp, C.; Gb, D.; Cc, D.; Gv, B. Effect of Hydrolyzed Protein-Based Mulching Coatings on the Soil Properties and Productivity in a Tunnel Greenhouse Crop System. Sci. Total Environ. 2018, 645, 1221–1229. [Google Scholar]
- Moreno, M.M.; Gonzalez-Mora, S.; Villena, J.; Campos, J.A.; Moreno, C. Deterioration Pattern of Six Biodegradable, Potentially Low-Environmental Impact Mulches in Field Conditions. J. Environ. Manag. 2017, 200, 490–501. [Google Scholar] [CrossRef]
- Treinyte, J.; Grazuleviciene, V.; Grigalaviciene, I.; Miskine, E.; Bridziuviene, D. Polymer Composites from Poly (Vinyl Alcohol), Horn Meal and Crude Glycerol for Mulching Coatings. Waste Biomass 2017, 8, 1225–1235. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.; Sun, Z.Y.; Samad, A.A. Rheology and thermal transition state of polyvinyl alcohol-cassava starch blends. Carbohydr. Polym. 2010, 81, 737–739. [Google Scholar] [CrossRef]
- Corato, U.D.; Dbari, I.; Viola, E.; Pugliese, M. Assessing the Main Opportunities of Integrated Biorefining from Agro-Bioenergy Co/By-Products and Agroindustrial Residues into High-Value Added Products Associated to Some Emerging Markets: A Review. Renew. Sust. Energy 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Qu, P.; Huang, H.; Wu, G.; Sun, E.; Chang, Z. Effects of Hydrolysis Degree of Soy Protein Isolate on the Structure and Performance of Hydrolyzed Soy Protein Isolate/Urea/Formaldehyde Copolymer Resin. J. Appl. Polym. Sci. 2015, 132, 41469. [Google Scholar] [CrossRef]
- Ma, H.; Liu, D.D.; Cheng, Y.; Raynaldo, F.A.; Dabbour, M.; Chao, J.P.; Ali, A.; Yang, S.S. Ultrasound Viscous Reduction Effects on The Proteolysis of Soy Protein Isolate at a Limited Degree of Hydrolysis: Changes in the Functional Characteristics and Protein Structure. Ultrason. Sonochem. 2024, 104, 106847. [Google Scholar]
- Qu, P.; Cao, Y.; Wu, G.; Tang, Y.; Xia, L. Preparation and Properties of Coir-Based Substrate Bonded by Modified Urea Formaldehyde Resins for Seedlings. BioResources 2018, 13, 4332–4345. [Google Scholar] [CrossRef]
- Lin, H.L.; Chen, X.Y.; Lei, H.; Zhou, X.J.; Du, G.B.; Essawy, H.; Xi, X.D.; Hou, D.F.; Song, J.X.; Cao, M. Synthesis and Characterization of a Bio-Aldehyde-Based Lignin Adhesive with Desirable Water Resistance. Int. J. Biol. Macromol. 2024, 264, 130020. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Reduction of Formaldehyde Emission from Urea-Formaldehyde Resin by Maleated Nanolignin. Int. J. Adhes. Adhes. 2024, 132, 103677. [Google Scholar] [CrossRef]
- Roumeli, E.; Papadopoulou, E.; Pavlidou, E.; Vourlias, G.; Bikiaris, D.; Paraskevopoulos, K.M.; Chrissafis, K. Synthesis, Characterization and Thermal Analysis of Urea–Formaldehyde/NanoSio2 Resins. Thermochim. Acta 2012, 527, 33–39. [Google Scholar] [CrossRef]
- Jin, T.; Zeng, H.Y.; Huang, Y.F.; Liu, L.; Yao, W.T.; Guo, H.Y.; Shi, S.L.; Du, G.B.; Zhang, L.P. Synthesis of Biomass Hyperbranched Polyamide Resin from Cellulose and Citric Acid for Wood Adhesive. Int. J. Biol. Macromol. 2023, 253, 126575. [Google Scholar] [CrossRef]
