Chemical Recycling of PLA and Its Copolyesters with Poly(Ethylene Azelate) via Microwave-Assisted Alkaline Hydrolysis and Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLA, PEAz, and Their Copolyesters
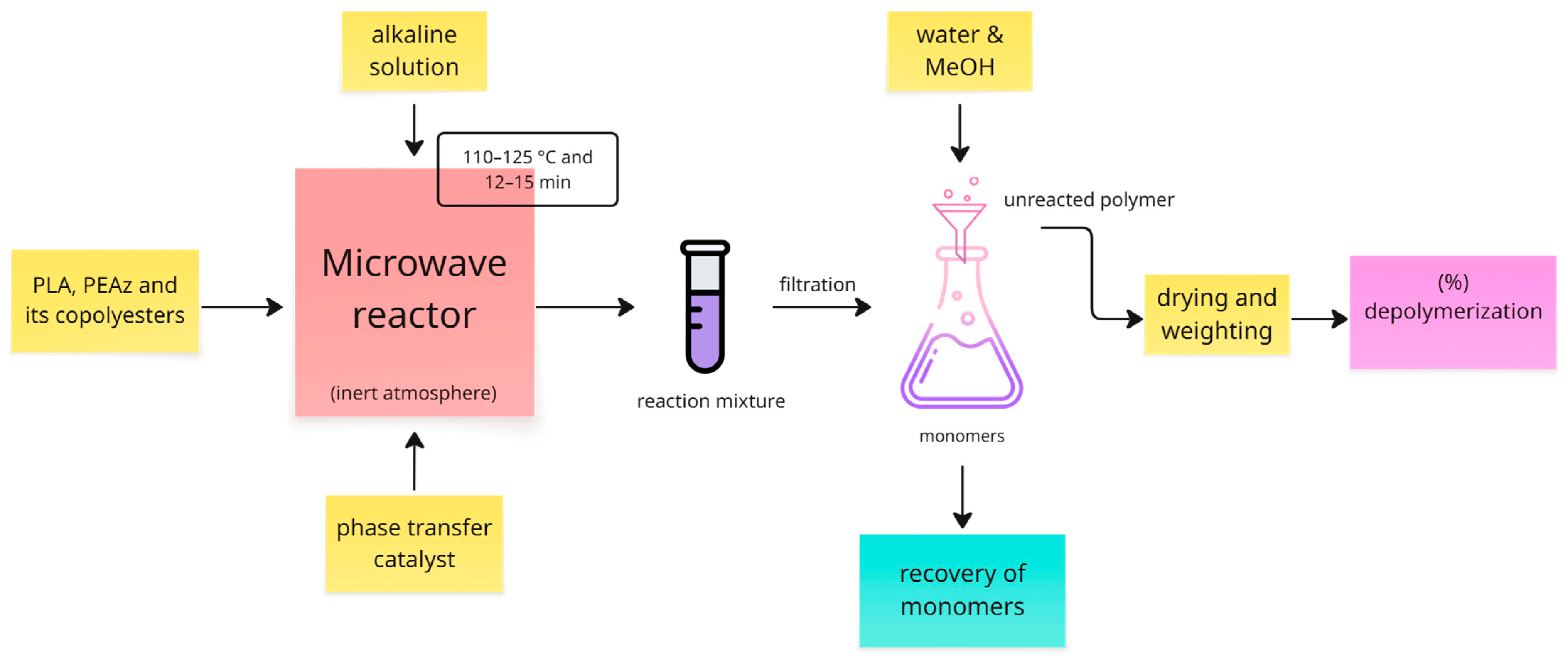
2.3. Alkaline Hydrolysis Assisted with Microwave Irradiation
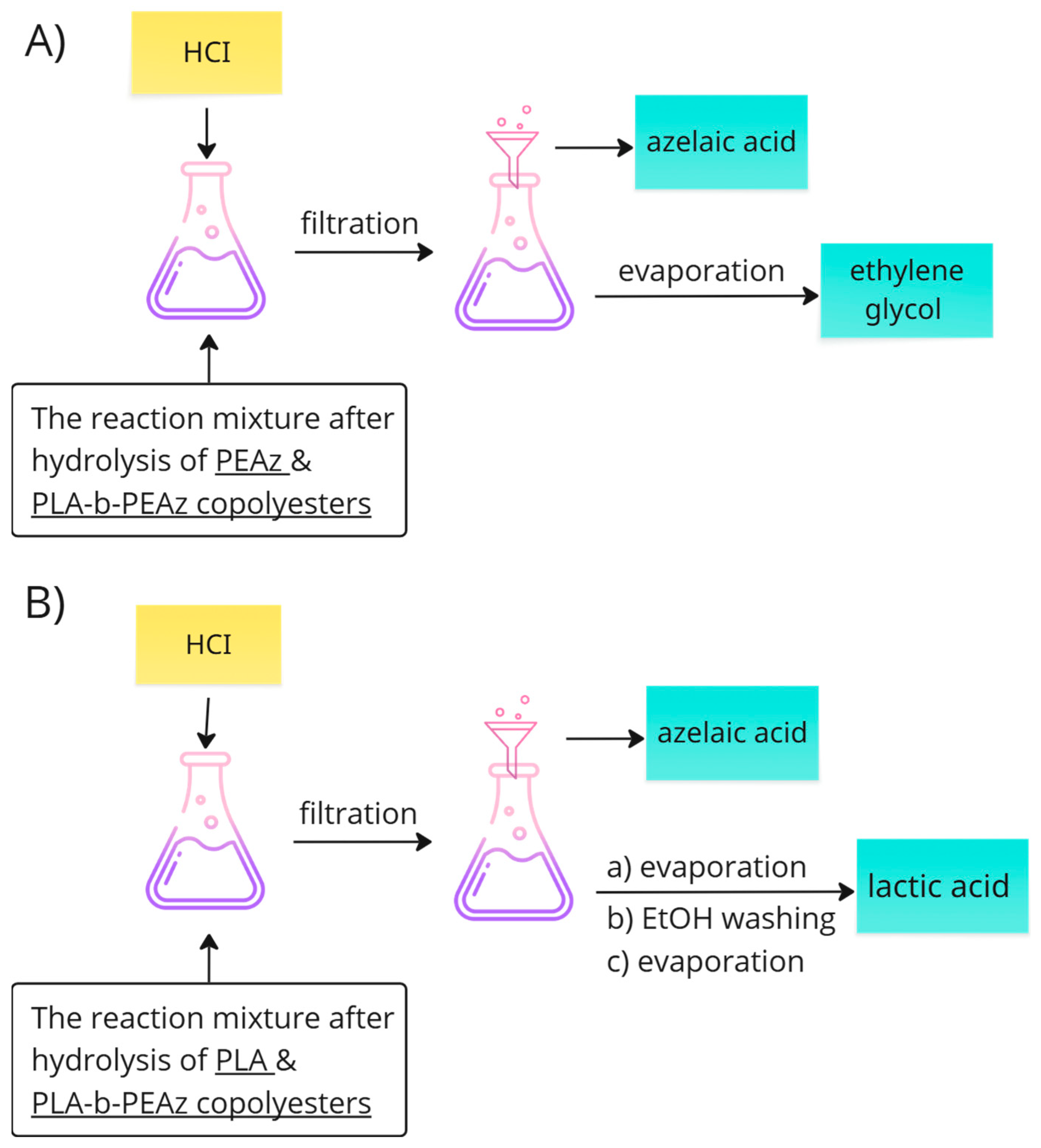
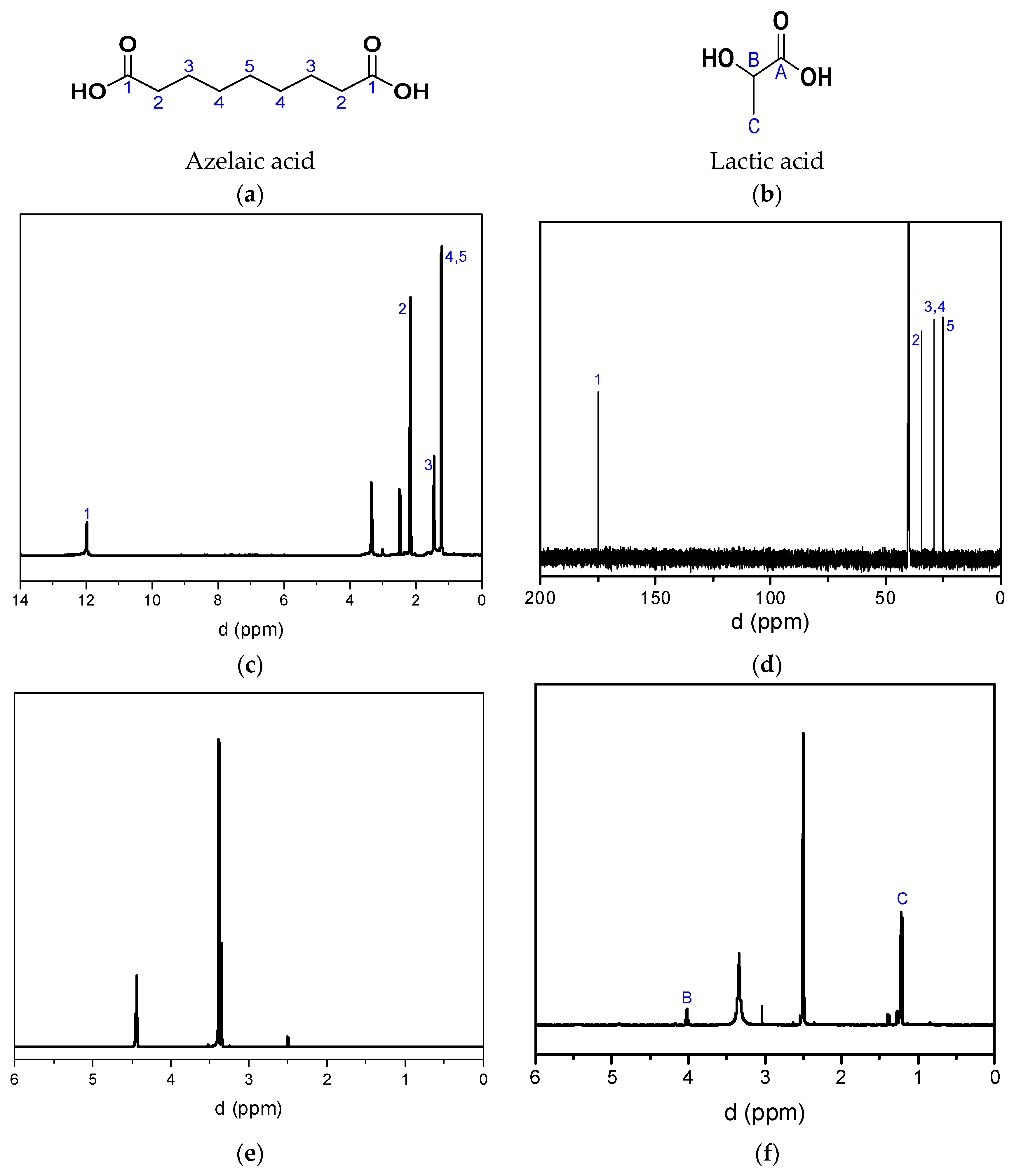
2.4. Recovery of Lactic Acid and Azelaic Acid
- In the case of poly(ethylene azelate), the HCI solution was added in order to reach the pH value close to 1–2. In acidic conditions the precipitation of azelaic acid immediately occurred and the acid was collected through filtration. After the evaporation of the solvents, ethylene glycol was retrieved, but only in the case of ethylene glycol.
- In the case of PLA and its copolyesters, to remove the (co)-polymer residues, the filtrate was acidified, causing azelaic acid to precipitate. It was then collected by filtration, and the filtrate was concentrated. Lactic acid was subsequently collected after the addition of EtOH (by concentration).
2.5. Enzymatic Hydrolysis
2.6. Characterizations
2.6.1. GPC—Gel Permeation Chromatography
2.6.2. NMR—Nuclear Magnetic Resonance
2.6.3. DSC—Differential Scanning Calorimetry
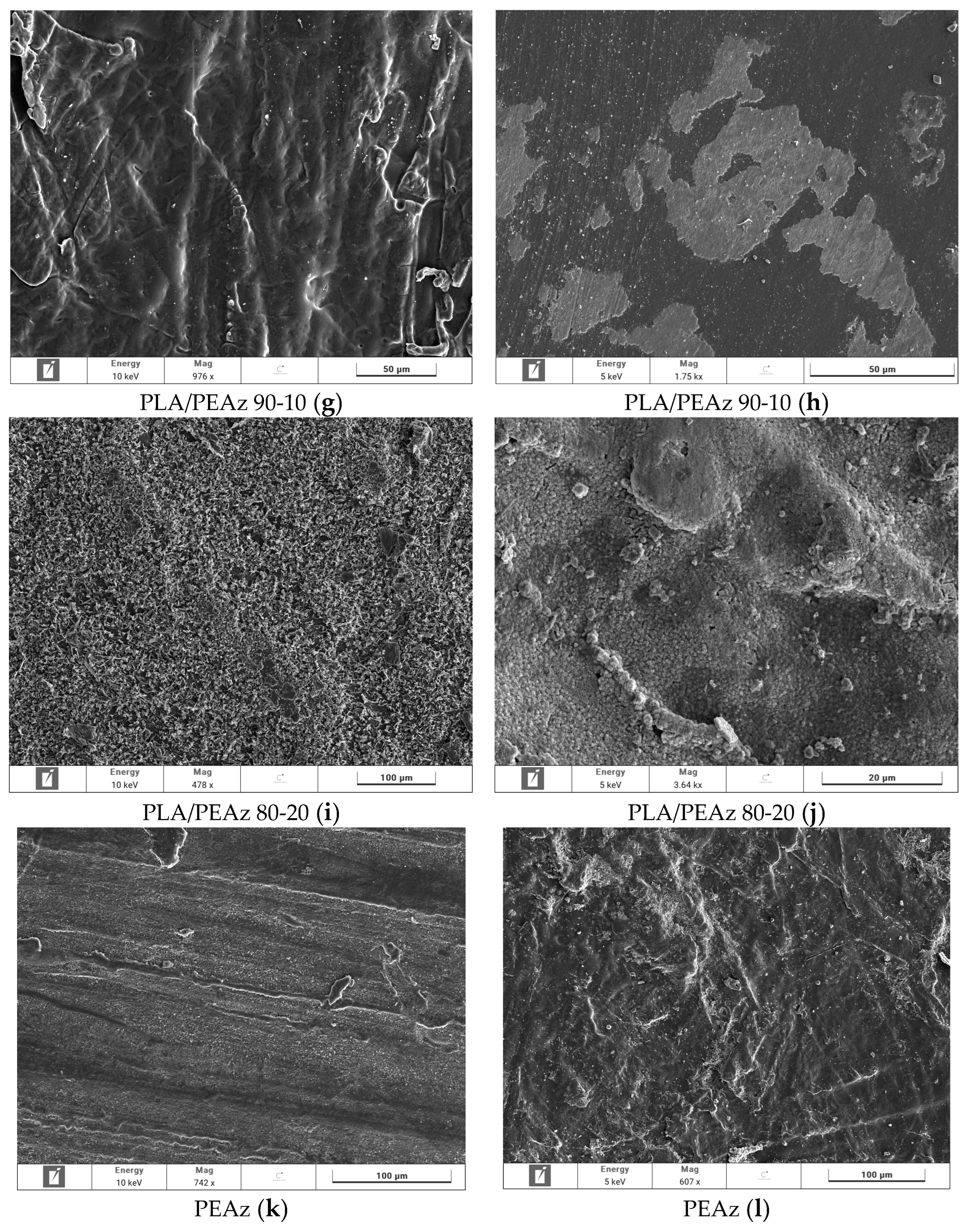
2.6.4. SEM—Scanning Electron Microscopy
3. Results and Discussion
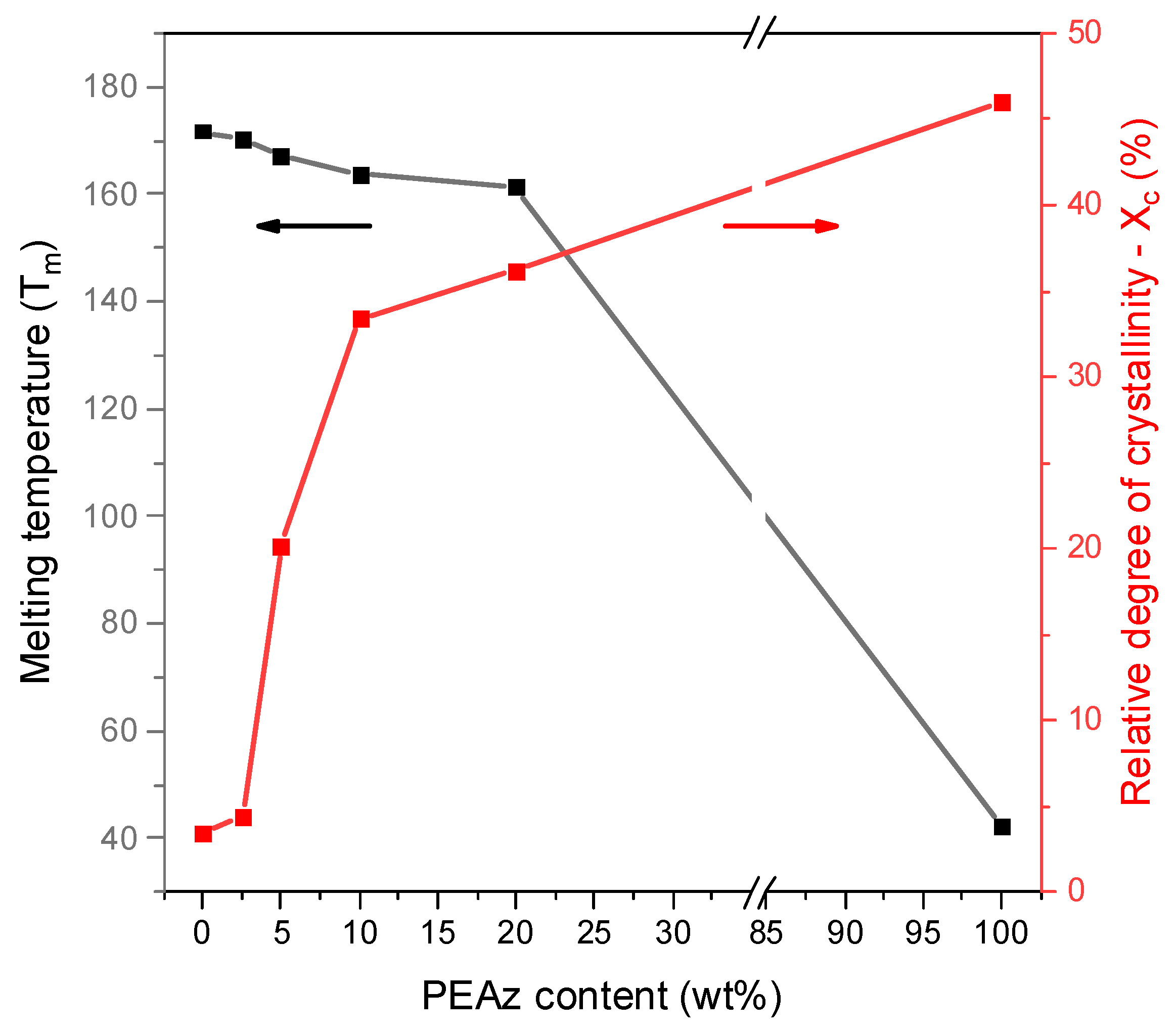
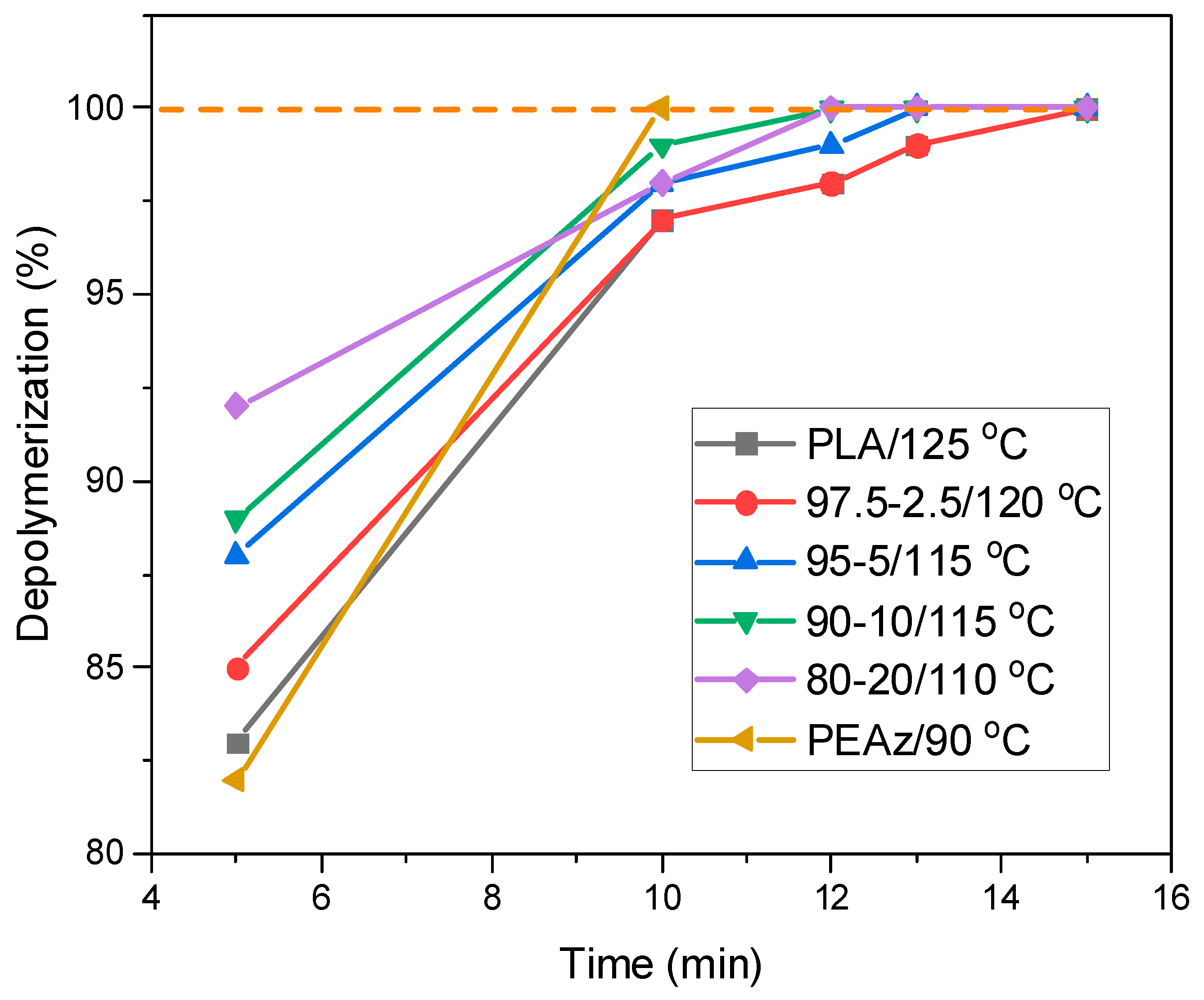
3.1. Synthesis and Microwave Hydrolysis of PLA and Its PEAz Copolymers
3.2. Enzymatic Hydrolysis of PLA and Its PEAz Copolymers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renedo, O.D.; Alonso-Lomillo, M.A.; Martinez, M.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.; Verma, A.; Appeltans, R.; Buntinx, M.; Ferraris, E.; Deferme, W. Printed Electronics (PE) As An enabling Technology to Realize Flexible Mass Customized Smart Applications. Proc. CIRP 2020, 96, 115–120. [Google Scholar] [CrossRef]
