Intelligent Biopolymer-Based Films: Promising New Solutions for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Film Preparation
- Chitosan-based films
- Chitosan–PVA-based films
- PVA-based films
- PLA-based films
2.2.2. Evaluation of Halochromic Properties
2.2.3. Evaluation of Antioxidant Activity/Capacity
2.2.4. Evaluation of the Moisture Content
2.2.5. Evaluation of Swelling Capacity and Solubility
2.2.6. Indicator Film Testing on Food Products
2.2.7. Film Characterization by Infrared Spectroscopy (ATR FT-IR)
2.2.8. Thermogravimetric Analysis
3. Results and Discussion
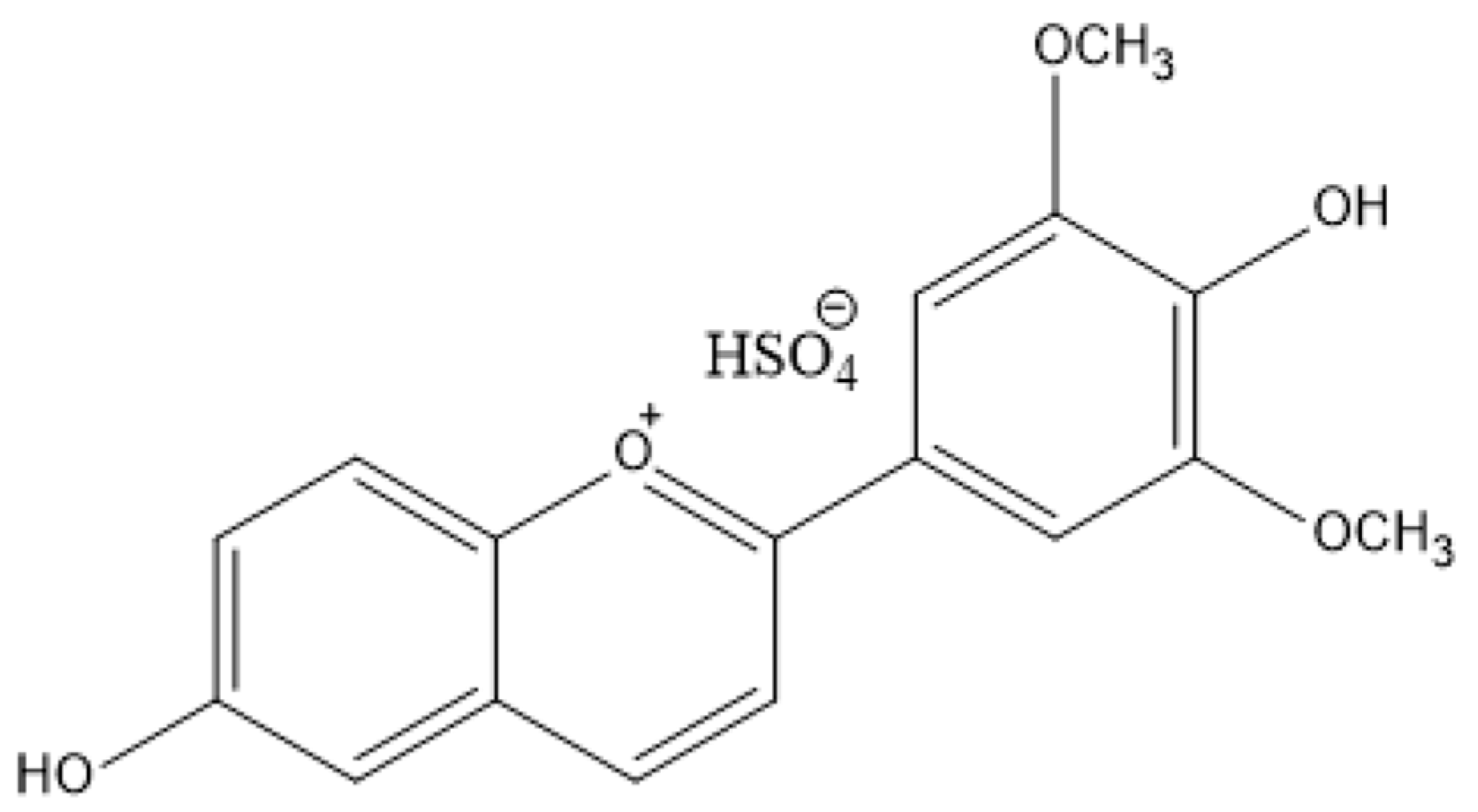
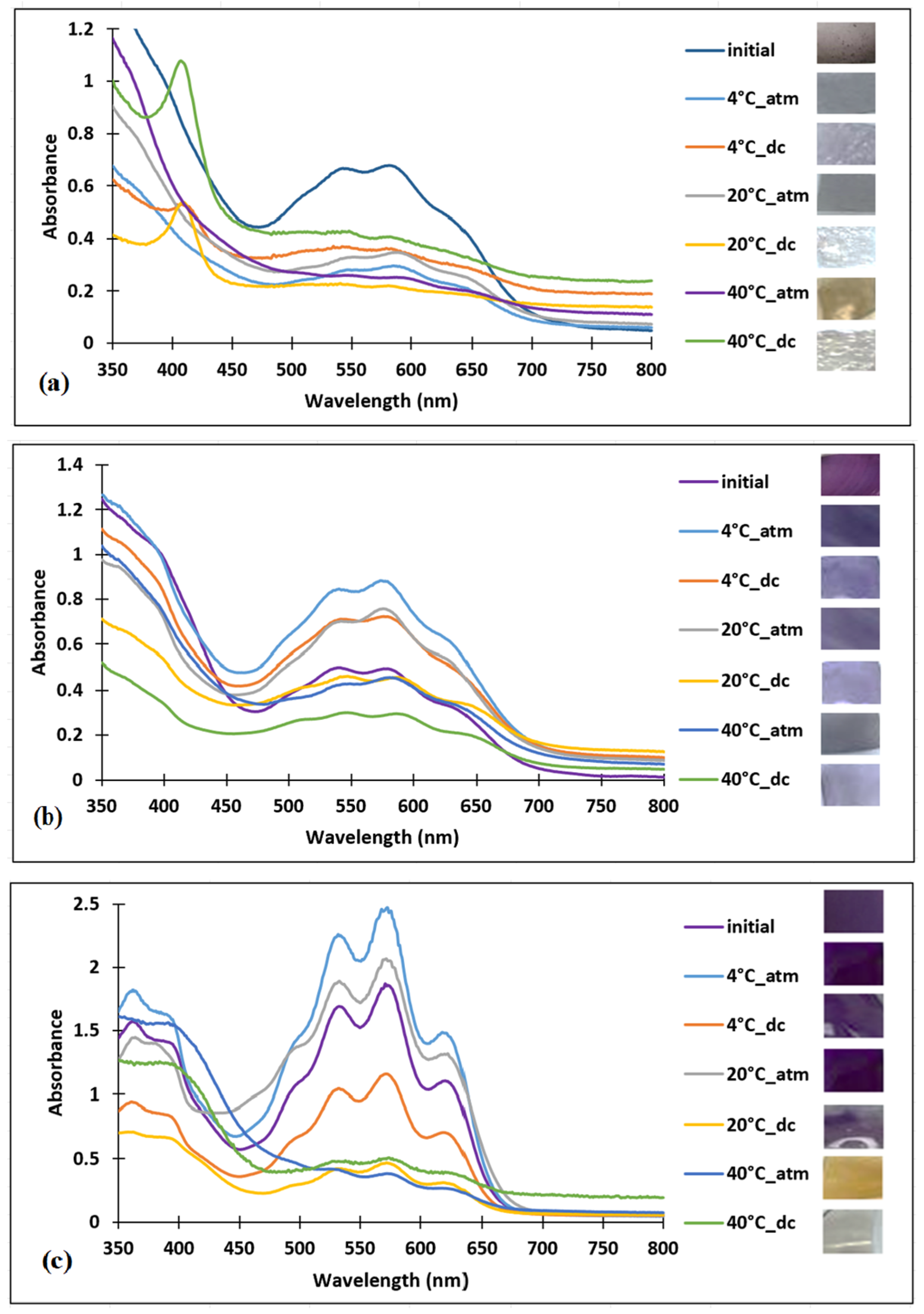
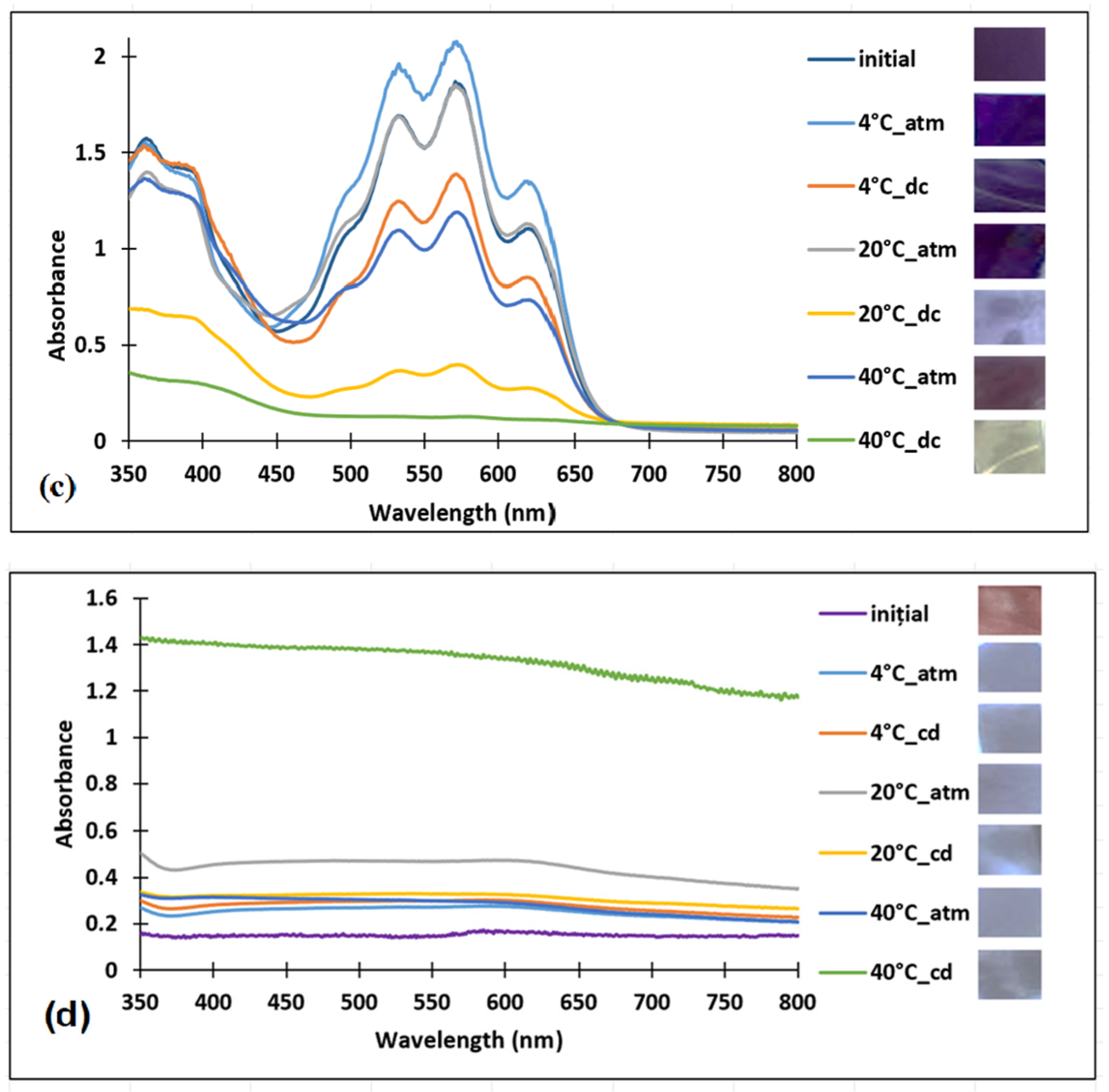
3.1. Preparation and Characterization of the Polymer Films Loaded with Anthocyanin-Inspired Dye
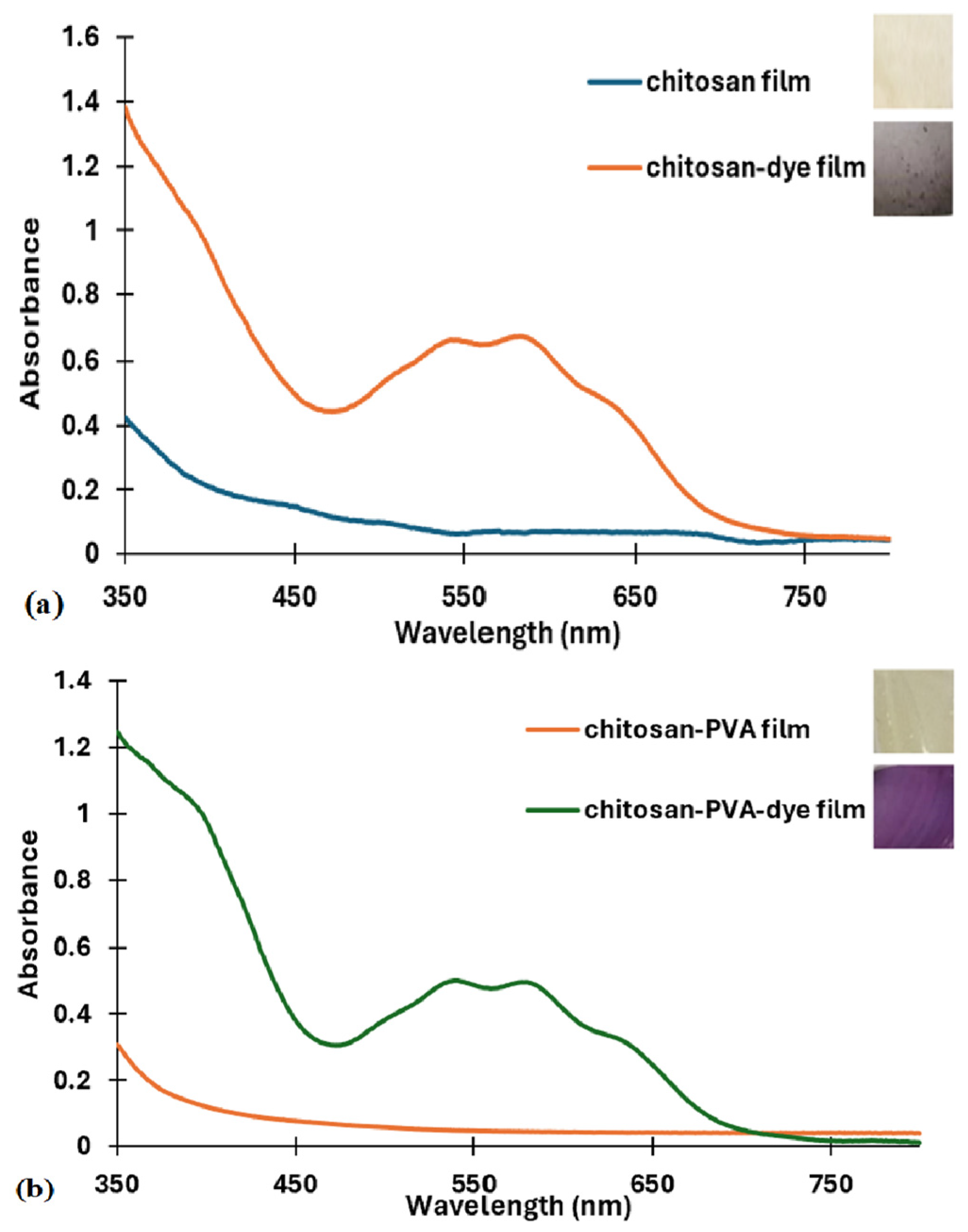
3.1.1. Validation of Dye Incorporation into the Polymer Matrix by FT-IR and UV–VIS Spectroscopy
3.1.2. Thermal Stability and Performance of Dye-Based Films
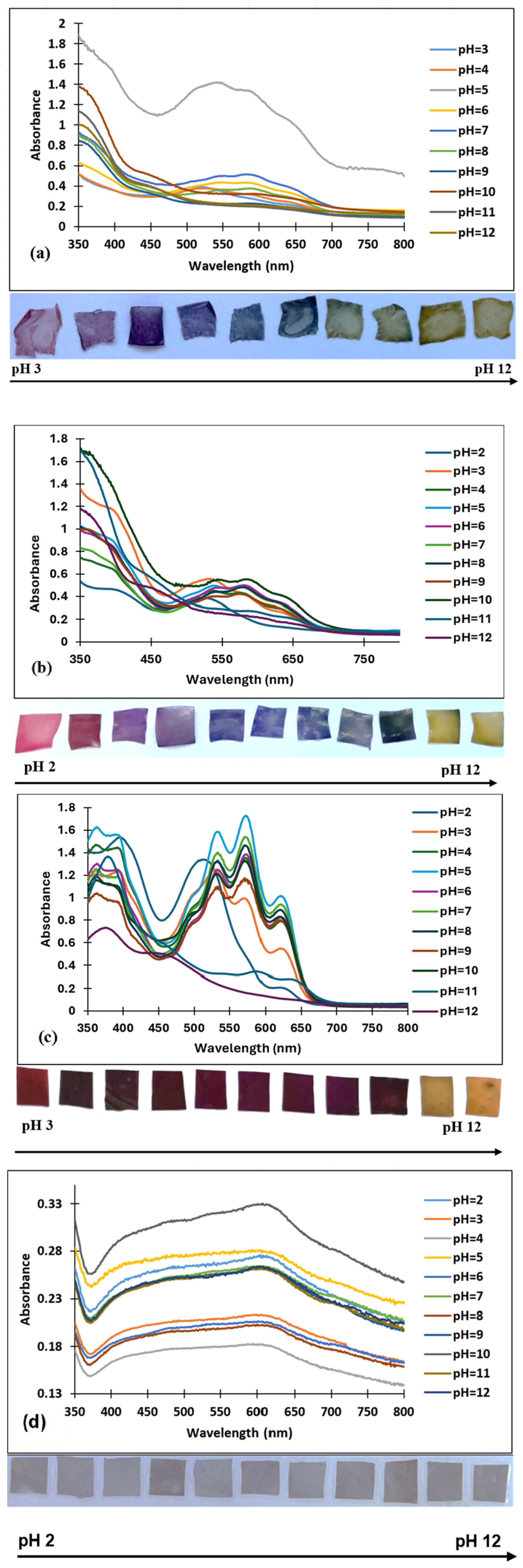
3.2. Sensitivity of the Films to pH Changes
3.3. Antioxidant Properties of the Polymer Film–Dye Composites
3.4. Evaluation of the Moisture Content
3.5. Evaluation of Swelling Capacity and Solubility