- Park, S.; Jeong, B.; Park, B.D. A Comparison of Adhesion Behavior of Urea-Formaldehyde Resins with Melamine-Urea-Formaldehyde Resins in Bonding Wood. Forests 2021, 12, 1037. [Google Scholar] [CrossRef]
- Du, Y.L.; Huang, G.Q.; Wang, H.O.; Xiao, J.X. Effect of High Coacervation Temperature on the Physicochemical Properties of Resultant Microcapsules Through Induction of Maillard Reaction Between Soybean Protein and Chitosan. J. Food Eng. 2018, 234, 91–97. [Google Scholar] [CrossRef]
- Lang, Q.; Liu, C.H.; Zhu, X.X.; Zhang, C.; Zhang, S.M.; Li, L.H.; Liu, S.; Chen, H.T. Fabrication and Characterization of Degradable Crop-Straw-Fiber Composite Film Using in Situ Polymerization with Melamine-Urea-Formaldehyde Prepolymer for Agricultural Film Mulching. Materials 2022, 15, 5170. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Zheng, R.; Shi, K.; Wang, B.L.; Pan, Y.X.; Ji, X.L. Macroporous Polyvinyl Alcohol-Formaldehyde-Silicon Composite Sponges with Designed Structure for High-Efficiency Water-In-Oil Emulsion Separation. J. Environ. Chem. Eng. 2024, 12, 111738. [Google Scholar] [CrossRef]
- Luo, J.L.; Zhang, J.Y.; Gao, Q.; Mao, A.; Li, J.Z. Toughening and Enhancing Melamine–Urea–Formaldehyde Resin Properties Via in Situ Polymerization of Dialdehyde Starch And Microphase Separation. Polymers 2019, 11, 1167. [Google Scholar] [CrossRef]
- Min, Y.; Haiyan, M.; Runzhou, H.; Zhenghao, G.; Pujian, T.; Lixin, S.; Qinglin, W.; Kai, S. Mechanical and Thermal Properties of r-High Density Polyethylene Composites Reinforced with Wheat Straw Particleboard Dust and Basalt Fiber. Int. J. Ploym. Sci. 2018, 2018, 5101937. [Google Scholar]
- Rozyanty, A.R.; Zhafer, S.F.; Shayfull, Z.; Nainggolan, I.; Musa, L.; Zheing, L.T. Effect Of Water and Mechanical Retting Process on Mechanical and Physical Properties of Kenaf Bast Fiber Reinforced Unsaturated Polyester Composites. Compos. Struct. 2021, 257, 113384. [Google Scholar] [CrossRef]
- Chen, C.A.; Yz, A.; Wh, A.; Cheng, Q.B.; Yi, A.; Na, Z.A. Incorporation of Xuan-Paper Waste Residue in Red Mud/Waste Polyethylene Composites. J. Hazard. Mater. 2020, 399, 123051. [Google Scholar] [CrossRef]
- Wang, A.N.; Yin, P.; Liu, X.G.; Xian, G.J. Comparative Analysis of Hydrothermal Properties of Ramie Fiber-Reinforced Phenolic Resin and Ramie Fiber-Reinforced Unsaturated Polyester Composites. Polym. Compos. 2023, 44, 5201–5213. [Google Scholar] [CrossRef]
- Ma, Y.J.; Luo, Y.X.; Zhang, Q.N.; Gao, Y.M.; Li, J.S.; Shah, S.; Wang, X.Z.; Zhang, X.Y. Biodegradable Films Prepared from Pulp Lignocellulose Adhesives of Urea Formaldehyde Resin Modified by Biosulfonate. Polymers 2022, 14, 2863. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.C.; Wang, Y.; Huang, W.B.; Lv, L.Z.; Zhu, G.B.; Qu, Q.T.; Liang, Y.R.; Zheng, W.; Zheng, H.H. A Novel Covalently Grafted Binder Through in-Situ Polymerization for High-Performance Si-Based Lithium-Ion Batteries. Electrochim. Acta 2021, 400, 139442. [Google Scholar] [CrossRef]
