Recycling of Epoxy/Fiberglass Composite Using Pyridine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
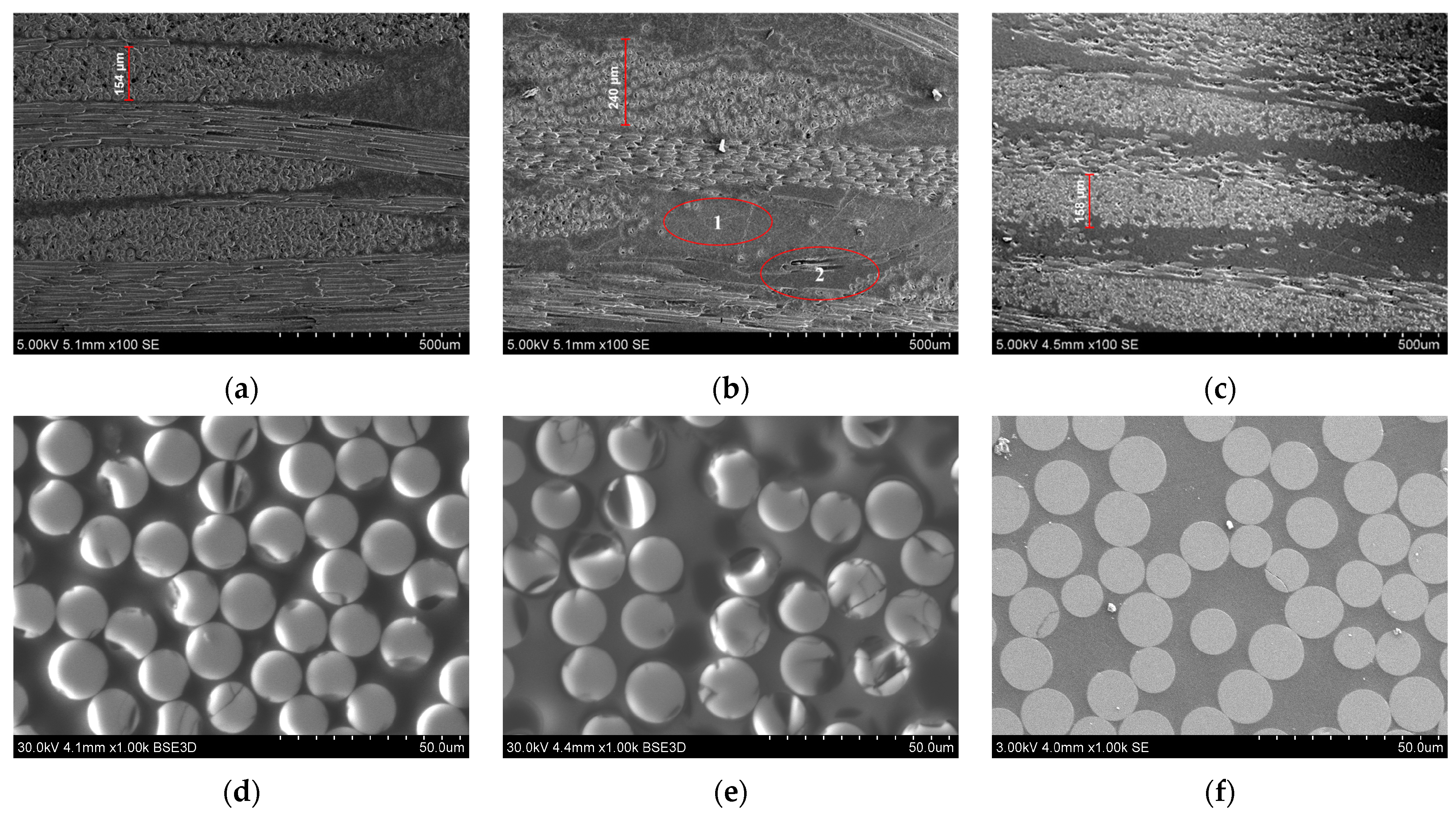
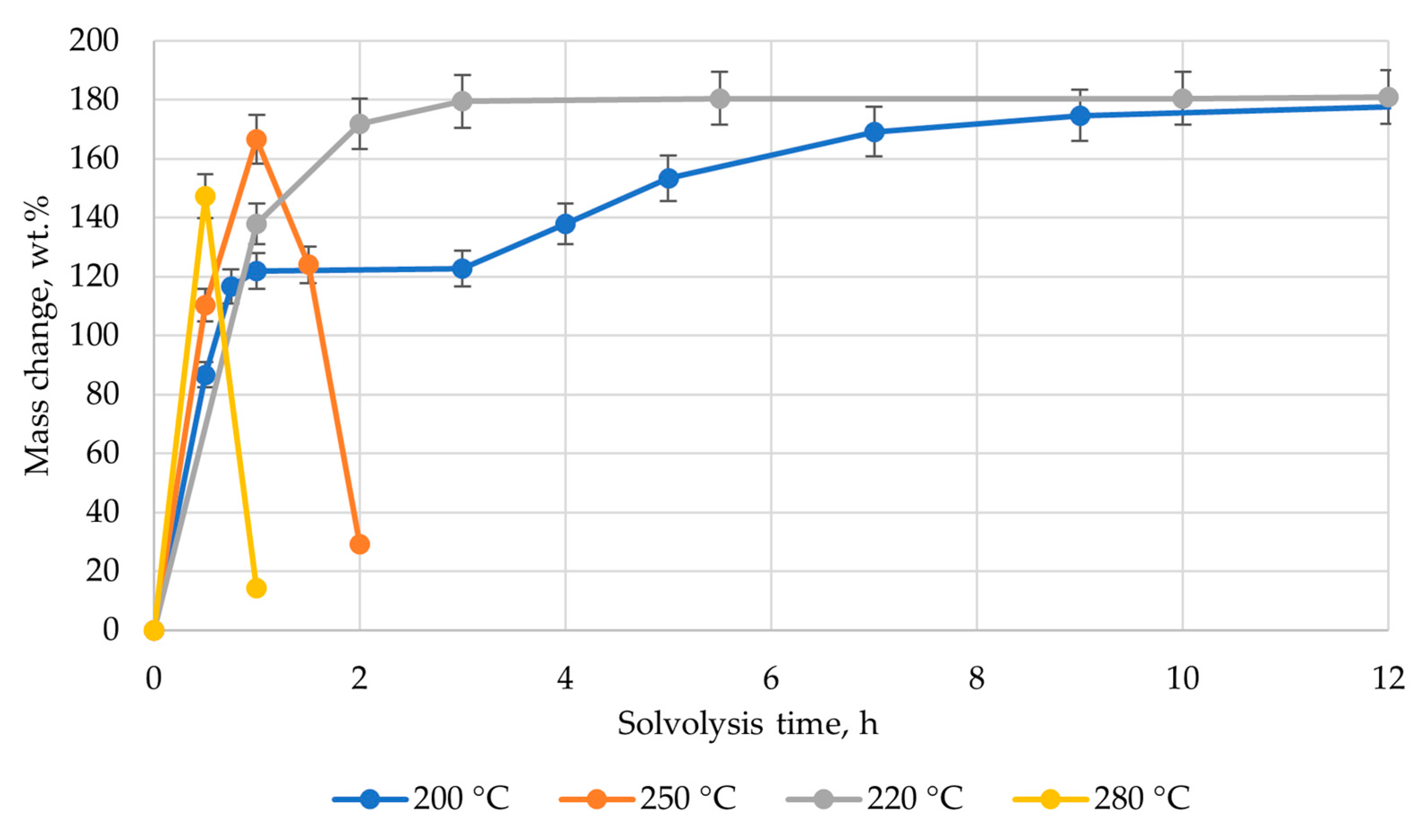
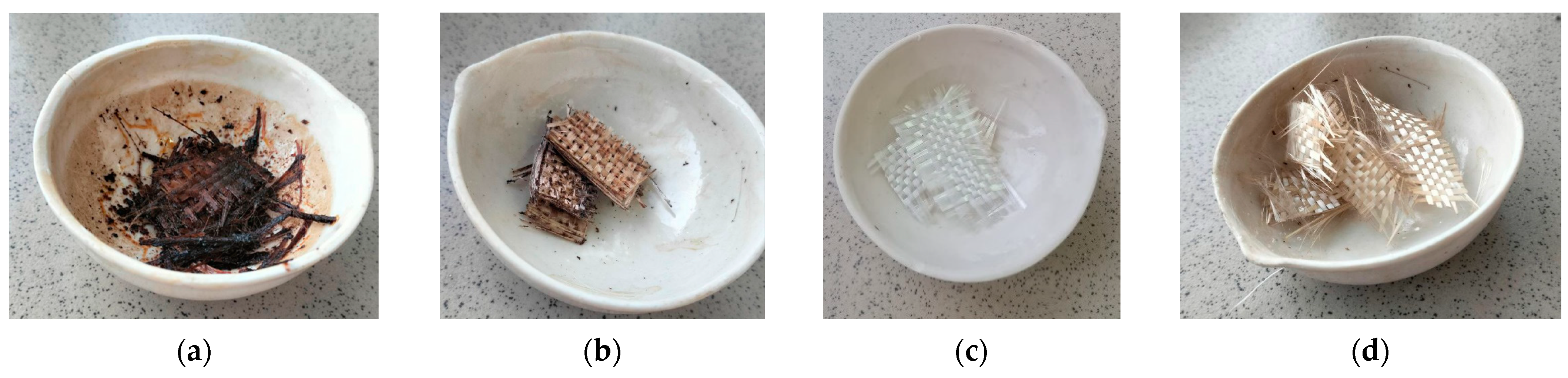
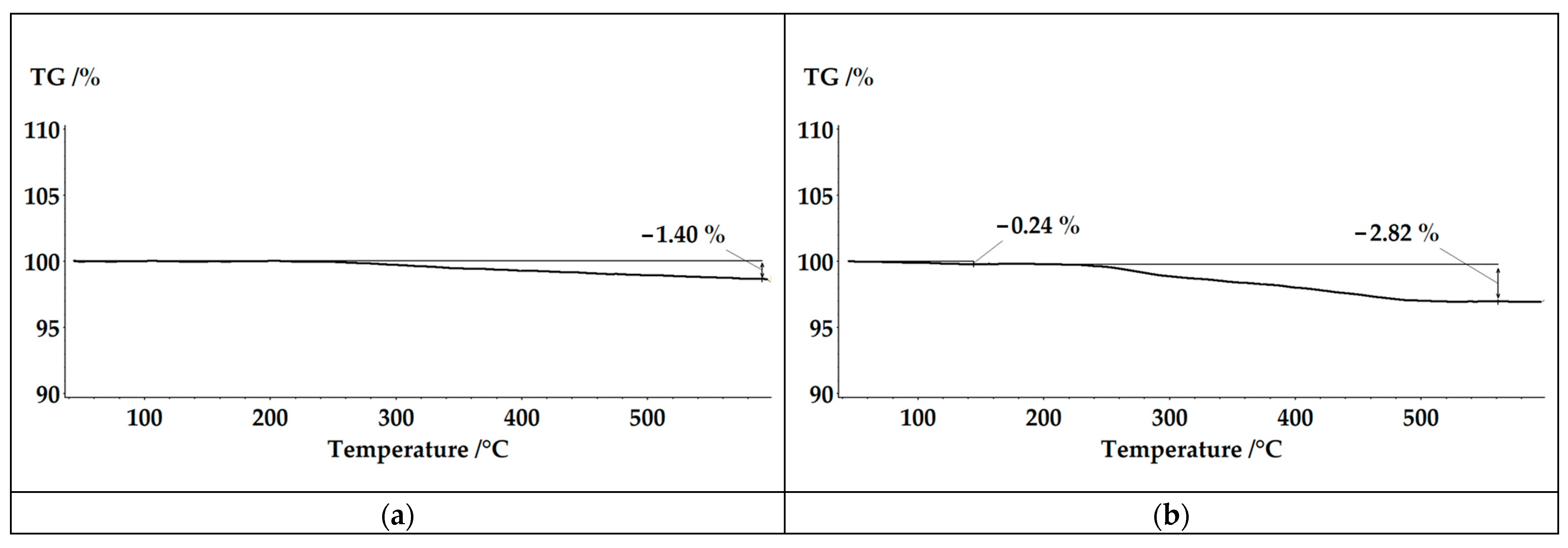
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bledzki, A.K.; Seidlitz, H.; Goracy, K.; Urbaniak, M.; Rösch, J.J. Recycling of Carbon Fiber Reinforced Composite Polymers—Review—Part 1: Volume of Production, Recycling Technologies, Legislative Aspects. Polymers 2021, 13, 300. [Google Scholar] [CrossRef]
- De, B.; Bera, M.; Bhattacharjee, D.; Ray, B.C.; Mukherjee, S. A Comprehensive Review on Fiber-Reinforced Polymer Composites: Raw Materials to Applications, Recycling, and Waste Management. Prog. Mater. Sci. 2024, 146, 101326. [Google Scholar] [CrossRef]
- Baley, C.; Davies, P.; Troalen, W.; Chamley, A.; Dinham-Price, I.; Marchandise, A.; Keryvin, V. Sustainable Polymer Composite Marine Structures: Developments and Challenges. Prog. Mater. Sci. 2024, 145, 101307. [Google Scholar] [CrossRef]
- Muflikhun, M.A.; Fiedler, B. Failure Prediction and Surface Characterization of GFRP Laminates: A Study of Stepwise Loading. Polymers 2022, 14, 4322. [Google Scholar] [CrossRef] [PubMed]
- Balıkoğlu, F.; Demircioğlu, T.K.; Yıldız, M.; Arslan, N.; Ataş, A. Mechanical Performance of Marine Sandwich Composites Subjected to Flatwise Compression and Flexural Loading: Effect of Resin Pins. J. Sandw. Struct. Mater. 2018, 22, 2030–2048. [Google Scholar] [CrossRef]
- Mattsson, C.; André, A.; Juntikka, M.; Tränkle, T.; Sott, R. Chemical Recycling of End-of-Life Wind Turbine Blades by Solvolysis/HTL. IOP Conf. Ser. Mater. Sci. Eng. 2020, 942, 012013. [Google Scholar] [CrossRef]
- Soutis, C. Fibre Reinforced Composites in Aircraft Construction. Prog. Aerosp. Sci. 2005, 41, 143–151. [Google Scholar] [CrossRef]
- Parveez, B.; Kittur, M.I.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; Umarfarooq, M.A. Scientific Advancements in Composite Materials for Aircraft Applications: A Review. Polymers 2022, 14, 5007. [Google Scholar] [CrossRef]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Investigation of Degradation of Composites Based on Unsaturated Polyester Resin and Vinyl Ester Resin. Materials 2022, 15, 1286. [Google Scholar] [CrossRef]
- Ahmad, H.; Shah, A.U.R.; Afaq, S.K.; Azad, M.M.; Arif, S.; Siddiqi, M.U.R.; Xie, L. Development and Characterization of Kevlar and Glass Fibers Reinforced Epoxy/Vinyl Ester Hybrid Resin Composites. Polym. Compos. 2024, 45, 8133–8146. [Google Scholar] [CrossRef]
- Majewski, P.; Florin, N.; Jit, J.; Stewart, R.A. End-of-Life Policy Considerations for Wind Turbine Blades. Renew. Sustain. Energy Rev. 2022, 164, 112538. [Google Scholar] [CrossRef]
- Chatziparaskeva, G.; Papamichael, I.; Voukkali, I.; Loizia, P.; Sourkouni, G.; Argirusis, C.; Zorpas, A.A. End-of-Life of Composite Materials in the Framework of the Circular Economy. Microplastics 2022, 1, 377–392. [Google Scholar] [CrossRef]