3.6. Evaluation of the Efficacy of Film–Dye Composite Films as an Indicator of the Freshness of Samples of Food Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2020, 40, e12725. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Pirouzifard, M.K.; Erfani, A. Smart film based on chitosan/Melissa officinalis essences/pomegranate peel extract to detect cream cheeses spoilage. Food Addit. Contam. Part A 2020, 37, 634–648. [Google Scholar] [CrossRef]
- Zeng, T.; Durif, F.; Robinot, E. Can eco-design packaging reduce consumer food waste? an experimental study. Technol. Forecast. Soc. Chang. 2021, 162, 120342. [Google Scholar] [CrossRef]
- Gomes, V.; Pires, A.S.; Mateus, N.; de Freitas, V.; Cruz, L. Pyranoflavylium-cellulose acetate films and the glycerol effect towards the development of pH-freshness smart label for food packaging. Food Hydrocoll. 2022, 127, 107501. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Liu, L.; Cui, Z.; Zhong, Y. Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods 2022, 11, 1884. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, L.; Yu, J.; Shao, P. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, H.; Zhao, J.; Humayun, M.; Wu, S.; Wang, C.; Zhi, Z.; Pang, J. A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness. Food Hydrocoll. 2022, 133, 107998. [Google Scholar] [CrossRef]
- Bilgiç, S.; Söğüt, E.; Seydim, A.C. Chitosan and Starch Based Intelligent Films with Anthocyanins from Eggplant to Monitor pH Variations. Turk. J. Agric. Food Sci. Technol. 2019, 7, 61–66. [Google Scholar] [CrossRef]
