Enhancing the Sensory Quality, Stability, and Shelf Life of Baobab Fruit Pulp Drinks: The Role of Hydrocolloids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials Collection
2.3. Sample Preparation
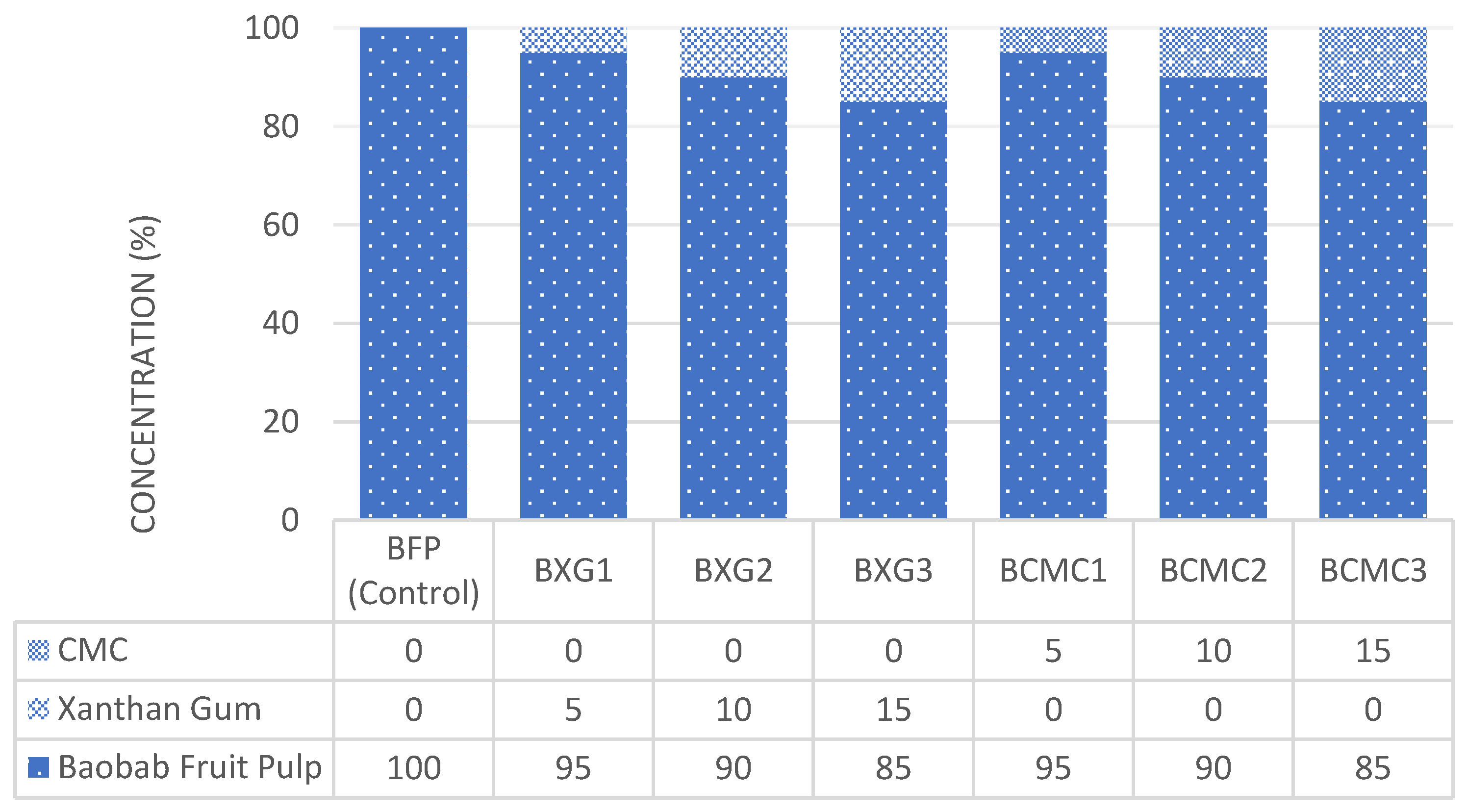
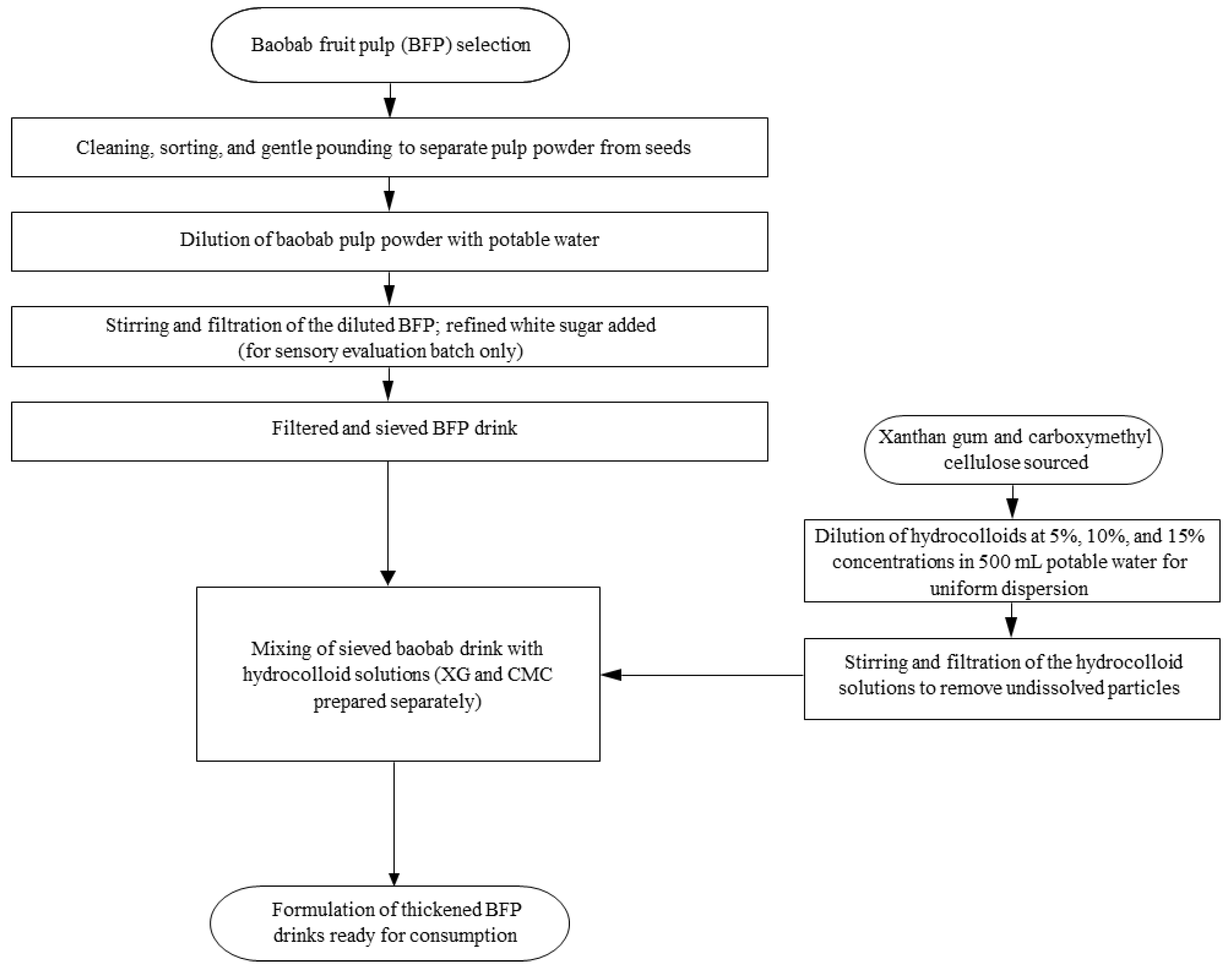
2.4. Extraction and Thickening of Baobab Fruit Pulp Drink
2.5. Physicochemical and Functional Properties
2.5.1. Titratable Acidity (TA) and pH Measurements
2.5.2. Total Soluble Solids (TSSs)
2.5.3. Bulk Density
2.5.4. Water Absorption Capacity (WAC)
2.5.5. Solubility and Swelling Power (SP)
2.5.6. Vitamin C Content
2.5.7. Viscosity
2.6. Microbial Analysis
2.7. Sensory Evaluation
2.8. Proximate Composition
2.8.1. Moisture Content
2.8.2. Ash Content
2.8.3. Crude Lipid Content
2.8.4. Crude Protein Content
2.8.5. Crude Fiber Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of the Powdered BFP
3.2. Functional Properties of the Studied BFP
3.3. Physicochemical Properties and Vitamin C Content
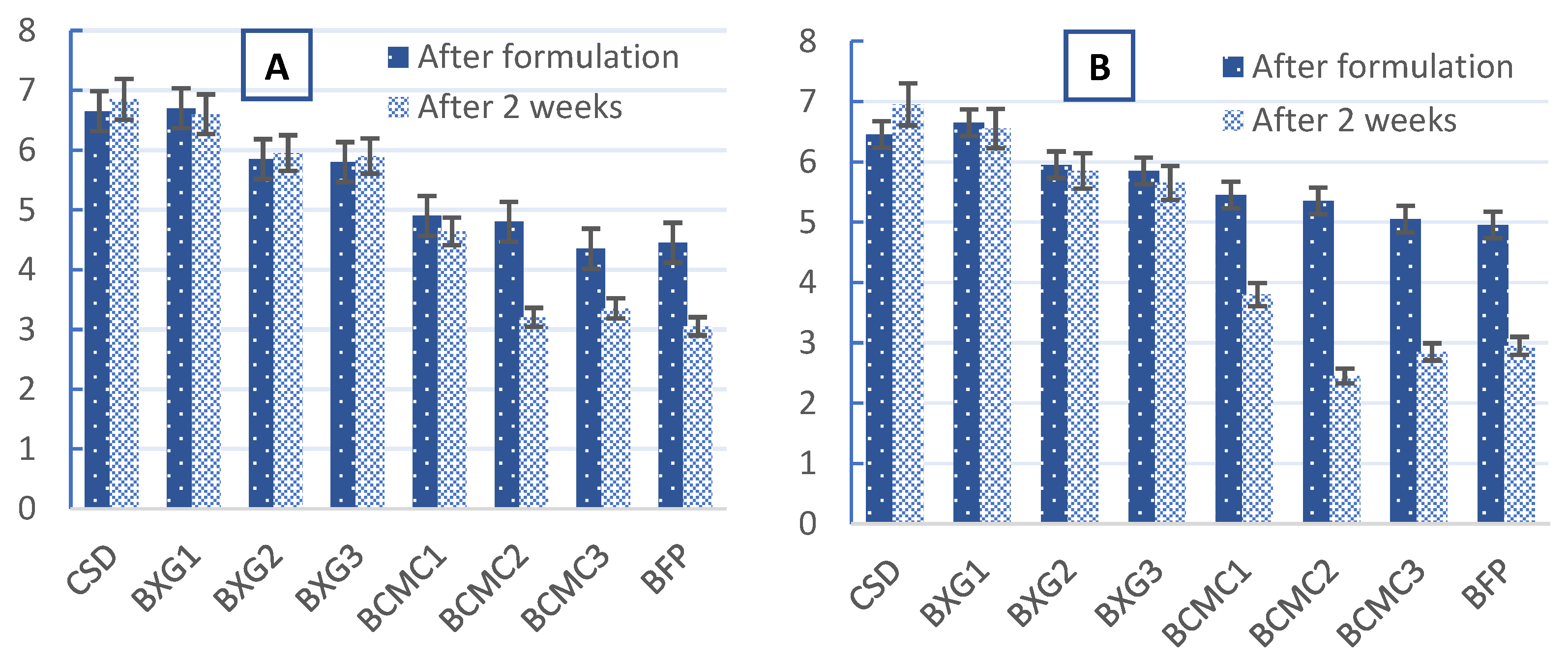
3.4. Sensory Scores of the BFP Drink Samples Immediately After Formulation and After 2 Weeks of Refrigeration at 4 °C
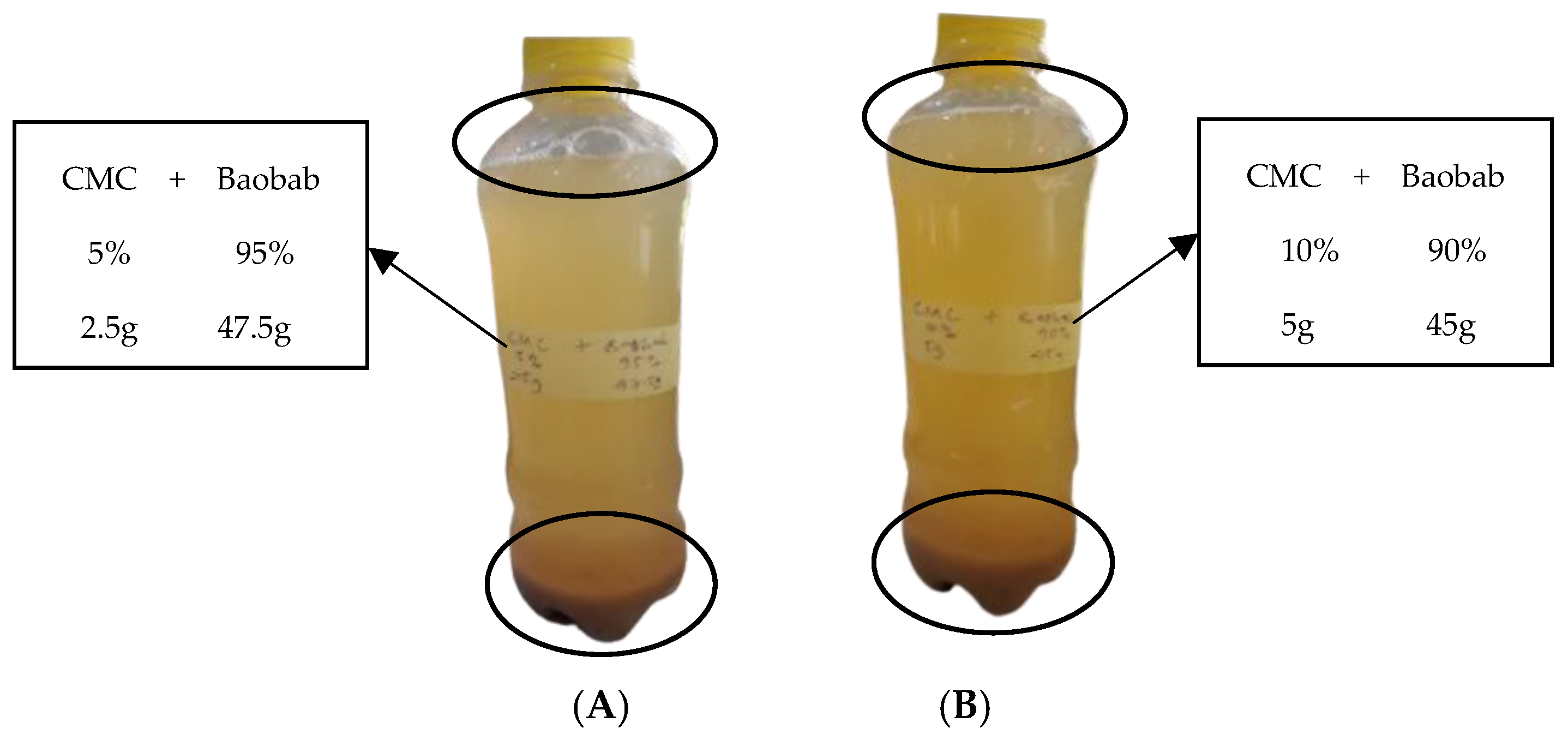
3.5. Microbial Growth in the BFP Drink
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BFP | Baobab fruit pulp |
| XG | Xanthan gum |
| CMC | Carboxymethyl cellulose |
| BXG | BFP: Xanthan gum |
| BCMC | BFP: Carboxymethyl cellulose |
| CFU | Colony Forming Unit |
| CSD | Commercial Synthetic Drink |
| Ca. | Circa |
| Kcal | Kilocalorie |
| USA | United States of America |
| EU | European Union |
| cP | Centipoises |
| IUPAC | International Union of Pure and Applied Chemistry |
| RPM | Revolution per minute |
| ICMSF | International Commission on Microbiological Specifications for Foods |
| APC | Aerobic plate count |
| TNTC | Too numerous to count |
References
- Ndjouenkeu, R.; Fombang, E.N.; Nkenmogne, I.E.K.; Panyoo, E.A.; Yadang, G. Technological and Nutraceutical Development Potentials of Some Traditional and Underutilized Plant Foods of the Savanna Region of Cameroon. In Nutritional and Health Aspects of Food in Western Africa; Elsevier: Amsterdam, The Netherlands, 2024; pp. 31–65. [Google Scholar]
- Nouruddeen, Z.; Mohd Ghazali, H.; Karim, R.; Mohammed, A. Baobab Tree (Adansonia digitata L.) Parts: Nutrition, Applications in Food and Uses in Ethno-Medicine—A Review. Ann. Nutr. Disord. Ther. 2014, 1, 1011. [Google Scholar]