- Joustra, J.; Flipsen, B.; Balkenende, R. Structural Reuse of High End Composite Products: A Design Case Study on Wind Turbine Blades. Resour. Conserv. Recycl. 2021, 167, 105393. [Google Scholar] [CrossRef]
- Hao, C.; Zhao, B.; Guo, X.; Zhang, S.; Fei, M.; Shao, L.; Liu, W.; Cao, Y.; Liu, T.; Zhang, J. Mild Chemical Recycling of Waste Wind Turbine Blade for Direct Reuse in Production of Thermoplastic Composites with Enhanced Performance. Resour. Conserv. Recycl. 2025, 215, 108159. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current Status of Recycling of Fibre Reinforced Polymers: Review of Technologies, Reuse and Resulting Properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Pietroluongo, M.; Padovano, E.; Frache, A.; Badini, C. Mechanical Recycling of an End-of-Life Automotive Composite Component. Sustain. Mater. Technol. 2020, 23, e00143. [Google Scholar] [CrossRef]
- Aldosari, S.M.; AlOtaibi, B.M.; Alblalaihid, K.S.; Aldoihi, S.A.; AlOgab, K.A.; Alsaleh, S.S.; Alshamary, D.O.; Alanazi, T.H.; Aldrees, S.D.; Alshammari, B.A. Mechanical Recycling of Carbon Fiber-Reinforced Polymer in a Circular Economy. Polymers 2024, 16, 1363. [Google Scholar] [CrossRef] [PubMed]
- Kooduvalli, K.; Unser, J.; Ozcan, S.; Vaidya, U.K. Embodied Energy in Pyrolysis and Solvolysis Approaches to Recycling for Carbon Fiber-Epoxy Reinforced Composite Waste Streams. Recycling 2022, 7, 6. [Google Scholar] [CrossRef]
- Kumar, B.G.; Singh, R.P.; Nakamura, T. Degradation of Carbon Fiber-Reinforced Epoxy Composites by Ultraviolet Radiation and Condensation. J. Compos. Mater. 2002, 36, 2713–2733. [Google Scholar] [CrossRef]
- Morin, C.; Loppinet-Serani, A.; Cansell, F.; Aymonier, C. Near- and Supercritical Solvolysis of Carbon Fibre Reinforced Polymers (CFRPs) for Recycling Carbon Fibers as a Valuable Resource: State of the Art. J. Supercrit. Fluids 2012, 66, 232–240. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Wang, L.; Lu, J.-X.; Dong, R.; Duan, H.; Yang, J. The Challenge of Recycling Fast-Growing Fibre-Reinforced Polymer Waste. Nat. Rev. Mater. 2025, 10, 81–82. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Seidlitz, H.; Krenz, J.; Goracy, K.; Urbaniak, M.; Rösch, J.J. Recycling of Carbon Fiber Reinforced Composite Polymers—Review—Part 2: Recovery and Application of Recycled Carbon Fibers. Polymers 2020, 12, 3003. [Google Scholar] [CrossRef]
- Ginder, R.S.; Ozcan, S. Recycling of Commercial E-Glass Reinforced Thermoset Composites via Two Temperature Step Pyrolysis to Improve Recovered Fiber Tensile Strength and Failure Strain. Recycling 2019, 4, 24. [Google Scholar] [CrossRef]
- Protsenko, A.E.; Pimenova, E.D.; Petrov, V.V. Recycling of Glass Fibers Sheets from Thermoset Reinforced Plastic Using Thermolysis Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 734, 012185. [Google Scholar] [CrossRef]
- Clough, R.L.; Gillen, K.T. Polymer Degradation under Ionizing Radiation: The Role of Ozone. J. Polym. Sci. A Polym. Chem. 1989, 27, 2313–2324. [Google Scholar] [CrossRef]