- Shao, P.; Liu, L.; Yu, J.; Lin, Y.; Gao, H.; Chen, H.; Sun, P. An overview of intelligent freshness indicator packaging for food quality and safety monitoring. Trends Food Sci. Technol. 2021, 118, 285–296. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Xu, F.; Yun, D.; Yong, H.; Liu, J. Development of pork and shrimp freshness monitoring labels based on starch/polyvinyl alcohol matrices and anthocyanins from 14 plants: A comparative study. Food Hydrocoll. 2022, 124, 107293. [Google Scholar] [CrossRef]
- Kuswandi, B.; Seftyani, M.; Pratoko, D.K. Edible colorimetric label based on immobilized purple sweet potato anthocyanins onto edible film for packaged mushrooms freshness monitoring. J. Food Sci. Technol. 2024, 61, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Trevisol, T.C.; Pelicioli, J.; Terrazas, M.B.; Monteiro, A.R.; Valencia, G.A. Preparation and Characterization of Colorimetric Indicator Films Based on Chitosan/Polyvinyl Alcohol and Anthocyanins from Agri-Food Wastes. J. Polym. Environ. 2021, 29, 1616–1629. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible film sensors containing red radish extract for meat spoilage observation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Barbosa, C.H.; Cerqueira, M.A.; Azevedo, A.G.; Barros, C.; Machado, A.V.; Coelho, A.; Furtado, R.; Correia, C.B.; Saraiva, M.; et