- Gorman, A.M.; Clayton, A.; O’Connell, T.; Johnson, D. A recyclable screen ink with state-of-the-art performance developed using a bottom-up, safety and sustainability-driven approach. MRS Adv. 2023, 8, 311–316. [Google Scholar] [CrossRef]
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A.C. A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mat. 2019, 32, e1905279. [Google Scholar] [CrossRef]
- Ali, W.; Ali, H.; Souissi, S.; Zinck, P. Are bioplastics an ecofriendly alternative to fossil fuel plastics? Environ. Chem. Lett. 2023, 21, 1991–2002. [Google Scholar] [CrossRef]
- Park, J.-Y.; Park, J.-S. The present status and future aspects of the market for printed electronics. J. Korean Inst. Inf. Commun. Eng. 2013, 17, 263–272. [Google Scholar]
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Altohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zuo, D.; Sun, J. Bioplastic production in terms of life cycle assessment: A state-of-the-art review. Environ. Sci. Ecotech. 2023, 19, 100254. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Bikiaris, D.N. Biobased plastics for the transition to a circular economy. Mater Lett. 2024, 362, 136174. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Comparing life cycle energy and GHG emissions of bio-based PET, recycled PET, PLA, and man-made cellulosics. Biofuels Bioprod. Biorefining 2012, 6, 625–639. [Google Scholar] [CrossRef]
- Palmieri, E.; Cancelliere, R.; Maita, F.; Micheli, L.; Maiolo, L. An ethyl cellulose novel biodegradable flexible substrate material for sustainable screen-printing. RSC Adv. 2024, 14, 18103–18108. [Google Scholar] [CrossRef]
