Biopolymeric Blends of Thermoplastic Starch and Polylactide as Sustainable Packaging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Thermoplastic Starch and Thermoplastic Starch–Citrate
2.1.2. Preparation of Binary Blends
2.2. Chemical Structure Characterization
2.3. Morphology
2.4. Differential Scanning Calorimetry Analysis (DSC)
2.5. Water Vapour Permeability (WVP)
2.6. Tensile Test of the Materials
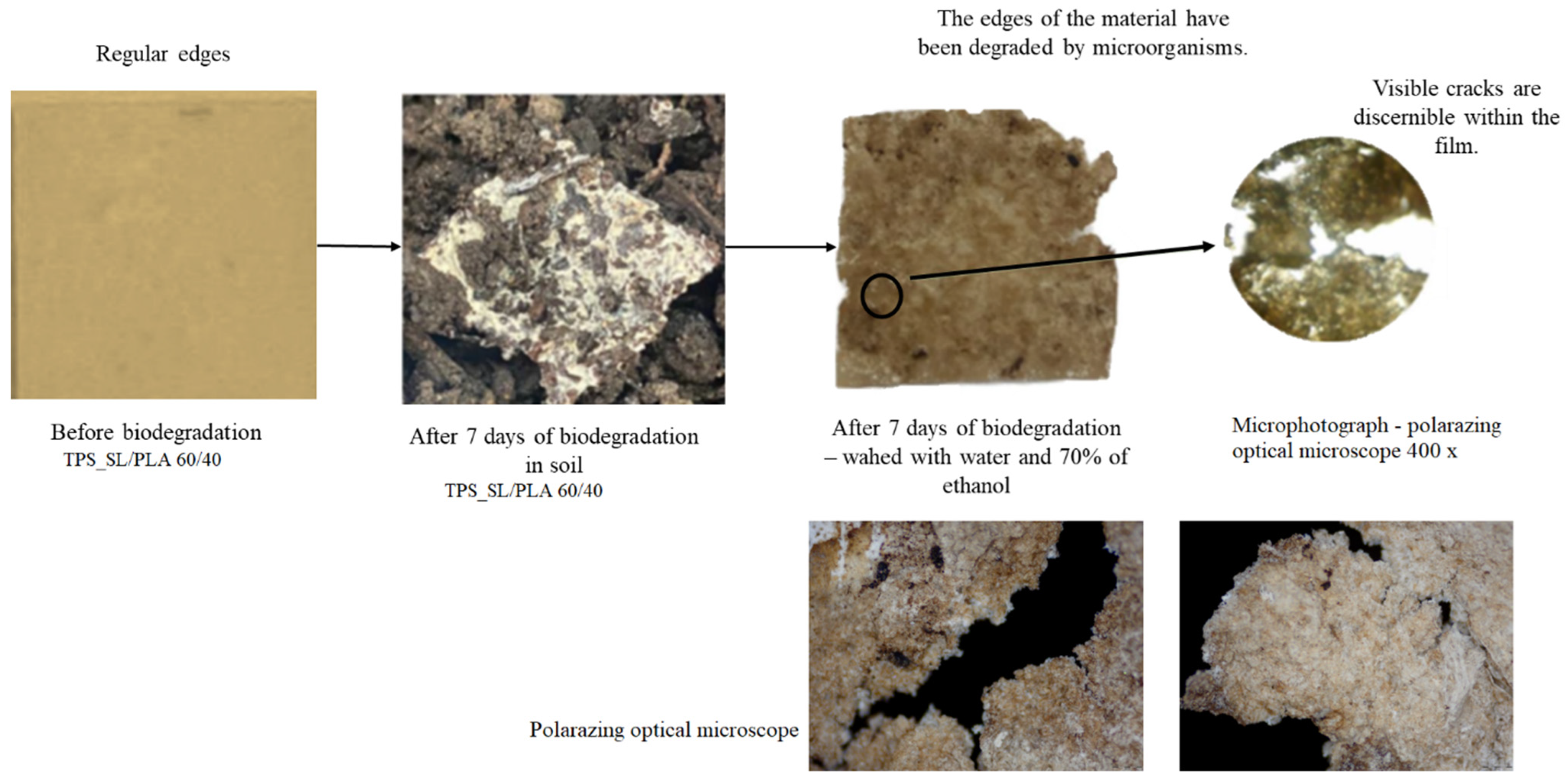
2.7. Determination of Biodegradability of Materials in Soil
2.8. Polarizing Optical Microscope (POM)
3. Results and Discussion
3.1. Characterization of Materials
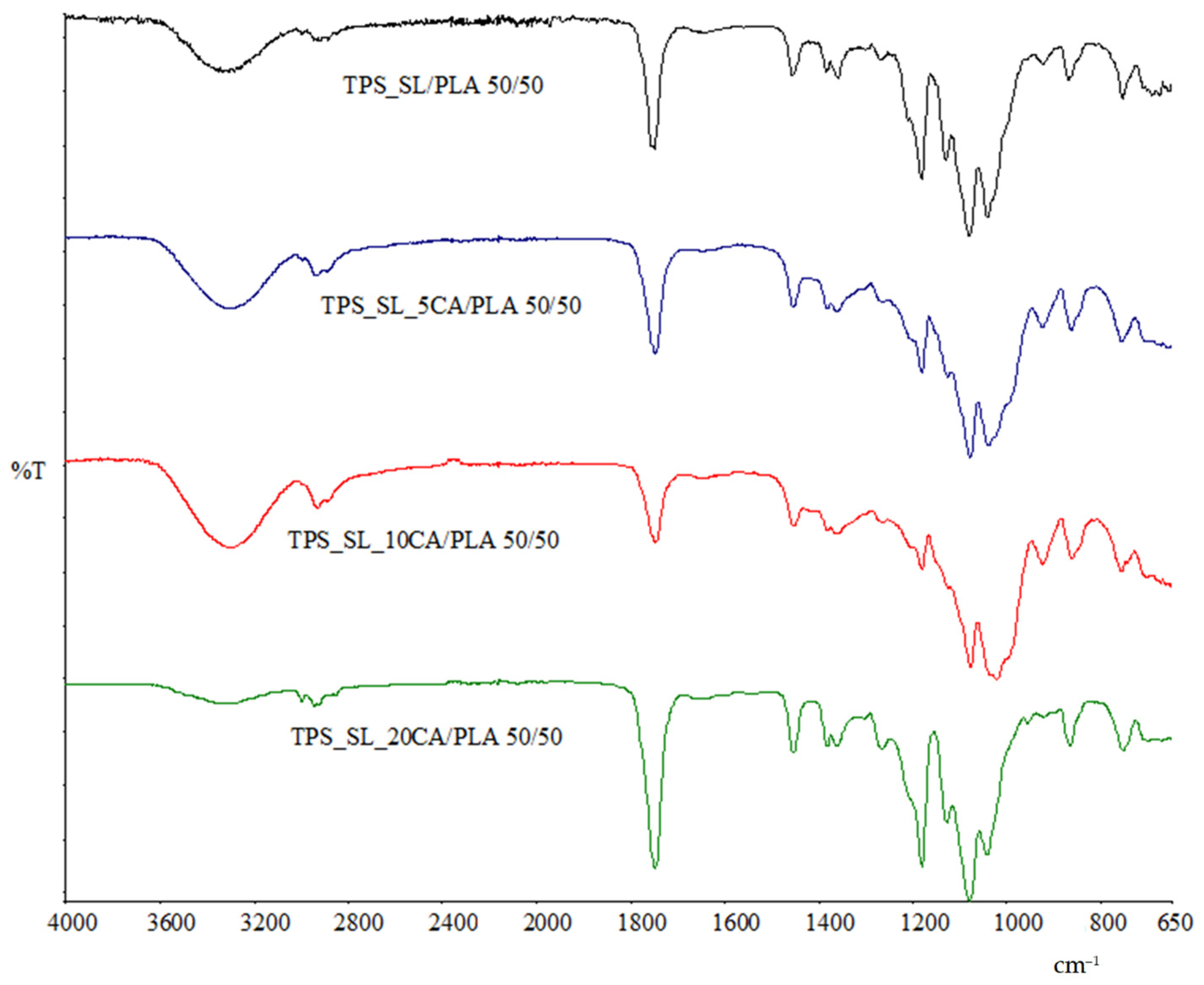
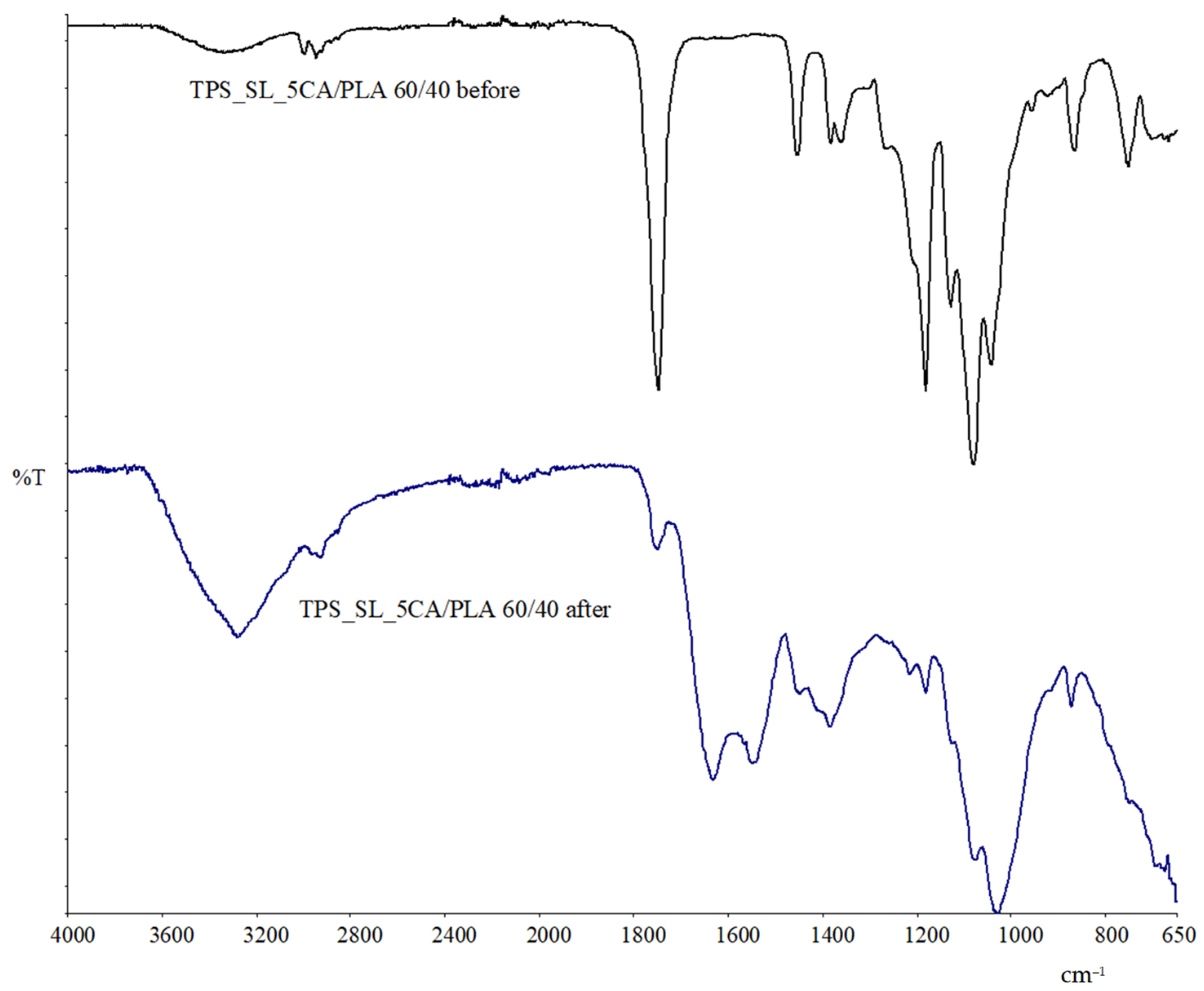