al. PLA films loaded with green tea and rosemary polyphenolic extracts as an active packaging for almond and beef. Food Packag. Shelf Life 2023, 36, 101041. [Google Scholar] [CrossRef]
- Wang, W.; Niu, B.; Liu, R.; Chen, H.; Fang, X.; Wu, W.; Wang, G.; Gao, H.; Mu, H. Development of bio-based PLA/cellulose antibacterial packaging and its application for the storage of shiitake mushroom. Food Chem. 2023, 429, 136905. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Xu, Y.; Ali, A.; Hussain, Z.; Duan, Q.; Liu, H.; Yu, L. Antimicrobial packaging materials of PLA/starch composites functionalized by pomegranate peel. J. Taiwan Inst. Chem. Eng. 2024, 156, 105371. [Google Scholar] [CrossRef]
- Shin, H.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. Preparation and characterization of ductile PLA/PEG blend films for eco-friendly flexible packaging application. Food Packag. Shelf Life 2022, 34, 100966. [Google Scholar] [CrossRef]
- Barik, M.; BhagyaRaj, G.V.S.; Dash, K.K.; Shams, R. A thorough evaluation of chitosan-based packaging film and coating for food product shelf-life extension. J. Agric. Food Res. 2004, 16, 101164. [Google Scholar] [CrossRef]