- Kang, P.-L.; Lin, Y.-H.; Settu, K.; Yen, C.-S.; Yeh, C.-Y.; Liu, J.-T.; Chen, C.-J.; Chang, S.-J. A Facile Fabrication of Biodegradable and Biocompatible Cross-Linked Gelatin as Screen Printing Substrates. Polymers 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Mettakoonpitak, J.; Khongsoun, K.; Wongwan, N.; Kaewbutdee, S.; Siripinyanond, A.; Kuharuk, A.; Henry, C.S. Simple biodegradable plastic screen-printing for microfluidic paper-based analytical devices. Sens. Actuators B Chem. 2021, 331, 129463. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid) a versatile biobased polymer of next decades with multifunctional properties. From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Delikonstantis, E.; Yfanti, V.-L.; Galvita, V.V.; Lemonidou, A.A.; Geem, K.V. An electricity-powered future for mixed plastic waste chemical recycling. Waste Manag. 2024, 193, 155–170. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Jones, M.D. A circular economy approach to plastic waste. Polym. Degrad. Stab. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Kombe, G.G. Global Trends and Strategic Pathways for Waste Plastics Depolymerization in the Circular Economy. Clean. Waste Syst. 2025, 11, 100292. [Google Scholar] [CrossRef]
- Peti, D.; Dobránsky, J.; Michalík, P. Recent Advances in Polymer Recycling: A Review of Chemical and Biological Processes for Sustainable Solutions. Polymers 2025, 17, 603. [Google Scholar] [CrossRef]
- Guselnikova, O.; Semyonov, O.; Sviridova, E.; Gulyaev, R.; Gorbunova, A.; Kogolev, D.; Trelin, A.; Yamauchi, Y.; Boukherroub, R.; Postnikov, P.S. “Functional upcycling” of polymer waste towards the design of new materials. Chem. Soc. Rev. 2023, 52, 4755–4832. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sust. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Qian, S.; Liu, Z.; Weng, Y.; Zhang, Y. Depolymerization and Re/Upcycling of Biodegradable PLA Plastics. ACS Omega 2024, 9, 13509–13521. [Google Scholar] [CrossRef] [PubMed]