- Sidibe, M.; Williams, J.T.; Hughes, A.; Haq, N.; Smith, R.W. Baobab, Adansonia digitata L., 1st ed.; International Centre for Underutilized Crops, University of Southampton: Southampton, UK, 2002. [Google Scholar]
- Coe, S.A.; Clegg, M.; Armengol, M.; Ryan, L. The Polyphenol-Rich Baobab Fruit (Adansonia digitata L.) Reduces Starch Digestion and Glycemic Response in Humans. Nutr. Res. 2013, 33, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M. An Updated Review of Adansonia digitata: A Commercially Important African Tree. S. Afr. J. Bot. 2011, 77, 908–919. [Google Scholar] [CrossRef]
- Wickens, G.E. The Baobabs: Pachycauls of Africa, Madagascar and Australia; Springer Science & Business Media: London, UK, 2008. [Google Scholar]
- Al-Khusaibi, M.; Al-Habsi, N.; Shafiur Rahman, M. Traditional Foods; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 3030246205. [Google Scholar]
- Saka, J.; Rapp, I.; Akinnifesi, F.; Ndolo, V.; Mhango, J. Physicochemical and Organoleptic Characteristics of Uapaca Kirkiana, Strychnos Cocculoides, Adansonia digitata and Mangiferia Indica Fruit Products. Int. J. Food Sci. Technol. 2007, 42, 836–841. [Google Scholar] [CrossRef]
- Nour, A.A.; Magboul, B.I.; Kheiri, N.H. Chemical Composition of Baobab Fruit (Adansonia digitata). Trop. Sci. 1980, 22, 383–388. [Google Scholar]
- Ahmed, A.E.M.; Mozzon, M.; Dawod, A.B.A.; Mustafa, E.O.A.; Mukarram, S.A.; Kovács, B. Potential of Baobab (Adansonia digitata L.) Dried Fruit Pulp in Contributing to Food and Nutrient Security in Sudan and Senegal. J. Agric. Food Res. 2025, 19, 101681. [Google Scholar] [CrossRef]
- Kaboré, D.; Sawadogo-Lingani, H.; Diawara, B.; Compaoré, C.S.; Dicko, M.H.; Jakobsen, M. A Review of Baobab (Adansonia digitata) Products: Effect of Processing Techniques, Medicinal Properties and Uses. Afr. J. Food Sci. 2011, 5, 833–844. [Google Scholar]
- Adedayo, M.R.; Olayemi, F.F.; Bamishaiye, E.I. Proximate and Mineral Composition of a Local Drink Made from Baobab Fruit (Adansonia digitata) Pulp. Adv. Biores. 2011, 2, 82–85. [Google Scholar]
- Gebauer, J.; El-Siddig, K.; Ebert, G. Baobab (Adansonia digitata L.) a Review on a Multipurpose Tree with Promising Future in the Sudan. Gartenbauwissenschaft 2002, 67, 155–160. [Google Scholar] [CrossRef]
- De Caluwe, E.; Halamoua, K.; Damme, P. Van Adansonia digitata L.—A Review of Traditional Uses, Phytochemistry and Pharmacology. Afr. Focus 2010, 23, 11–51. [Google Scholar] [CrossRef]