- Sheng, R.; Ding, Q.Y.; Ren, Z.H.; Li, D.N.; Fan, S.C.; Cai, L.L.; Quan, X.F.; Wang, Y.; Yi, M.T.; Zhang, Y.X.; et al. Interfacial and Micellization Behavior of Binary Mixture of Amino Sulfonate Amphoteric Surfactant and Octadecyltrimethyl Ammonium Bromide: Effect of Short Chain Alcohol and Its Chain Length. J. Mol. Liq. 2021, 334, 116064. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma Radiation-Induced Crosslinked Composite Membranes Based on Polyvinyl Alcohol/Chitosan/Agno3/Vitamin E for Biomedical Applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Jovanović, V.; Samaržija-Jovanović, S.; Petković, B.; Milićević, Z.; Marković, G.; Marinović-Cincović, M. Biocomposites Based on Cellulose and Starch Modified Urea-Formaldehyde Resin: Hydrolytic, Thermal, and Radiation Stability. Polym. Compos. 2019, 40, 1287–1294. [Google Scholar] [CrossRef]
- Liu, K.; Su, C.; Ma, W.; Li, H.; Zeng, Z.; Li, L. Free Formaldehyde Reduction in Urea-Formaldehyde Resin Adhesive: Modifier Addition Effect and Physicochemical Property Characterization. BioResources 2020, 15, 2339–2355. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Chen, C.; Chang, J.; Li, L. Formaldehyde Emission Behavior of Plywood with Phenol-Formaldehyde Resin Modified by Bio-Oil Under Radiant Floor Heating Condition. Build. Environ. 2018, 144, 565–572. [Google Scholar] [CrossRef]
- Ristic, M.; Samarzija-Jovanovix, S.; Jovanovic, V.; Kostic, M.; Jovanovié, T.E.T.; Markovié, G.; Marinovié-Cincovié, M.; Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; et al. Hydrolytic and Thermal Stability of Urea-Formaldehyde Resins Based on Tannin and Betaine Bio-Fillers. J. Vinyl. Addit. Technol. 2023, 29, 1082–1092. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Gautam, M.; Maurya, H.K. Recent Development of Biodegradation Techniques of Polymer. Int. J. Res. Granthaalayah 2018, 6, 414–452. [Google Scholar] [CrossRef]
- Kadea, S.; Kittikorn, T.; Hedthong, R.; Malakul, R.; Chumprasert, S. Utilization of Microfibrillated Cellulose as a Filler in Adhesive for Improvement of Mechanical–Thermal Performance and Biodegradation of Wood Pulp/Pla Laminate Biocomposite. J. Polym. Environ. 2024, 32, 1741–1751. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.; Parka, B.D.; Kim, J.S.; Causin, V. Converting Crystalline Thermosetting Urea–Formaldehyde Resins to Amorphous Polymer Using Modified Nanoclay. J. Ind. Eng. Chem. 2020, 87, 78–89. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Beaujean, M.; Pasch, H.; Rode, K.; Dalet, P. Acetals Nduced Strength Increase of Melamine–Urea–Formaldehyde (Muf) Polycondensation Adhesives. Ii. Solubility and Colloidal State Disruption. J. Appl. Polym. Sci. 2010, 86, 1855–1862. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.; Zhao, X.; Ye, L. Reactive Toughening of Urea–Formaldehyde Resin with Poly(Vinyl Alcohol) by Formation of Interpenetrating Networks. Polym. Eng. Sci. 2018, 58, 2031–2038. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Jian, Z.; Lu, M. Crystallization Behavior of Stable Urea Formaldehyde Resin Dispersed by Polyvinyl Alcohol. Iran. Polym. J. 2015, 24, 13–20. [Google Scholar] [CrossRef]