- Kotnarowska, D. Influence of Ultraviolet Radiation and Aggressive Media on Epoxy Coating Degradation. Prog. Org. Coatings 1999, 37, 149–159. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite Material Recycling Technology—State-of-the-Art and Sustainable Development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Schwarz, S.; Höftberger, T.; Burgstaller, C.; Hackl, A.; Schwarzinger, C. Pyrolytic Recycling of Carbon Fibers from Prepregs and Their Use in Polyamide Composites. Open J. Compos. Mater. 2020, 10, 92–105. [Google Scholar] [CrossRef]
- Abdou, T.R.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of Polymeric Composites from Industrial Waste by Pyrolysis: Deep Evaluation for Carbon Fibers Reuse. Waste Management 2021, 120, 1–9. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Recycling of Carbon Fiber-Reinforced Plastic Using Supercritical and Subcritical Fluids. J. Mater. Cycles Waste Manag. 2017, 19, 15–20. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical Recycling of Carbon Fibers Reinforced Epoxy Resin Composites in Oxygen in Supercritical Water. Mater. Des. 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Vallee, M.; Tersac, G.; Destais-Orvoen, N.; Durand, G. Chemical Recycling of Class A Surface Quality Sheet-Molding Composites. Ind. Eng. Chem. Res. 2004, 43, 6317–6324. [Google Scholar] [CrossRef]
- Jiang, T.-W.; Reddy, K.S.K.; Chen, Y.-C.; Wang, M.-W.; Chang, H.-C.; Abu-Omar, M.M.; Lin, C.-H. Recycling Waste Polycarbonate to Bisphenol A-Based Oligoesters as Epoxy-Curing Agents, and Degrading Epoxy Thermosets and Carbon Fiber Composites into Useful Chemicals. ACS Sustain. Chem. Eng. 2022, 10, 2429–2440. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Opiri, M.M.; Ezzir, A.; Li, G.; Warner, I.M. Recycling Thermoset Epoxy Resin Using Alkyl-Methyl-Imidazolium Ionic Liquids as Green Solvents. ACS Appl. Polym. Mater. 2021, 3, 5588–5595. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Chen, J.; Xiong, J.; Wang, D.; Wang, S.; Wu, S.; Guo, B. Tuning the Mechanical and Dynamic Properties of Imine Bond Crosslinked Elastomeric Vitrimers by Manipulating the Crosslinking Degree. Polym. Chem. 2020, 11, 1348–1355. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Liu, Y.; Wu, S.; Guo, B. Mechanically Robust, Self-Healable, and Reprocessable Elastomers Enabled by Dynamic Dual Cross-Links. Macromolecules 2019, 52, 3805–3812. [Google Scholar] [CrossRef]
- El Ghazzaoui, H.; Salle, E.L.G.L.; Bellettre, J. Recyclage D’un Composite à base D’une Résine Thermodurcissable Par de L’eau en Condition Subcritique. Mater. Tech. 2012, 100, 517–524. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Q.; Yuan, X.-X.; van Kasteren, J.M.N.; Wang, Y.-Z. Highly Efficient Solvolysis of Epoxy Resin Using Poly (Ethylene Glycol)/NaOH Systems. Polym. Degrad. Stab. 2012, 97, 1101–1106. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, H.Y.; Bae, S.Y.; Kumazawa, H. Characteristics and Kinetics of Degradation of Polystyrene in Supercritical Water. J. Appl. Polym. Sci. 2006, 101, 695–700. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; Dodds, C.; Hyde, J.; García-Serna, J.; Poliakoff, M.; Lester, E.; Cocero, M.J.; Kingman, S.; Pickering, S.; Wong, K.H. Chemical Recycling of Carbon Fibre Reinforced Composites in Nearcritical and Supercritical Water. Compos. Part A Appl. Sci. Manuf. 2008, 39, 454–461. [Google Scholar] [CrossRef]