- Oyelek, G.O.; Salam, M.A.; Adetoro, R.O. Some Aspects of Nutrient Analysis of Seed, Pulp and Oil of Baobab (Adansonia digitata L.). IOSR J. Environ. Sci. Toxicol. Food Technol. 2012, 1, 32–50. [Google Scholar] [CrossRef]
- Magaia, T.; Uamusse, A.; Sjohol, I.; Skog, K. Dietary Fiber, Organic Acids and Minerals in Selected Wild Edible Fruits of Mozambique. Springerplus 2013, 2, 88. [Google Scholar] [CrossRef] [PubMed]
- Tembo, D.T.; Holmes, M.J.; Marshall, L.J. Effect of Thermal Treatment and Storage on Bioactive Compounds, Organic Acids and Antioxidant Activity of Baobab Fruit (Adansonia digitata) Pulp from Malawi. J. Food Compos. Anal. 2017, 58, 40–51. [Google Scholar] [CrossRef]
- Osman, M.A. Chemical and Nutrient Analysis of Baobab (Adansonia digitata) Fruit and Seed Protein Solubility. Plant Foods Hum. Nutr. 2004, 59, 29–33. [Google Scholar] [CrossRef]
- Abdalla, A.A.; Mohammed, M.A.; Mudawi, H.A. Production and Quality Assessment of Instant Baobab (Adansonia digitata L.). Adv. J. Food Sci. Technol. 2010, 2, 125–133. [Google Scholar]
- Abdalla, A.A.; Yagoup, N.E.H.; Mudawi, H.A. Production and Quality Evaluation of Baobab (Adansonia digitata) Beverages. J. Appl. Sci. Res. 2010, 6, 729–741. [Google Scholar]
- Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Shafiur Rahman, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air-and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef]
- Copeland, L. Assessing the Glycemic Impact of Starch by in Vitro Methods. Food Biosci. 2024, 60, 104434. [Google Scholar] [CrossRef]
- Alba, K.; Offiah, V.; Laws, A.P.; Falade, K.O.; Kontogiorgos, V. Baobab Polysaccharides from Fruits and Leaves. Food Hydrocoll. 2020, 106, 105–874. [Google Scholar] [CrossRef]
- Saka, J.D.K.; Mwendo-Phiri, E.; Akinnifesi, F.K. Community Processing and Nutritive Value of Some Miombo Indigenous Fruits in Central and Southern Malawi. In Proceedings of the 14th Southern African Regional Review and Planning Workshop, Harare, Zimbabwe, 3–7 September 2001; Akinnifesi, F.K., AGumya, A., Eds.; International Centre for Research in Agroforestry, ICRAF, Harare: Harare, Zimbabwe, 2002; pp. 164–169. [Google Scholar]
- Igboeli, L.; Addy, E.; Salami, L. Effects of Some Processing Techniques on the Antinutrient Contents of Baobab Seeds (Adansonia digitata). Bioresour. Technol. 1997, 59, 29–31. [Google Scholar] [CrossRef]