- Hisham, F.; Akmal, M.M.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer chitosan: Potential sources, extraction methods, and emerging applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Cong, M.; Qi, Y.; Wan, K.; Jiang, G.; Liu, X. Characterization of chitosan film incorporated pine bark extract and application in carp slices packaging. Int. J. Biol. Macromol. 2024, 271, 132609. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag. Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Zúniga, M.M.G.; Ding, R.; Oh, E.; Nguyen, T.B.; Trung, T.T.; Nam, J.-D.; Suhr, J. Avocado seed starch utilized in eco-friendly, UV-blocking, and high-barrier polylactic acid (PLA) biocomposites for active food packaging applications. Int. J. Biol. Macromol. 2024, 265, 130837. [Google Scholar] [CrossRef]
- Bhat, V.G.; Masti, S.P.; Narasagoudr, S.S.; Chougale, R.B.; SK, P.K.; Dalbanjan, N.P.; Malabadi, R.B. Chitosan, Poly(vinyl alcohol) and Chitosan/Poly(vinyl alcohol) based active films loaded with white turmeric powder for food packaging applications. Food Biosci. 2024, 60, 104402. [Google Scholar] [CrossRef]
- Shetty, G.R.; Rao, B.L. Preparation and characterization of silk fibroin-polyvinyl alcohol (PVA) blend films for food packaging materials. Mater. Today Proc. 2022, 55, 194–200. [Google Scholar] [CrossRef]
- Souza, R.D.; Lopes, E.R.; Ramos, E.M.; de Oliveira, T.V.; de Oliveira, C.P. Active packaging: Development and characterization of polyvinyl alcohol (PVA) and nitrite film for pork preservation. Food Chem. 2024, 437, 137811. [Google Scholar] [CrossRef]