- Okajima, I.; Watanabe, K.; Sako, T. Chemical Recycling of Carbon Fiber Reinforced Plastic with Supercritical Alcohol. J. Adv. Res. Phys. 2012, 3, 1–4. [Google Scholar]
- Piñero-Hernanz, R.; García-Serna, J.; Dodds, C.; Hyde, J.; Poliakoff, M.; Cocero, M.J.; Kingman, S.; Pickering, S.; Lester, E. Chemical Recycling of Carbon Fibre Composites Using Alcohols under Subcritical and Supercritical Conditions. J. Supercrit. Fluids 2008, 46, 83–92. [Google Scholar] [CrossRef]
- Borjan, D.; Knez, Ž.; Knez, M. Recycling of Carbon Fiber-Reinforced Composites—Difficulties and Future Perspectives. Materials 2021, 14, 4191. [Google Scholar] [CrossRef] [PubMed]
- Protsenko, A.E.; Petrov, V.V. Strengthening of Glass Fibers Obtained by Recycling of Polymer Composite Material. Strength. Technol. Coat. 2022, 18, 347–351. [Google Scholar] [CrossRef]
- Protsenko, A.E.; Protsenko, A.N.; Shakirova, O.G.; Petrov, V.V. Recycling of Epoxy/Fiberglass Composite Using Supercritical Ethanol with (2,3,5-Triphenyltetrazolium)2[CuCl4] Complex. Polymers 2023, 15, 1559. [Google Scholar] [CrossRef]
- Protsenko, A.E.; Petrov, V.V. Recycling of the Polymer Composite Fillers in Amino Alcohol Medium. J. Phys. Conf. Ser. 2022, 2353, 012009. [Google Scholar] [CrossRef]
- Protsenko, A.E.; Petrov, V.V. Recycling of Fiberglass Fillers Obtained from Polymer Composites Based on an Epoxy Vinyl Ester Binder. Mech. Compos. Mater. 2022, 58, 537–544. [Google Scholar] [CrossRef]
- Protsenko, A.E.; Telesh, V.V. Inhibition and Cathalysis as a Method to Improve the Mechanical Properties of a Fiberglass-Reinforced Plastic. Mech. Compos. Mater. 2015, 51, 555–560. [Google Scholar] [CrossRef]
- Marsh, G. Composites Consolidate in Commercial Aviation. Reinf. Plast. 2016, 60, 302–305. [Google Scholar] [CrossRef]
- Fleischer, J.; Teti, R.; Lanza, G.; Mativenga, P.; Möhring, H.C.; Caggiano, A. Composite Materials Parts Manufacturing. CIRP Ann. 2018, 67, 603–626. [Google Scholar] [CrossRef]
- Rubino, F.; Nisticò, A.; Tucci, F.; Carlone, P. Marine Application of Fiber Reinforced Composites: A Review. J. Mar. Sci. Eng. 2020, 8, 26. [Google Scholar] [CrossRef]
- Thomsen, O.T. Sandwich Materials for Wind Turbine Blades—Present and Future. J. Sandw. Struct. Mater. 2009, 11, 7–26. [Google Scholar] [CrossRef]
- ISO 175:2010; Plastics—Methods of Test for the Determination of the Effects of Immersion in Liquid Chemicals. ISO: Geneva, Switzerland, 2010.
- AISO 11566:1996; Carbon Fibre—Determination of the Tensile Properties of Single-Filament Specimens. ISO: Geneva, Switzerland, 1996.
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2010.