- Krüger, A.; Ferrero, C.; Zaritzky, N.E. Modelling Corn Starch Swelling in Batch Systems: Effect of Sucrose and Hydrocolloids. J. Food Eng. 2003, 58, 125–133. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A Review: Interaction of Starch/Non-Starch Hydrocolloid Blending and the Recent Food Applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Alam, M.; Kaur, S.; Dar, B.N.; Nanda, V. Classification, Techno-functional Properties, and Applications of Diverse Hydrocolloids in Fruits-based Products: A Concise Review. J. Food Sci. 2025, 90, e70119. [Google Scholar] [CrossRef]
- Asiamah, E.; Buckman, E.S.; Peget, F.; Akonor, P.T.; Padi, A.; Boateng, C.; Affrifah, N.S. Effect of Xanthan Gum and Carboxymethyl Cellulose on Structure, Functional and Sensorial Properties of Yam Balls. Heliyon 2022, 8, e11200. [Google Scholar] [CrossRef]
- Bahaji, A.; Li, J.; Sánchez-López, Á.M.; Baroja-Fernández, E.; Muñoz, F.J.; Ovecka, M.; Almagro, G.; Montero, M.; Ezquer, I.; Etxeberria, E. Starch Biosynthesis, Its Regulation and Biotechnological Approaches to Improve Crop Yields. Biotechnol. Adv. 2014, 32, 87–106. [Google Scholar] [CrossRef]
- Lee, H.-C.; Brant, D.A. Rheology of Concentrated Isotropic and Anisotropic Xanthan Solutions. 1. A Rodlike Low Molecular Weight Sample. Macromolecules 2002, 35, 2212–2222. [Google Scholar] [CrossRef]
- Lim, T.W.; Lim, R.L.H.; Pui, L.P.; Tan, C.P.; Ho, C.W. Synergistic Enhancing Effect of Xanthan Gum, Carboxymethyl Cellulose and Citric Acid on the Stability of Betacyanins in Fermented Red Dragon Fruit (Hylocereus polyrhizus) Drink during Storage. Heliyon 2023, 9, e21025. [Google Scholar] [CrossRef]
- Abedi, F.; Sani, A.M.; Karazhiyan, H. Effect of Some Hydrocolloids Blend on Viscosity and Sensory Properties of Raspberry Juice-Milk. J. Food Sci. Technol. 2014, 51, 2246–2250. [Google Scholar] [CrossRef]
- Vicent, V.; Rweyemamu, L. Rheological and Physicochemical Properties of Soy-Moringa Beverages: The Role of Xanthan Gum Stabilisation. Tanzan. J. Sci. 2025, 51, 241–255. [Google Scholar] [CrossRef]
- Suriya, K.; Jomduang, S. The Optimal Formula of Longan Energy Gel Drink Containing of High Bioactive Compounds and Antioxidant Activities. Int. J. Food Eng. 2019, 5, 256–261. [Google Scholar] [CrossRef]
- Adedokun, T.O.; Matemu, A.; Höglinger, O.; Mlyuka, E.; Adedeji, A. Evaluation of Functional Attributes and Storage Stability of Novel Juice Blends from Baobab, Pineapple, and Black-Plum Fruits. Heliyon 2022, 8, e09340. [Google Scholar] [CrossRef]