- Achilias, D.S.; Antonakou, E.V.; Roupakias, C.; Megalokonomos, P.; Lappas, A. Recycling techniques of polyolefins from plastic wastes. Glob. NEST J. 2008, 10, 114–122. [Google Scholar]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Harasymchuk, I.; Kočí, V.; Vitvarová, M. Chemical recycling: Comprehensive overview of methods and technologies. Int. J. Sust. Eng. 2024, 17, 124–148. [Google Scholar] [CrossRef]
- Hirao, K.; Nakatsuchi, Y.; Ohara, H. Alcoholysis of Poly(l-lactic acid) under microwave irradiation. Polym. Degrad. Stab. 2010, 95, 925–928. [Google Scholar] [CrossRef]
- Nim, B.; Opaprakasit, M.; Petchsuk, A.; Opaprakasit, P. Microwave-assisted chemical recycling of polylactide (PLA) by alcoholysis with various diols. Polym. Degrad. Stab. 2020, 181, 109363. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Kolokotsiou, L.; Vouvoudi, E.; Redhwi, H.; Al-Arfaj, A.A.; Achilias, D.S. Depolymerization of PLA by Phase Transfer Catalysed Alkaline Hydrolysis in a Microwave Reactor. J. Polym. Environ. 2020, 28, 1664–1672. [Google Scholar] [CrossRef]
- Pingale, N.D.; Shulka, S.R. Microwave assisted ecofriendly recycling of poly (ethylene terephthalate) bottle waste. Eur. Polym. J. 2008, 44, 4151. [Google Scholar] [CrossRef]
- Parab, Y.; Pingale, N.; Shukla, S.R. Aminolytic depolymerization of poly (ethylene terephthalate) bottle waste by conventional and microwave irradiation heating. J. Appl. Polym. Sci. 2012, 125, 1103–1107. [Google Scholar] [CrossRef]