- Chi, M.; Gargouri, R.; Schrader, T.; Damak, K.; Maâlej, R.; Sierka, M. Atomistic Descriptors for Machine Learning Models of Solubility Parameters for Small Molecules and Polymers. Polymers 2022, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Askadskii, A.A. Development of Studies Concerning Analysis of the Porous Structure and Solubility of Polymers. Polym. Sci. Ser. A 2012, 54, 849–858. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Kolmakova, L.K.; Tager, A.A.; Slonimskii, G.L.; Korshak, V.V. The assessment of the cohesive energy density between low molecular weight liquids and polymers. Polym. Sci. USSR 1977, 19, 1159–1169. [Google Scholar] [CrossRef]
- Xu, S.; Dong, X.; Zhao, Y.; Han, J.; Ji, Y.; Kuang, R.; Zhang, S.; Ma, S. Preparation of Environmentally Friendly Anticorrosive Coatings with Aniline Trimer-Modified Waterborne Polyurethane. Coatings 2024, 14, 1380. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Purslow, D. Matrix Fractography of Fibre-Reinforced Epoxy Composites. Composites 1986, 17, 289–303. [Google Scholar] [CrossRef]
- Zhu, P.; Yang, Y.Z.; Chen, Y.; Qian, G.R.; Liu, Q. Influence Factors of Determining Optimal Organic Solvents for Swelling Cured Brominated Epoxy Resins to Delaminate Waste Printed Circuit Boards. J. Mater. Cycles Waste Manag. 2018, 20, 245–253. [Google Scholar] [CrossRef]
- Iglesias, J.G.; González-Benito, J.; Aznar, A.J.; Bravo, J.; Baselga, J. Effect of Glass Fiber Surface Treatments on Mechanical Strength of Epoxy Based Composite Materials. J. Colloid Interface Sci. 2002, 250, 251–260. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Degradation of a Model Epoxy Resin by Solvolysis Routes. Polym. Degrad. Stab. 2015, 118, 96–103. [Google Scholar] [CrossRef]
- Xue, G.; Ishida, H.; Koenig, J.L. An Investigation of the Polymerization of Pyridine with Epoxy Compounds. Polymer 1986, 27, 1134–1137. [Google Scholar] [CrossRef]
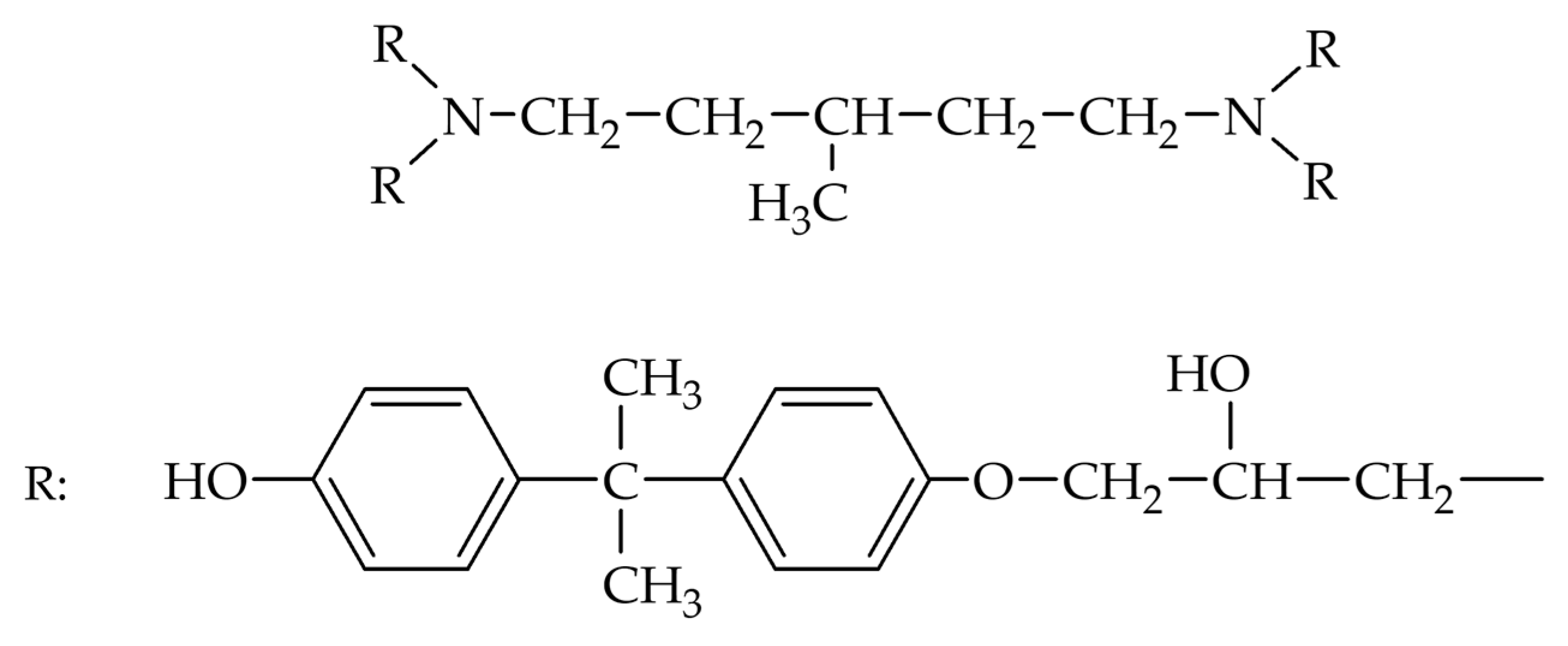
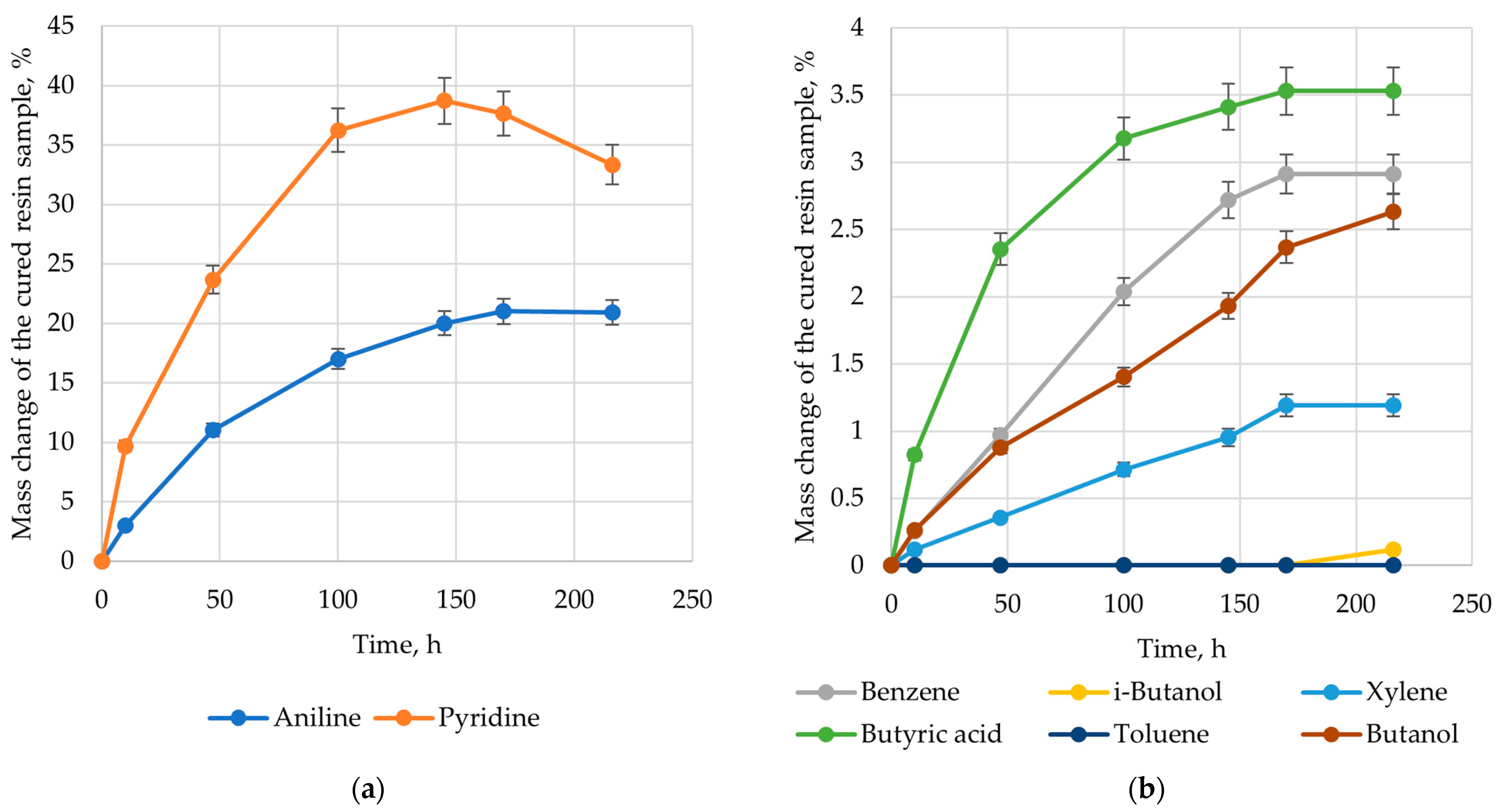

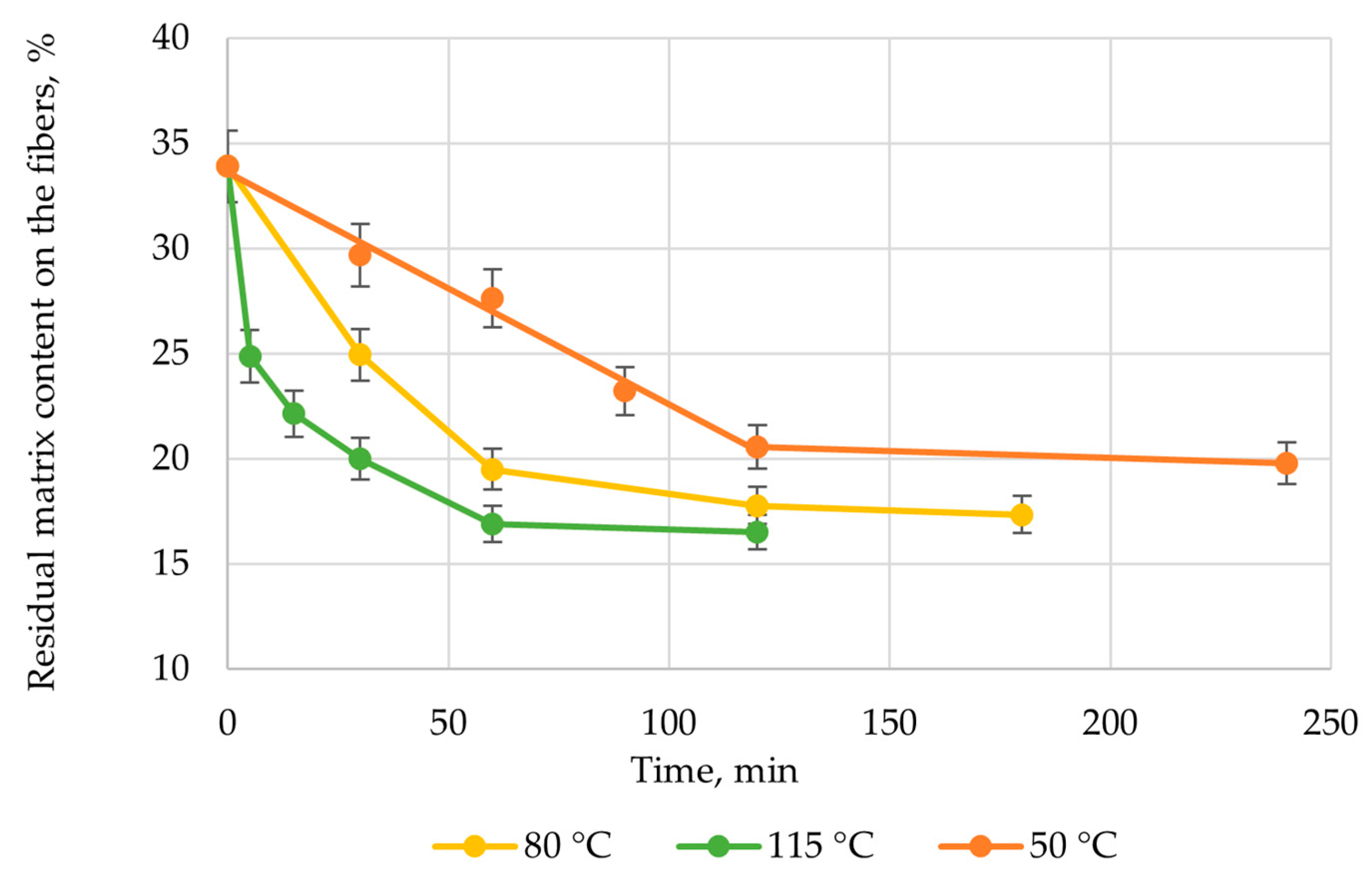