- do Nascimento Saldanha, G.R.; da Rocha Viana, J.D.; Portela, D.H.M.; Araujo, K.L.; da Silva Araújo, Í.M.; dos Santos Garruti, D.; de Moraes, I.V.M.; Wurlitzer, N.J. Development of a Dietary Fiber-Rich, High-Value Food Ingredient from Cashew Juice Processing for Use in Plant-Based Products. J. Food Meas. Charact. 2025, 19, 1386–1404. [Google Scholar] [CrossRef]
- Changes in AOAC® Official Methods of Analysis. J. AOAC Int. 1996, 79, 363–367. [CrossRef]
- Leach, H.W. Structure of starch granule I. Swelling and solubility patterns of various starches. J. Cereal Chem. 1959, 36, 534–544. [Google Scholar]
- Nielsen, S.S. Vitamin C Determination by Indophenol Method. In Nielsen’s Food Analysis Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2024; pp. 153–156. [Google Scholar]
- AOAC Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists Inc.: Rockville, MD, USA, 2020.
- Röβle, C.; Gormley, T.R.; Brunton, N.; Butler, F. Quality and Antioxidant Properties of Fresh-Cut Apple Wedges from 10 Cultivars During Modified Atmosphere Packaging Storage. Food Sci. Technol. Int. 2011, 17, 267–276. [Google Scholar] [CrossRef]
- Assogbadjo, A.E.; Kyndt, T.; Sinsin, B.; Gheysen, G.; Van Damme, P. Patterns of Genetic and Morphometric Diversity in Baobab (Adansonia digitata) Populations Across Different Climatic Zones of Benin (West Africa). Ann. Bot. 2006, 97, 819–830. [Google Scholar] [CrossRef]
- Adejuyitan, J.A.; Abioye, A.O.; Otunola, E.T.; Oyewole, Y.N. An Evaluation of Some Properties of Baobab Fruit Powder and Ogi Mixes. Transnatl. J. Sci. Technol. 2012, 2, 99–102. [Google Scholar]
- Woodroof, J.K.; Phillips, G.F. Carbonated and Non-Carbonated Beverages, 3rd ed.; Avi Publishing Company, Inc.: Westport, CT, USA, 1978. [Google Scholar]
- Nwachukwu, E.; Ezeigbo, C.G. Changes in the Microbial Population of Pasteurized Soursop Juice Treated with Benzoate and Lime during Storage. Afr. J. Microbiol. Res. 2013, 7, 3992–3995. [Google Scholar]
- Chadare, F.J.; Linnemann, A.R.; Hounhouigan, J.D.; Nout, M.J.R.; Van Boekel, M. Baobab Food Products: A Review on Their Composition and Nutritional Value. Crit. Rev. Food Sci. Nutr. 2008, 49, 254–274. [Google Scholar] [CrossRef]
- Sourri, P.; Tassou, C.C.; Nychas, G.-J.E.; Panagou, E.Z. Fruit Juice Spoilage by Alicyclobacillus: Detection and Control Methods—A Comprehensive Review. Foods 2022, 11, 747. [Google Scholar] [CrossRef]