- Wu, S.J.; Yang, Y.J.; Cheng, Y.; Wang, S.D.; Zhou, Z.B.; Zhang, P.; Zhu, X.Q.; Wang, B.D.; Zhang, H.; Xie, S.; et al. Fluorogenic Detection of Mercury Ion in Aqueous Environment Using Hydrogel-Based Aie Sensing Films. Aggregate 2023, 4, e287. [Google Scholar] [CrossRef]
- Filipović, V.; Bristow, K.L.; Filipović, L.; Wang, Y.S.; Sintim, H.Y.; Flury, M.; Šimůnek, J. Sprayable Biodegradable Polymer Membrane Technology for Cropping Systems: Challenges and Opportunities. Environ. Sci. Technol. 2020, 54, 4709–4711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wu, Q.; Fan, B.H.; Zheng, X.R.; Zhang, J.Z.; Li, W.H.; Guo, L. Effects of Mulching Biodegradable Films Under Drip Irrigation on Soil Hydrothermal Conditions and Cotton (Gossypium hirsutum L.) Yield. Agric. Water Manag. 2019, 213, 477–485. [Google Scholar] [CrossRef]
- Thrän, J.; Garcia-Garcia, G.; Parra-López, C.; Ufarte, A.; García-García, C.; Parra, S.; Sayadi-Gmada, S. Environmental And Economic Assessment of Biodegradable and Compostable Alternatives for Plastic Materials in Greenhouses. Waste Manag. 2024, 175, 92–100. [Google Scholar] [CrossRef]
- Yang, J.J.; Qin, R.Z.; Shi, X.P.; Wei, H.H.; Sun, G.J.; Li, F.M.; Zhang, F. The Effects of Plastic Film Mulching and Straw Mulching on Licorice Root Yield and Soil Organic Carbon Content in a Dryland Farming. Sci. Total Environ. 2022, 826, 154113. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Kaur, S.; Baldwin, T.C.; Radecka, I.; Jiang, G.Z.; Bretz, I.; Duale, K.; Adamus, G.M. Kowalczuk, Effective Control against Broadleaf Weed Species Provided by Biodegradable Pbat/Pla Mulch Film Embedded with the Herbicide 2-Methyl-4-Chlorophenoxyacetic Acid (Mcpa). Acs Sustain. Chem. Eng. 2020, 8, 5360–5370. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.Y.; Shi, H.H.; Zhang, Y.H.; Hu, Q.; Sun, Y.N. Modeling Effects of Biodegradable Film Mulching on Evapotranspiration and Crop Yields in Inner Mongoli. Agric. Water Manag. 2023, 275, 107996. [Google Scholar] [CrossRef]
- Meier, M.A.R. Plant Oils: The Perfect Renewable Resource for Polymer Science. Eur. Polym. J. 2012, 47, 837–852. [Google Scholar]
- Bridziuviene, D.; Fataraite-Urboniene, E.; Rainosalo, E.; Rajan, R.; Cesoniene, L.; Grazuleviciene, V. Forestry Wastes Filled Polymer Composites for Agricultural Use. J. Clean. Prod. 2018, 205, 388–406. [Google Scholar]
- Vaicekauskaite, J.; Ostrauskaite, J.; Treinyte, J.; Grazuleviciene, V.; Bridziuviene, D.; Rainosalo, E. Biodegradable Linseed Oil-Based Cross-Linked Polymer Composites Filled with Industrial Waste Materials for Mulching Coatings. J. Polym. Environ. 2019, 27, 395–404. [Google Scholar] [CrossRef]