- Benninga, J.; Gebben, B.; Folkersma, R.; Voet, V.S.D.; Loos, K. Rapid Microwave-Assisted Chemical Recycling of Poly(p-Phenylene Terephthalamide). Am. Chem. Soc. 2025, 147, 7191–7195. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Trash to Treasure: Microwave-Assisted Conversion of Polyethylene to Functional Chemicals. Ind. Eng. Chem. Res. 2017, 56, 14814–14821. [Google Scholar] [CrossRef]
- Ioannidis, R.O.; Terzopoulou, Z.; Zamboulis, A.; Bikiaris, N.D.; Noordam, M.J.; Nikolaidis, N. Novel biobased, flexible blocky copolyesters based on poly(lactic acid) and poly(ethylene azelate). Mater. Adv. 2025, 6, 2975–2989. [Google Scholar] [CrossRef]
- Wong, M.K.; Lock, S.S.M.; Chan, Y.H.; Yeoh, S.J.; Tan, I.S. Towards sustainable production of bio-based ethylene glycol: Progress, perspective and challenges in catalytic conversion and purification. Chem. Eng. J. 2023, 468, 143699. [Google Scholar] [CrossRef]
- Todea, A.; Deganutti, C.; Spennato, M.; Asaro, F.; Zingone, G.; Milizia, T.; Gardossi, L. Azelaic acid: A bio-based building block for biodegradable polymers. Polymers 2021, 13, 4091. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Achilias, D.S.; Karagiannidis, N. Synthesis, crystallization, and enzymatic degradation of the biodegradable polyester poly(ethylene azelate). Macromol. Chem. Phys. 2010, 211, 2585–2595. [Google Scholar] [CrossRef]
- Hajilou, N.; Mostafayi, S.S.; Yarin, A.L.; Shokuhfar, T. A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives. AppliedChem 2025, 5, 1. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Lactic acid production by hydrolysis of poly(lactic acid) in aqueous solutions: An experimental and kinetic study. J. Polym. Environ. 2013, 21, 275–279. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.-S.; Thakur, V.-K. E-waste Recycling and Utilization: A Review of Current Technologies and Future Perspectives. Curr. Opin. Green Sustain. Chem. 2024, 47, 100900. [Google Scholar] [CrossRef]