- Wardani, N.I.; Alahmad, W.; Varanusupakul, P. A review of utilizing anthocyanins as natural reagents for eco-friendly solid-state colorimetric sensors: A green perspective. Green Anal. Chem. 2024, 9, 100117. [Google Scholar] [CrossRef]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J. Food Sci. Technol. 2022, 59, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]
- Parlak, M.E.; Sahin, O.I.; Dundar, A.N.; Saricaoglu, F.T.; Smaoui, S.; Goksen, G.; Koirala, P.; Al-Asmari, F.; Nirmal, N.P. Natural colorant incorporated biopolymers-based pH-sensing films for indicating the food product quality and safety. Food Chem. 2024, 439, 138160. [Google Scholar] [CrossRef]
- Gomes, V.; Bermudez, R.; Mateus, N.; Guedes, A.; Lorenzo, J.M.; de Freitas, V.; Cruz, L. FoodSmarTag: An innovative dynamic labeling system based on pyranoflavylium-based colorimetric films for real-time monitoring of food freshness. Food Hydrocoll. 2023, 143, 108914. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Păușescu, I.; Todea, A.; Dreavă, D.-M.; Boboescu, T.; Pațcan, B.; Pațcan, L.; Albulescu, D.; Badea, V.; Peter, F.; Tőtős, R.; et al. Experimental and Computational Studies on Bio-Inspired Flavylium Salts as Sensitizers for Dye-Sensitized Solar Cells. Materials 2022, 15, 6985. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.M.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.D.; Huang, X.W.; Jiang, C.P.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Lu, R.; Sameen, D.E.; Qin, W.; Wu, D.; Dai, J.; Li, S.; Liu, Y. Development of polylactic acid films with selenium microparticles and its application for food packaging. Coatings 2020, 10, 280. [Google Scholar] [CrossRef]
- Carmody, W.R. Easily prepared wide range buffer series. J. Chem. Educ. 1961, 38, 559. [Google Scholar] [CrossRef]
- Portes, E.; Gardrat, C.; Castellan, A.; Coma, V. Environmentally friendly films based on chitosan and tetrahydrocurcuminoid derivatives exhibiting antibacterial and antioxidative properties. Carbohydr. Polym. 2009, 76, 578–584. [Google Scholar] [CrossRef]


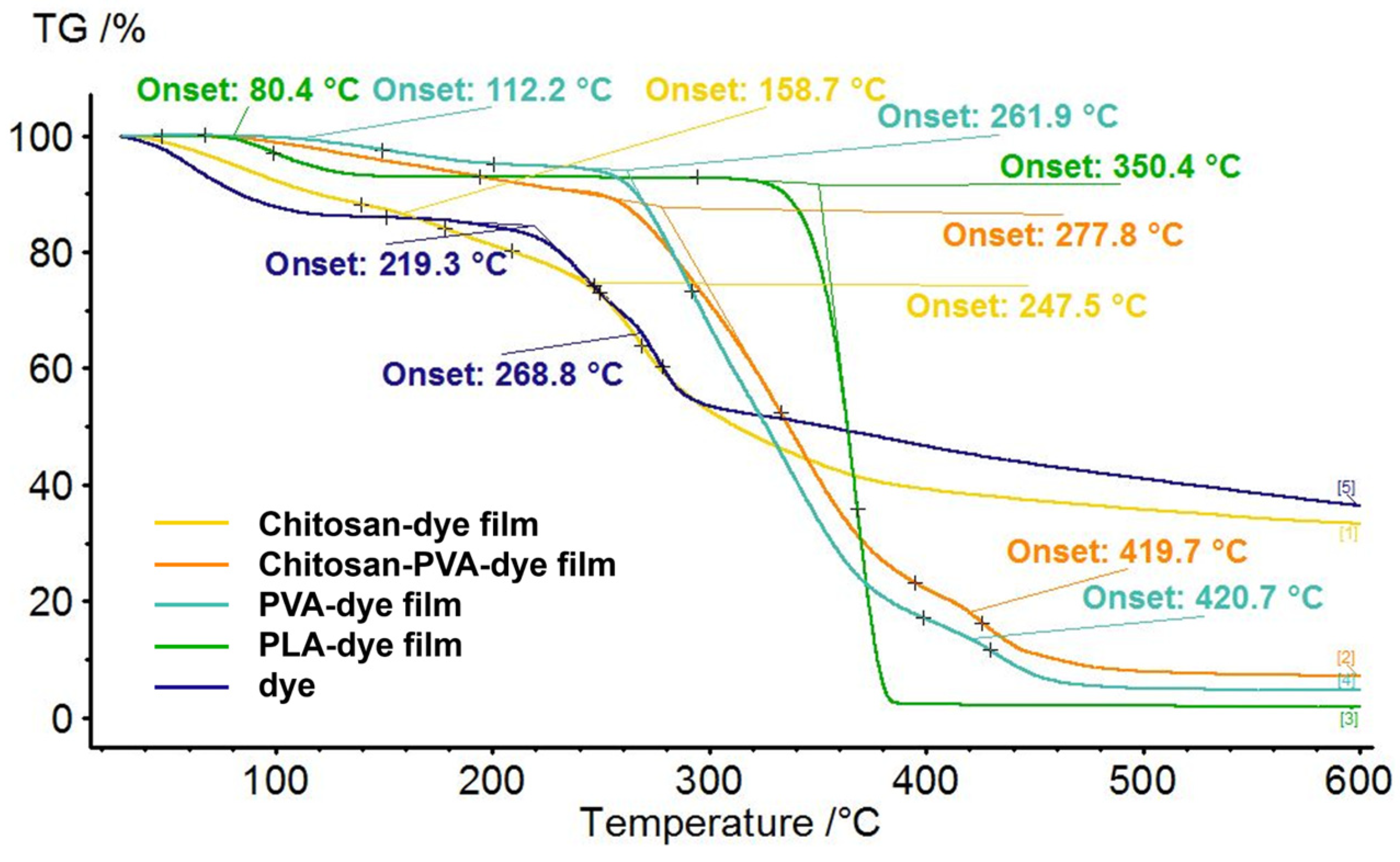


| Compound | Weight Loss [%] | |||
|---|---|---|---|---|
| 1st Stage | 2nd Stage | 3rd Stage | Final | |
| Chitosan–dye | 4.21 | 16.09 | - | 66.74 |
| Chitosan–PVA–dye | 40.76 | 6.81 | - | 92.92 |
| PVA–dye | 2.48 | 21.88 | 5.48 | 95.32 |
| PLA–dye | 2.86 | 57.09 | - | 98.17 |
| Dye | 11.83 | 12.96 | - | 63.65 |
| Polymeric Film | AA [%] |
|---|---|
| Chitosan–dye | 99.92 ± 1.78 |
| Chitosan–PVA–dye | 99.82 ± 2.07 |
| PVA–dye | 99.13 ± 3.21 |
| PLA–dye | 95.72 ± 2.86 |
| Polymeric Film | Moisture Content [%] |
|---|---|