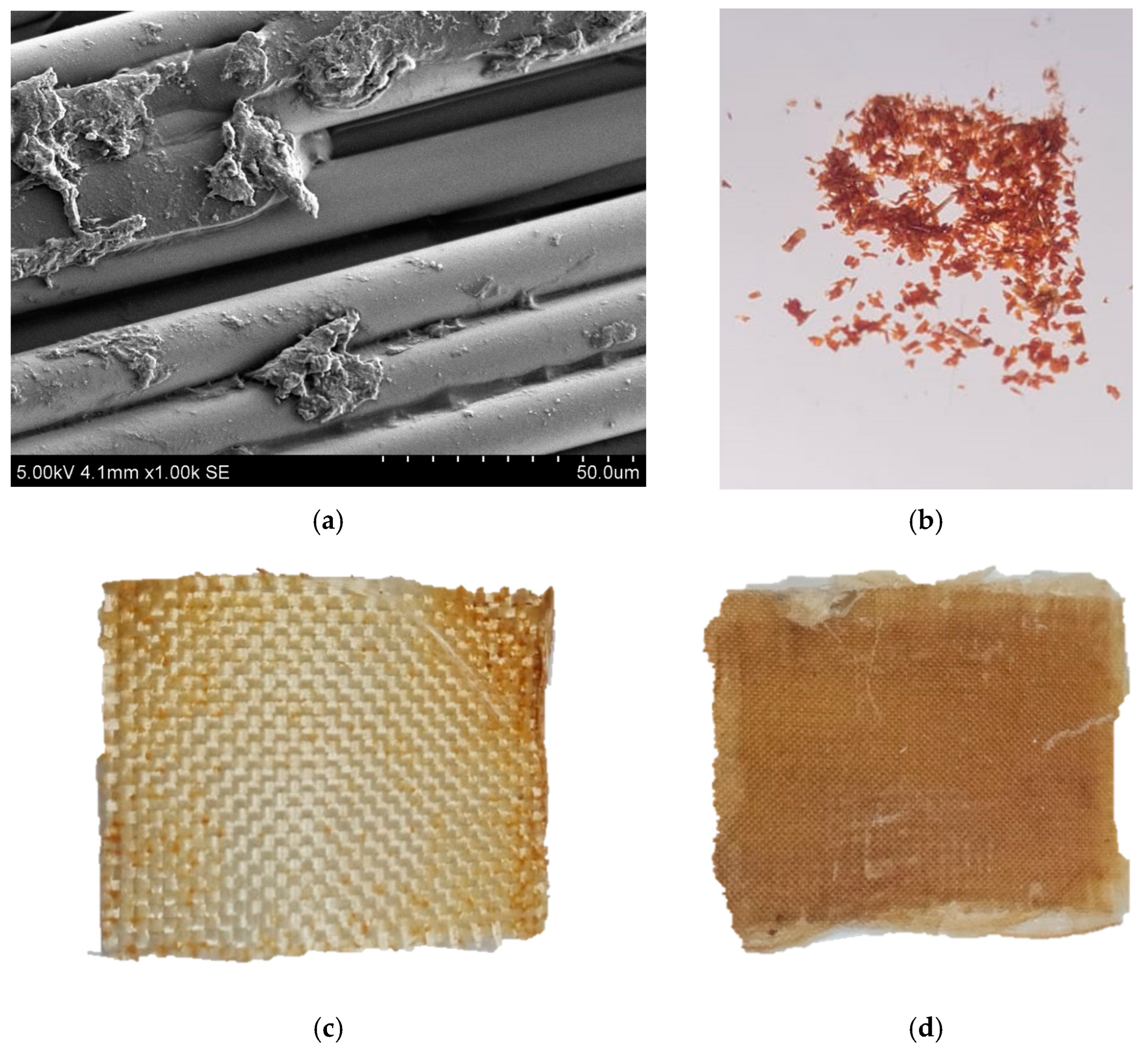
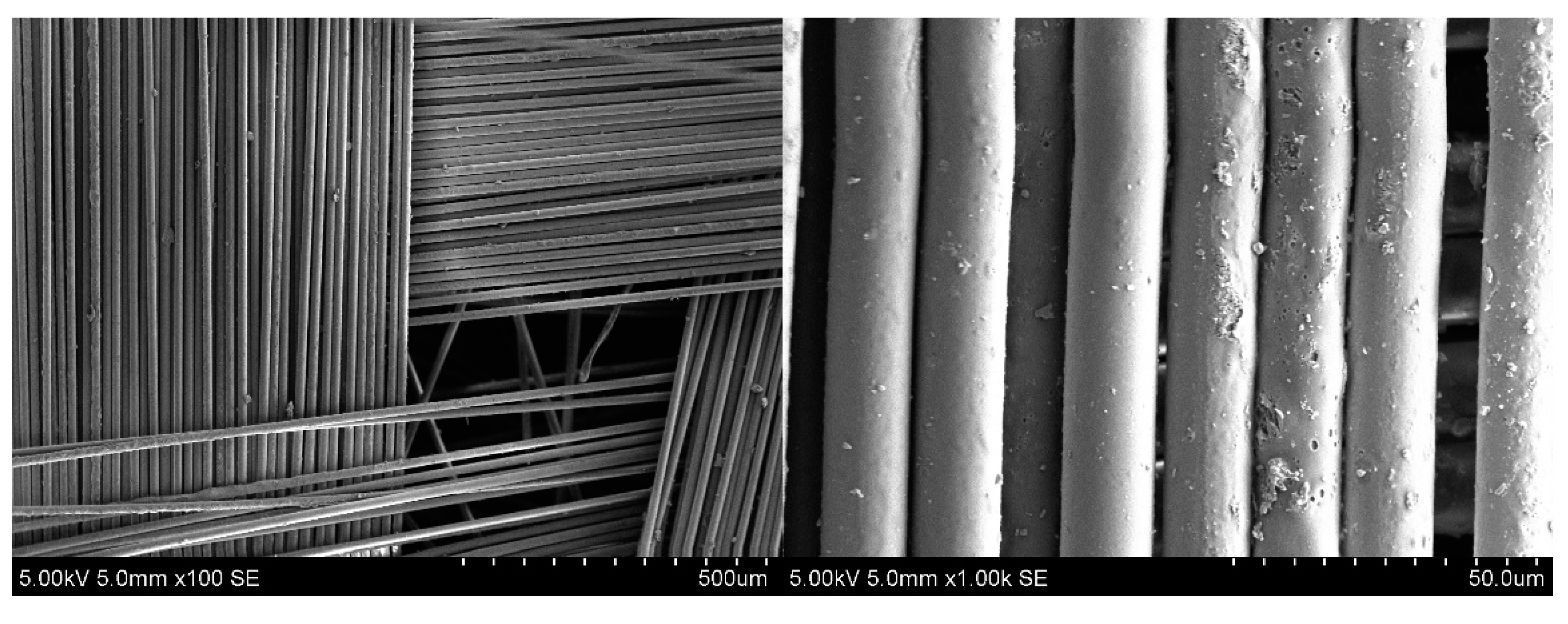
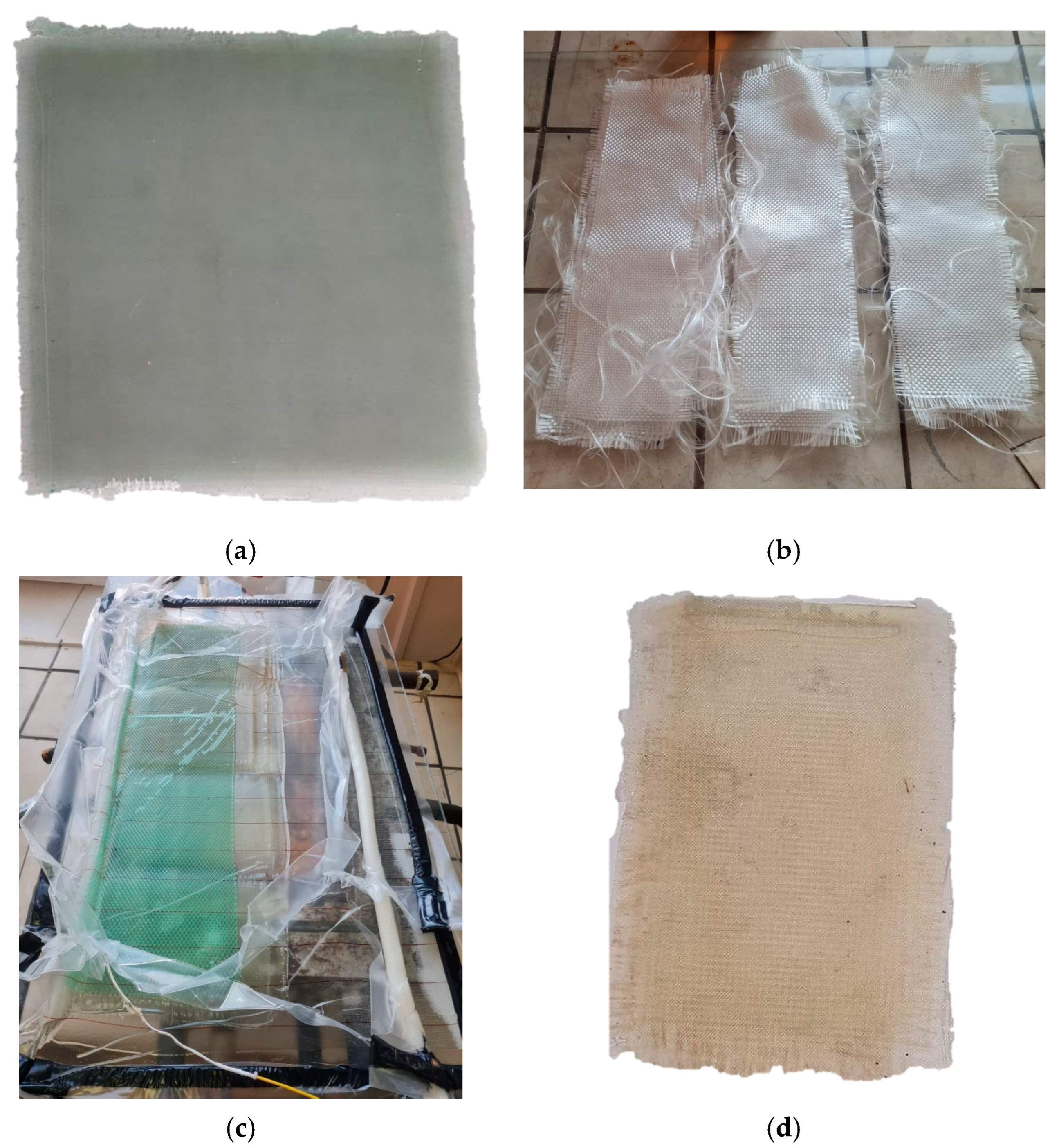
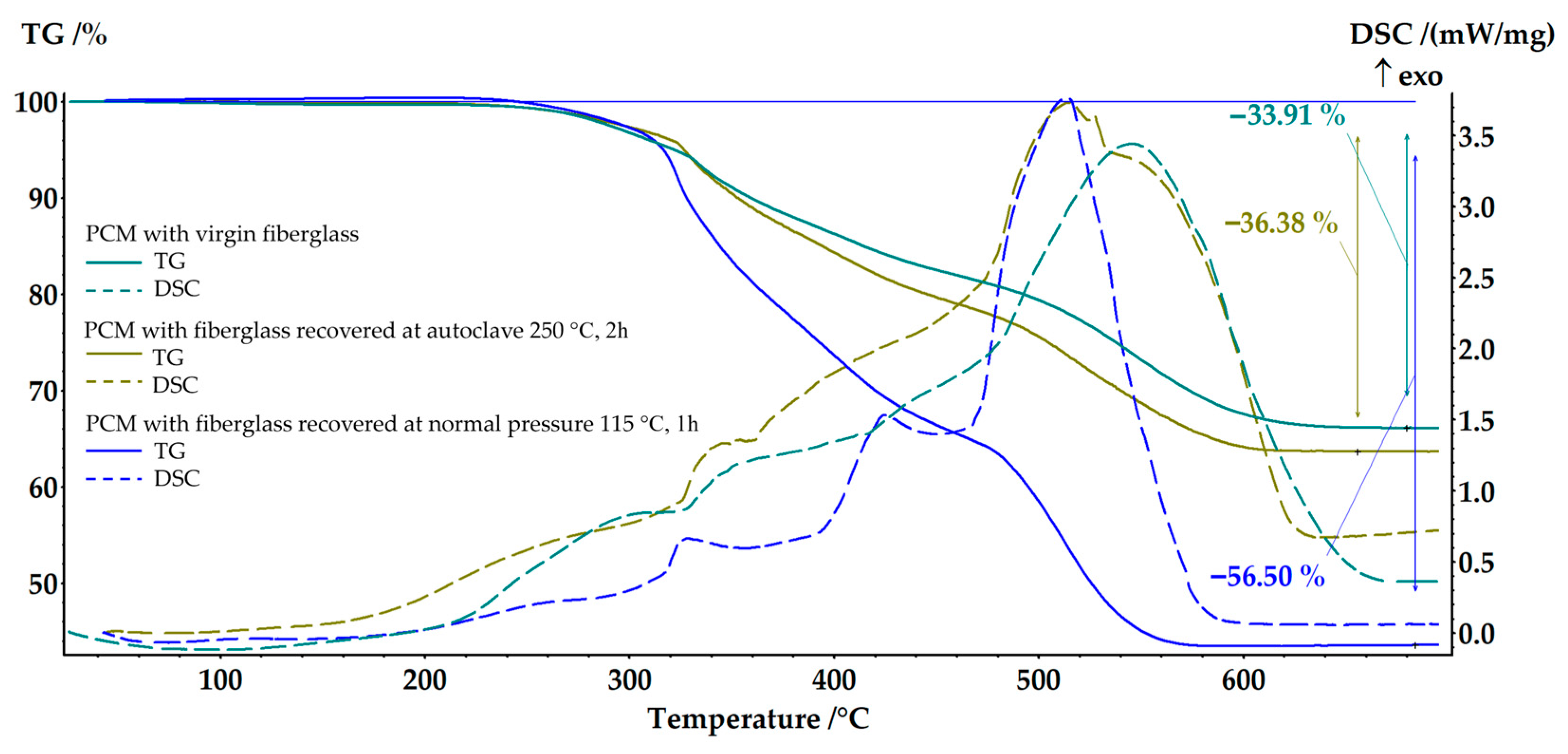
| Solvent | Δ, (cal/cm3)0.5 | Δδ, (cal/cm3)0.5 |
|---|---|---|
| Fragment of epoxy polymer | 10.095 | - |
| Aniline | 10.95 | 0.855 |
| Benzene | 9.3 | −0.795 |
| Xylene | 8.7 | −1.395 |
| Toluene | 8.9 | −1.195 |
| Pyridine | 10.1 | 0.005 |
| i-Butanol | 10.9 | 0.805 |
| Butyric acid | 11.2 | 1.105 |
| n-Butanol | 11.3 | 1.205 |
| Methanol | 14.8 | 4.705 |
| Ethanol | 13.0 | 2.905 |
| Acetic acid | 12.8 | 2.705 |
| Phenol | 12.1 | 2.005 |
| No. | T, °C | Time, h | Glass Fiber Tensile Strength, MPa | TG, wt.% |
|---|---|---|---|---|
| 1 | 200 | 10 | - | 18.5 |
| 2 | 220 | 5 | - | 11.9 |
| 3 | 250 | 2 | 2715 | 1.4 |
| 4 | 280 | 1 | 2645 | 2.8 |
| 0 | - | 2918 | 2.1 | |
| boiling | 1 | 2654 | 20.4 |
| Filler | Treatment | FGRP Flexural Strength, MPa |
|---|---|---|
| Origin fabric | - | 413 |
| Acetone treatment | 551.6 | |