- Palanisamy, K.; Subburaj, R.-G. Integration of electronic waste management: A review of current global generation, health impact, and technologies for value recovery and its pertinent management technique. Environ. Sci. Pollut. Res. 2023, 30, 63347–63367. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Jayakumar, A.; Velu, R. A Comprehensive Review on Printed Electronics: A Technology Drift towards a Sustainable Future. Nanomaterials 2022, 12, 4251. [Google Scholar] [CrossRef] [PubMed]
- Klonos, P.A.; Ioannidis, R.O.; Pitsavas, A.; Bikiaris, N.D.; Makri, S.P.; Koutsourea, S.; Grigoropoulos, A.; Deligkiozi, I.; Zoikis-Karathanasis, A.; Kyritsis, A.; et al. Segmental Mobility, Interfacial Polymer, Crystallization and Conductivity Study in Polylactides Filled with Hybrid Lignin-CNT Particles. Nanomaterials 2025, 15, 660. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Infante, C.; Orden, M.U.; Urreaga, J.M. Mechanical recycling of poly(lactic acid): Evaluation of a chain extender and a peroxide as additives for upgrading the recycled plastic. J. Clean. Prod. 2019, 219, 46–56. [Google Scholar] [CrossRef]
- Kumar, R.; Sadeghi, K.; Jang, J.; Seo, J. Mechanical, chemical, and bio-recycling of biodegradable plastic: A review. Sc. of the Tot. Env. 2023, 882, 163446. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Bikiaris, D.N.; Valera, M.A.; Mangas, A. Synthesis, properties, and enzymatic hydrolysis of poly(Lactic acid)-co-poly(propylene adipate) block copolymers prepared by reactive extrusion. Polymers 2021, 13, 4121. [Google Scholar] [CrossRef]
- Shalem, A.; Yehezkeli, O.; Fishman, A. Enzymatic degradation of polylactic acid (PLA). Appl. Microbiol. Biotechnol. 2024, 108, 1–20. [Google Scholar] [CrossRef]