- Owusu-Apenten, R.; Vieira, E. Fruits and Vegetables. In Elementary Food Science; Springer International Publishing: Cham, Switzerland, 2022; pp. 513–536. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Fasogbon, M.B. Chemical and Antioxidant Properties of Snake Tomato (Trichosanthes cucumerina) Juice and Pineapple (Ananas comosus) Juice Blends and Their Changes during Storage. Food Chem. 2017, 220, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Akkarachaneeyakorn, S.; Tinrat, S. Effects of Types and Amounts of Stabilizers on Physical and Sensory Characteristics of Cloudy Ready-to-Drink Mulberry Fruit Juice. Food Sci. Nutr. 2015, 3, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Boonditsataporn, L.; Vatthanakul, S. Effect of Carboxymethylcellulose and Xanthan Gum on the Physicochemical and Sensory Properties of Passion Fruit Topping Sauces. Int. J. Agric. Technol. 2022, 18, 35–48. [Google Scholar]
- Stadlmayr, B.; Wanangwe, J.; Waruhiu, C.G.; Jamnadass, R.; Kehlenbeck, K. Nutritional Composition of Baobab (Adansonia digitata L.) Fruit Pulp Sampled at Different Geographical Locations in Kenya. J. Food Compos. Anal. 2020, 94, 103617. [Google Scholar] [CrossRef]
- Tembo, D.T.W. Optimisation of Vitamin C Extraction and Physicochemical Properties of Adansonia digitata, Parinari curatellifolia, Strychnos cocculoides and Ziziphus mauritiana Fruits of Malawi. Master’s Thesis, University of Malawi, Chancellor College, Zomba, Malawi, 2008. [Google Scholar]
- USDA. FoodData Central. United States Department of Agriculture. Available online: https://fdc.nal.usda.gov/ (accessed on 10 May 2025).
- Monsen, E.R. Dietary Reference Intakes for the Antioxidant Nutrients: Vitamin C, Vitamin E, Selenium, and Carotenoids. J. Acad. Nutr. Diet. 2000, 100, 637. [Google Scholar]
- Carr, A.C.; Frei, B. Toward a New Recommended Dietary Allowance for Vitamin C Based on Antioxidant and Health Effects in Humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef]
- German Nutrition Society. New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, H.; Song, K.; Lee, J. Sensory and Rheological Characteristics of Thickened Liquids Differing Concentrations of a Xanthan Gum-based Thickener. J. Texture Stud. 2017, 48, 571–585. [Google Scholar] [CrossRef]
- Genovese, D.B.; Lozano, J.E. The Effect of Hydrocolloids on the Stability and Viscosity of Cloudy Apple Juices. Food Hydrocoll. 2001, 15, 1–7. [Google Scholar] [CrossRef]
- Gössinger, M.; Buchmayer, S.; Greil, A.; Griesbacher, S.; Kainz, E.; Ledinegg, M.; Leitner, M.; Mantler, A.; Hanz, K.; Bauer, R. Effect of Xanthan Gum on Typicity and Flavour Intensity of Cloudy Apple Juice. J. Food Process Preserv. 2018, 42, e13737. [Google Scholar] [CrossRef]