| Acetone after wash of recovered fabrics | 616.4 | |
| Solvolysis liquid and acetone wash | 597.6 | |
| Recovered | Pyridine, 1 h at the boiling point | 295.4 |
| 2 h 250 °C | 539 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protsenko, A.E.; Protsenko, A.N.; Shakirova, O.G.; Petrov, V.V. Recycling of Epoxy/Fiberglass Composite Using Pyridine. Polymers 2025, 17, 1513. https://doi.org/10.3390/polym17111513
Protsenko AE, Protsenko AN, Shakirova OG, Petrov VV. Recycling of Epoxy/Fiberglass Composite Using Pyridine. Polymers. 2025; 17(11):1513. https://doi.org/10.3390/polym17111513
Chicago/Turabian StyleProtsenko, Alexander E., Alexandra N. Protsenko, Olga G. Shakirova, and Victor V. Petrov. 2025. "Recycling of Epoxy/Fiberglass Composite Using Pyridine" Polymers 17, no. 11: 1513. https://doi.org/10.3390/polym17111513
APA StyleProtsenko, A. E., Protsenko, A. N., Shakirova, O. G., & Petrov, V. V. (2025). Recycling of Epoxy/Fiberglass Composite Using Pyridine. Polymers, 17(11), 1513. https://doi.org/10.3390/polym17111513