- Siracusa, C.; Quartinello, F.; Soccio, M.; Manfroni, M.; Lotti, N.; Dorigato, A.; Guebitz, G.M.; Pellis, A. On the Selective Enzymatic Recycling of Poly(pentamethylene 2,5-furanoate)/Poly(lactic acid) Blends and Multiblock Copolymers. ACS Sustain. Chem. Eng. 2023, 11, 9751–9760. [Google Scholar] [CrossRef]
- Achilias, D.S. Thermo-chemical recycling of plastics as a sustainable approach to the plastic waste issue. Euro-Mediterr. J. Environ. Integr. 2025, 1–14. [Google Scholar] [CrossRef]
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Was. Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M. Chemical aspects of polymer recycling. Adv. Ind. Eng. Pol. Res. 2021, 4, 133–150. [Google Scholar] [CrossRef]




| Medium | Catalyst | Temperature (°C) | Time (min) | PLA Depolymerization (%) |
|---|---|---|---|---|
| NaOH 10% w/v | (2DME)-TPPB | 80 | 5 | 56 ± 2 |
| NaOH 10% w/v | HTMAC | 80 | 5 | 64 ± 3 |
| NaOH 10% w/v | HTMAB | 80 | 5 | 69 ± 5 |
| H2O | HTMAB | 80 | 5 | 22 ± 1 |
| Medium | Catalyst (0.05 g) | Temperature (°C) | Time (min) | PLA Depolymerization (%) |
|---|---|---|---|---|
| NaOH 5% w/v | HTMAB | 80 | 5 | 42 ± 3 |
| NaOH 10% w/v | HTMAB | 80 | 5 | 69 ± 5 |
| NaOH 15% w/v | HTMAB | 80 | 5 | 57 ± 3 |
| NaOH 20% w/v | HTMAB | 80 | 5 | 48 ± 2 |
| Medium | Catalyst (HTMAB) | Temperature (°C) | Time (min) | PLA Depolymerization (%) |
|---|---|---|---|---|
| NaOH 10% w/v | No catalyst | 80 | 5 | 23 ± 4 |
| NaOH 10% w/v | 0.025 g | 80 | 5 | 54 ± 6 |
| NaOH 10% w/v | 0.05 g | 80 | 5 | 69 ± 5 |
| NaOH 10% w/v | 0.10 g | 80 | 5 | 70 ± 2 |
| NaOH 10% w/v | 0.15 g | 80 | 5 | 72 ± 3 |
| NaOH 10% w/v | 0.20 g | 80 | 5 | 73 ± 2 |
| Medium | Catalyst (0.05 g) | Temperature (°C) | Time (min) | PLA Depolymerization (%) |
|---|---|---|---|---|