- Roberts, D.D.; Elmore, J.S.; Langley, K.R.; Bakker, J. Effects of Sucrose, Guar Gum, and Carboxymethylcellulose on the Release of Volatile Flavor Compounds under Dynamic Conditions. J. Agric. Food Chem. 1996, 44, 1321–1326. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Sensory Evaluation Practices; Academic Press: Cambridge, MA, USA, 2020; ISBN 0128153350. [Google Scholar]
- Wronkowska, M.; Zieliński, H.; Szmatowicz, B.; Ostaszyk, A.; Lamparski, G.; Majkowska, A. Effect of Roasted Buckwheat Flour and Hull Enrichment on the Sensory Qualities, Acceptance and Safety of Innovative Mixed Rye/Wheat and Wheat Bakery Products. J. Food Process Preserv. 2019, 43, e14025. [Google Scholar] [CrossRef]
- Végh, R.; Csóka, M.; Stefanovits-Bányai, É.; Juhász, R.; Sipos, L. Biscuits Enriched with Monofloral Bee Pollens: Nutritional Properties, Techno-Functional Parameters, Sensory Profile, and Consumer Preference. Foods 2022, 12, 18. [Google Scholar] [CrossRef]
- Su, Y.; Wang, H.; Wu, Z.; Zhao, L.; Huang, W.; Shi, B.; He, J.; Wang, S.; Zhong, K. Sensory Description and Consumer Hedonic Perception of Ultra-High Temperature (UHT) Milk. Foods 2022, 11, 1350. [Google Scholar] [CrossRef]
- Hossain, M.; Shishir, M.R.I.; Saifullah, K.U.S.; Safeuzzaman, R.M. Production and Investigation of Biochemical and Organoleptic Changes of Mixed Fruit Juice during Storage Period. Int. J. Food Sci. Nutr. Diet. 2016, 5, 271–277. [Google Scholar]
- Begam, S.; Das, P.; Karmoker, P. Processing of Mixed Fruit Juice from Mango, Orange and Pineapple. Fundam. Appl. Agric. 2018, 3, 440–445. [Google Scholar] [CrossRef]
- Huang, S.; An, S.; Kannan, P.R.; Wahab, A.; Ali, S.; Xiaoqing, L.; Suhail, M.; Iqbal, M.Z.; Kong, X. Development and Characterization of Biodegradable Antibacterial Hydrogels of Xanthan Gum for Controlled Ciprofloxacin Release. Int. J. Biol. Macromol. 2025, 309, 142637. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Yang, Y.; Shen, X.; Huang, M.; Liu, S.; Tang, X. Retention and Release Properties of Cinnamon Essential Oil in Antimicrobial Films Based on Chitosan and Gum Arabic. Food Hydrocoll. 2018, 84, 84–92. [Google Scholar] [CrossRef]
- Pegg, A.M. The Application of Natural Hydrocolloids to Foods and Beverages. In Natural Food Additives, Ingredients and Flavourings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 175–196. [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods). Microorganisms in Foods 7: Microbiological Testing in Food Safety Management; Springer: New York, NY, USA, 2002. [Google Scholar]