| Chitosan–dye | 17.20 ± 2.97 |
| Chitosan–PVA–dye | 6.66 ± 1.89 |
| PVA–dye | 6.26 ± 3.45 |
| PLA–dye | 20.80 ± 3.32 |
| Polymeric Film | Water Solubility [%] | Swelling Capacity [%] |
|---|---|---|
| Chitosan–dye | 86.98 ± 4.13 | - |
| Chitosan–PVA–dye | 59.46 ± 3.65 | 81.76 ± 4.76 |
| PVA–dye | 52.72 ± 3.72 | 64.95 ± 3.77 |
| PLA–dye | 0.00 ± 0.00 | 0.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dăescu, D.I.; Dreavă, D.M.; Todea, A.; Peter, F.; Păușescu, I. Intelligent Biopolymer-Based Films: Promising New Solutions for Food Packaging Applications. Polymers 2024, 16, 2256. https://doi.org/10.3390/polym16162256
Dăescu DI, Dreavă DM, Todea A, Peter F, Păușescu I. Intelligent Biopolymer-Based Films: Promising New Solutions for Food Packaging Applications. Polymers. 2024; 16(16):2256. https://doi.org/10.3390/polym16162256
Chicago/Turabian StyleDăescu, Diana Ionela, Diana Maria Dreavă, Anamaria Todea, Francisc Peter, and Iulia Păușescu. 2024. "Intelligent Biopolymer-Based Films: Promising New Solutions for Food Packaging Applications" Polymers 16, no. 16: 2256. https://doi.org/10.3390/polym16162256
APA StyleDăescu, D. I., Dreavă, D. M., Todea, A., Peter, F., & Păușescu, I. (2024). Intelligent Biopolymer-Based Films: Promising New Solutions for Food Packaging Applications. Polymers, 16(16), 2256. https://doi.org/10.3390/polym16162256