| NaOH 10% w/v | HTMAB | 60 | 10 | 61 ± 4 |
| NaOH 10% w/v | HTMAB | 80 | 10 | 86 ± 2 |
| NaOH 10% w/v | HTMAB | 100 | 10 | 93 ± 3 |
| NaOH 10% w/v | HTMAB | 120 | 10 | 97 ± 2 |
| Medium | Catalyst (0.05 g) | Temperature (°C) | Time (min) | PLA Depolymerization (%) |
|---|---|---|---|---|
| NaOH 10% w/v | HTMAB | 125 | 2 | 67 ± 3 |
| NaOH 10% w/v | HTMAB | 125 | 5 | 84 ± 2 |
| NaOH 10% w/v | HTMAB | 125 | 10 | 98 ± 2 |
| NaOH 10% w/v | HTMAB | 125 | 15 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, R.O.; Bikiaris, N.D.; Vouvoudi, E.; Zamboulis, A.; Nikolaidis, N.; Bikiaris, D.N. Chemical Recycling of PLA and Its Copolyesters with Poly(Ethylene Azelate) via Microwave-Assisted Alkaline Hydrolysis and Enzymatic Hydrolysis. Polymers 2025, 17, 1374. https://doi.org/10.3390/polym17101374
Ioannidis RO, Bikiaris ND, Vouvoudi E, Zamboulis A, Nikolaidis N, Bikiaris DN. Chemical Recycling of PLA and Its Copolyesters with Poly(Ethylene Azelate) via Microwave-Assisted Alkaline Hydrolysis and Enzymatic Hydrolysis. Polymers. 2025; 17(10):1374. https://doi.org/10.3390/polym17101374
Chicago/Turabian StyleIoannidis, Rafail O., Nikolaos D. Bikiaris, Evangelia Vouvoudi, Alexandra Zamboulis, Nikolaos Nikolaidis, and Dimitrios N. Bikiaris. 2025. "Chemical Recycling of PLA and Its Copolyesters with Poly(Ethylene Azelate) via Microwave-Assisted Alkaline Hydrolysis and Enzymatic Hydrolysis" Polymers 17, no. 10: 1374. https://doi.org/10.3390/polym17101374
APA StyleIoannidis, R. O., Bikiaris, N. D., Vouvoudi, E., Zamboulis, A., Nikolaidis, N., & Bikiaris, D. N. (2025). Chemical Recycling of PLA and Its Copolyesters with Poly(Ethylene Azelate) via Microwave-Assisted Alkaline Hydrolysis and Enzymatic Hydrolysis. Polymers, 17(10), 1374. https://doi.org/10.3390/polym17101374