| Parameters 2 | |||||
|---|---|---|---|---|---|
| Sample Codes 1 | pH | TA (g Ascorbic Acid/100 mL) | TSS (°Brix) | Viscosity (cps) at 30 °C | Vitamin C (mg/L) |
| BXG1 | 3.7 ± 0.1 C | 4.1 ± 0.1 A | 8.3 ± 0.2 BC | 25.5 ± 0.2 A | 681 ± 1.0 C |
| BXG2 | 3.5 ± 0.1 B | 4.1 ± 0.5 A | 7.6 ± 0.9 BC | 27.2 ± 0.4 C | 755 ± 35.4 B |
| BXG3 | 4.0 ± 0.1 B | 3.8 ± 1.1 B | 8.5 ± 0.5 B | 30.8 ± 0.9 D | 653 ± 0.0 C |
| BCMC1 | 3.6 ± 0.1 BC | 3.7 ± 0.3 B | 9.1 ± 0.1 B | 26.3 ± 0.1 B | 560 ± 0.0 D |
| BCMC2 | 3.6 ± 0.0 C | 3.9 ± 0.1 B | 9.0 ± 0.0 B | 28.0 ± 0.1 C | 560 ± 0.0 D |
| BCMC3 | 3.6 ± 0.1 C | 3.9 ± 1.0 B | 8.5 ± 0.5 C | 29.8 ± 0.3 D | 656 ± 5.7 C |
| BFP | 3.10 ± 0.1 A | 4.2 ± 0.0 A | 7.4 ± 0.3 D | 20.9 ± 0.9 E | 801 ± 11.0 A |
| CSD | 4.2 ± 0.1 D | 2.7 ± 0.0 C | 12.4 ± 0.1 A | 24.05 ± 0.21 A | 290 ± 14.1 E |
| Sample Codes 1 | Total Plate Count (TPC) (cfu/mL) | |
|---|---|---|
| Immediately After Formulation | After 2 Weeks | |
| BXG1 | No Growth | 0.9 × 101 |
| BXG2 | No Growth | 1.9 × 101 |
| BXG3 | No Growth | 1.2 × 101 |
| BCMC1 | 0.9 × 101 | 5.2 × 102 |
| BCMC2 | 0.5 × 101 | 3.6 × 102 |
| BCMC3 | 0.2 × 101 | 4.2 × 102 |
| BFP | 1.5 × 101 | 5.6 × 102 |
| CSD | No Growth | 3.6 × 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, A.I.; Rilwan, A.; Nouruddeen, Z.B.; Ejiohuo, O.; Al-Habsi, N. Enhancing the Sensory Quality, Stability, and Shelf Life of Baobab Fruit Pulp Drinks: The Role of Hydrocolloids. Polymers 2025, 17, 1396. https://doi.org/10.3390/polym17101396
Muhammad AI, Rilwan A, Nouruddeen ZB, Ejiohuo O, Al-Habsi N. Enhancing the Sensory Quality, Stability, and Shelf Life of Baobab Fruit Pulp Drinks: The Role of Hydrocolloids. Polymers. 2025; 17(10):1396. https://doi.org/10.3390/polym17101396
Chicago/Turabian StyleMuhammad, Abdullahi Idris, Abdulrashid Rilwan, Zahrau Bamalli Nouruddeen, Ovinuchi Ejiohuo, and Nasser Al-Habsi. 2025. "Enhancing the Sensory Quality, Stability, and Shelf Life of Baobab Fruit Pulp Drinks: The Role of Hydrocolloids" Polymers 17, no. 10: 1396. https://doi.org/10.3390/polym17101396
APA StyleMuhammad, A. I., Rilwan, A., Nouruddeen, Z. B., Ejiohuo, O., & Al-Habsi, N. (2025). Enhancing the Sensory Quality, Stability, and Shelf Life of Baobab Fruit Pulp Drinks: The Role of Hydrocolloids. Polymers, 17(10), 1396. https://doi.org/10.3390/polym17101396