- Treinyte, J.; Grazuleviciene, V.; Paleckiene, R.; Ostrauskaite, J.; Cesoniene, L. Biodegradable Polymer Composites as Coating Materials for Granular Fertilizers. J. Polym. Environ. 2017, 26, 543–554. [Google Scholar] [CrossRef]
- Cao, H.; Jia, M.; Song, J.; Xun, M.; Yang, H. Rice-Straw Mat Mulching Improves the Soil Integrated Fertility Index of Apple Orchards on Cinnamon Soil and Fluvo-Aquic Soil. Sci. Hortic. 2020, 278, 109837. [Google Scholar] [CrossRef]
- Cao, Y.E.; Gao, Y.M.; Li, J.S.; Tian, Y.Q. Straw Composts, Gypsum and Their Mixtures Enhance Tomato Yields under Continuous Saline Water Irrigation. Agric. Water Manag. 2019, 223, 105721. [Google Scholar] [CrossRef]




| Sample | pH Value | Viscosity /mPa·s | Solid Content /% | Curing Time/s | Free Formaldehyde Content/% | Water Absorption/% |
|---|---|---|---|---|---|---|
| W6UF | 8.17 ± 0.01 a | 78.47 ± 0.17 a | 41.16 ± 0.08 d | 70.0 ± 0.9 b | 0.290 ± 0.001 c | 38.3 ± 1.7 a |
| W4UF | 7.97 ± 0.01 b | 67.93 ± 0.03 b | 43.95 ± 0.19 c | 67.0± 0.7 c | 0.260 ± 0.003 d | 39.8 ± 2.7 a |
| W2UF | 8.02 ± 0.04 ab | 23.67 ± 0.09 d | 47.09 ± 0.16 b | 63.0 ± 0.4 d | 0.310 ± 0.005 b | 40.6 ± 1.3 a |
| W8UF | 8.09 ± 0.05 ab | 45.80 ± 0.15 c | 39.01 ± 0.11 e | 74.0 ± 0.6 a | 0.290 ± 0.004 c | 41.5 ± 0.6 a |
| UF | 7.77 ± 0.04 c | 18.57 ± 0.03 e | 49.76 ± 0.06 a | 56.0 ± 0.3 e | 0.370 ± 0.003 a | 24.2 ± 0.6 b |
| Treatment | Root Length Density/(cm·cm−3) | Root Surface Area Density/(cm2·cm−3) | Root Volume Density /(cm3·cm−3) | Root Diameter /(mm) | Specific Root Length/(m·g−1) | Root Shoot Ratio |
|---|---|---|---|---|---|---|
| LDPE | 0.382 ± 0.005 b | 0.101 ± 0.009 a | 0.0090 ± 0.0006 a | 0.75 ± 0.02 a | 47.2 ± 2.0 c | 0.188 ± 0.021 bc |
| NM | 0.283 ± 0.010 c | 0.058 ± 0.003 b | 0.0020 ± 0.0003 c | 0.63 ± 0.03 b | 57.8 ± 3.7 ab | 0.164 ± 0.002 c |
| BioT1 | 0.421 ± 0.010 a | 0.089 ± 0.005 a | 0.0040 ± 0.0004 b | 0.74 ± 0.02 a | 66.7 ± 3.1 a | 0.214 ± 0.021 abc |
| BioT2 | 0.24 ± 0.008 d | 0.047 ± 0.002 b | 0.0010 ± 0.0001 cd | 0.60 ± 0.03 b | 51.6 ± 3.7 bc | 0.246 ± 0.006 a |
| BioT3 | 0.140 ± 0.007 e | 0.028 ± 0.001 c | 0.0010 ± 0.0001 d | 0.67 ± 0.03 ab | 33.5 ± 1.5 d | 0.231 ± 0.018 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.; Ma, Y.; Zhang, X. Urea–Formaldehyde Strengthened by Polyvinyl Alcohol: Impact on Mulch Film Properties and Cucumber Cultivation. Polymers 2025, 17, 1277. https://doi.org/10.3390/polym17091277
Shen T, Ma Y, Zhang X. Urea–Formaldehyde Strengthened by Polyvinyl Alcohol: Impact on Mulch Film Properties and Cucumber Cultivation. Polymers. 2025; 17(9):1277. https://doi.org/10.3390/polym17091277
Chicago/Turabian StyleShen, Tingting, Yongjie Ma, and Xueyan Zhang. 2025. "Urea–Formaldehyde Strengthened by Polyvinyl Alcohol: Impact on Mulch Film Properties and Cucumber Cultivation" Polymers 17, no. 9: 1277. https://doi.org/10.3390/polym17091277
APA StyleShen, T., Ma, Y., & Zhang, X. (2025). Urea–Formaldehyde Strengthened by Polyvinyl Alcohol: Impact on Mulch Film Properties and Cucumber Cultivation. Polymers, 17(9), 1277. https://doi.org/10.3390/polym17091277