3.1.1. Results of FTIR—ATR Spectroscopy
3.1.2. Scanning Electron Microscopy (SEM) Results
3.1.3. Differential Scanning Calorimetry (DSC) Results
3.1.4. Water Vapour Permeability (WVP) Test Results
3.1.5. Results of Mechanical Properties
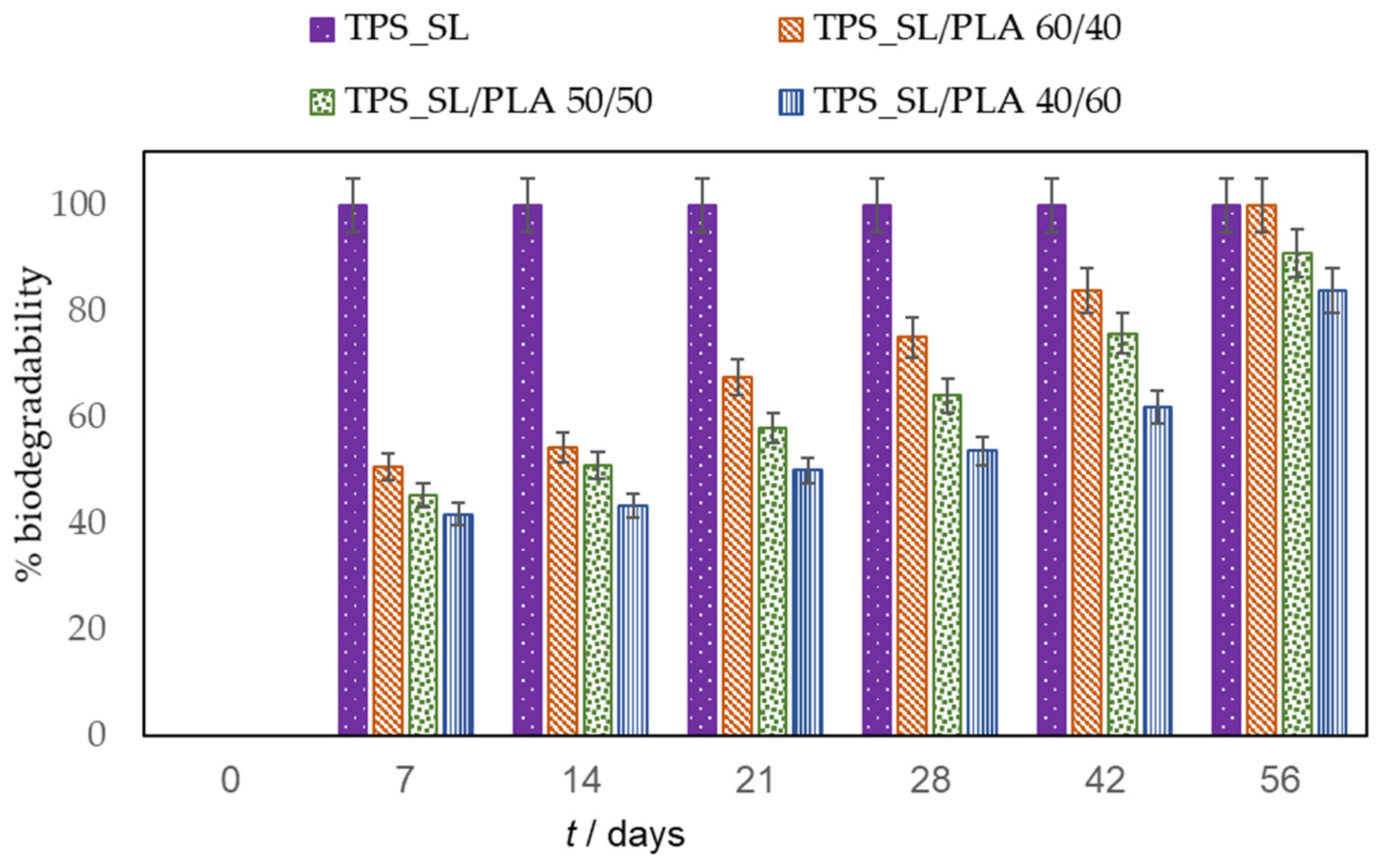
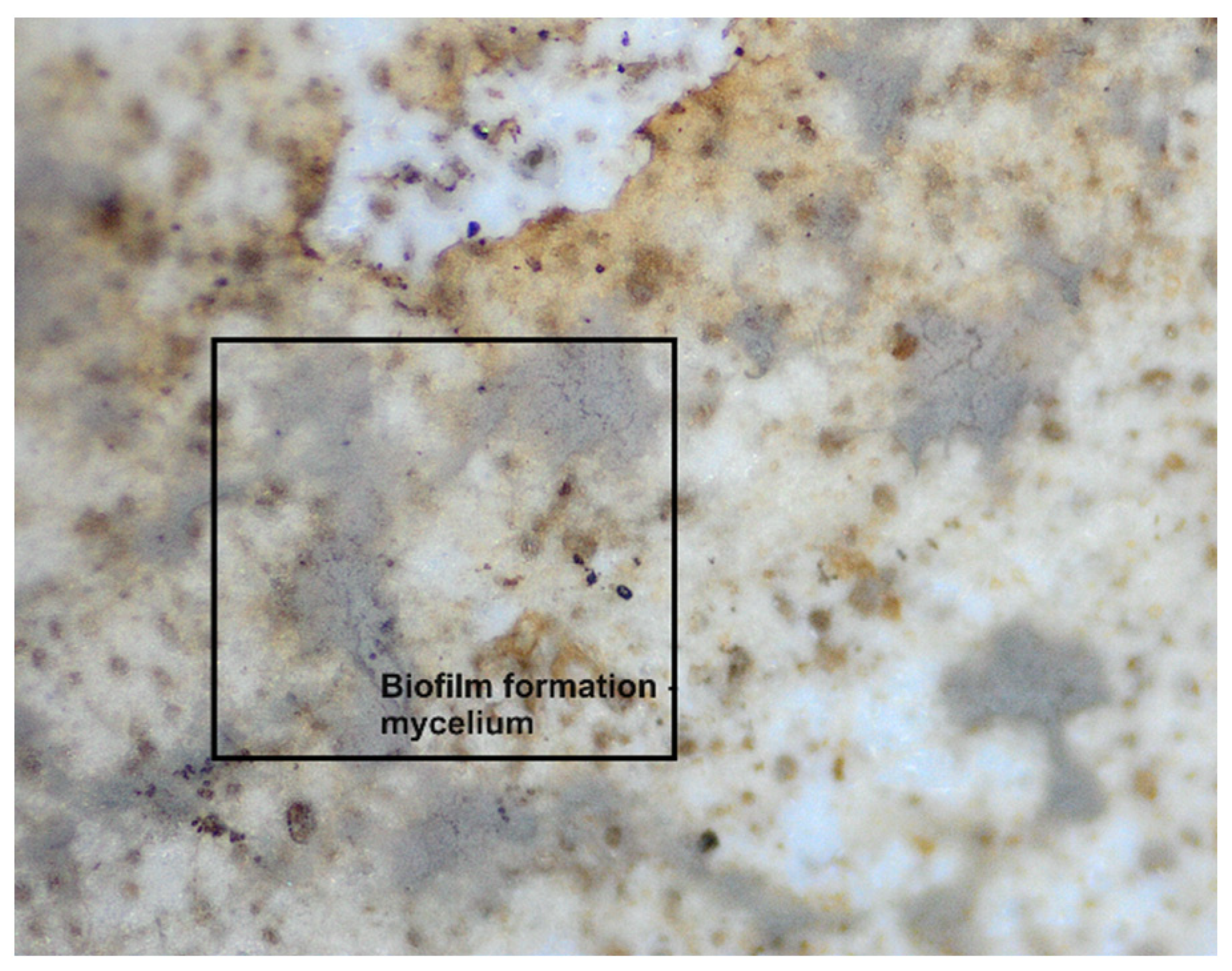
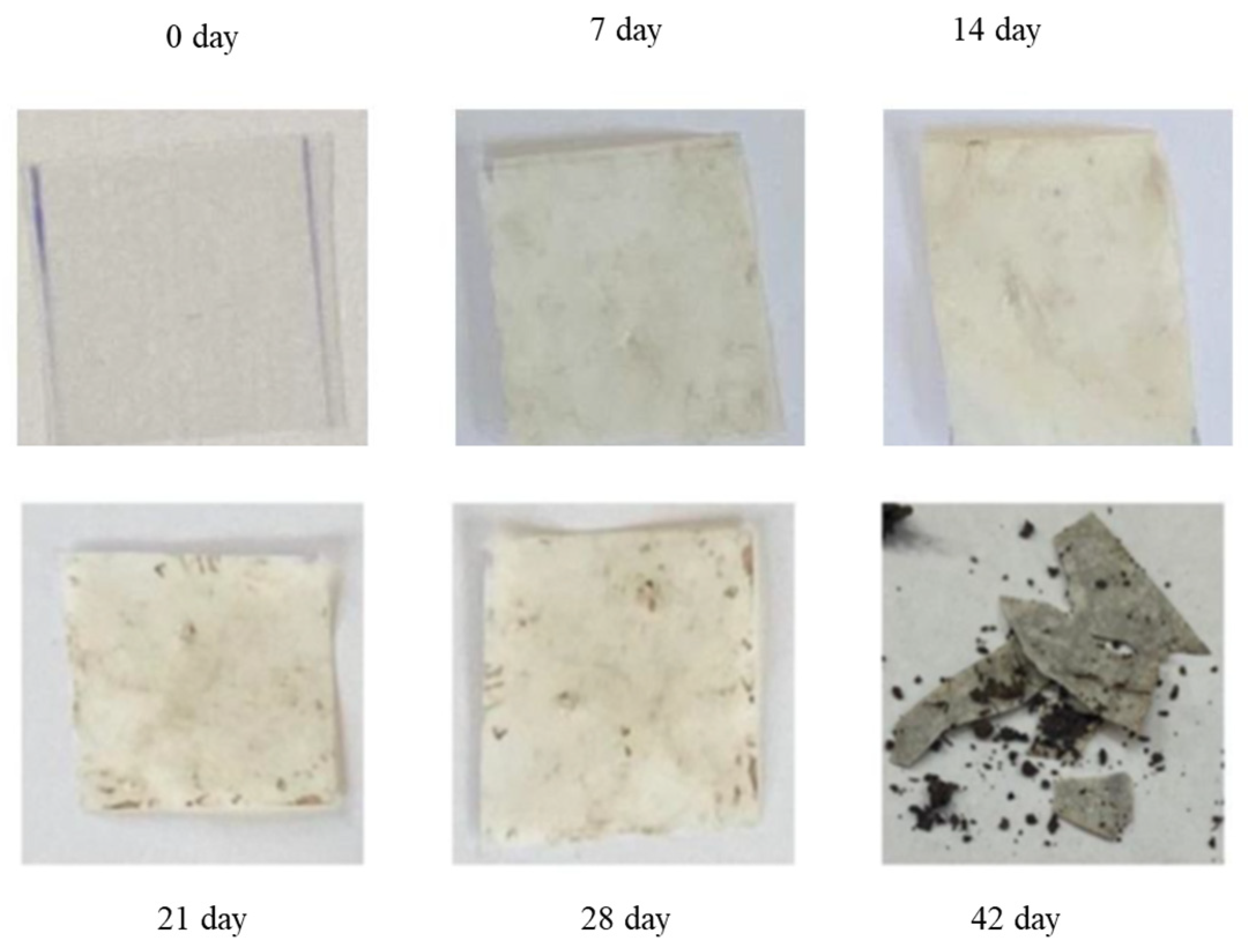
3.2. Assessing the Biodegradability Potential of Materials
Exploring Material Transformation Post-Biodegradation through FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Plastics—The Facts 2021. Plastics Europe, 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 4 February 2024).
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef] [PubMed]
- Jayarathna, S.; Andersson, M.; Andersson, R. Recent Advances in Starch-Based Blends and Composites for Bioplastics Applications. Polymers 2022, 14, 4557. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-García, A.; Osorio, B.H.; Aguirre-Loredo, R.Y.; Calambas, H.L.; Caicedo, C. Miscibility study of thermoplastic starch/polylactic acid blends: Thermal and superficial properties. Carbohydr. Polym. 2022, 293, 119744. [Google Scholar] [CrossRef] [PubMed]
- Mironescu, M.; Lazea-Stoyanova, A.; Barbinta-Patrascu, M.E.; Virchea, L.-I.; Rexhepi, D.; Mathe, E.; Georgescu, C. Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health. Polymers 2021, 13, 1190. [Google Scholar] [CrossRef] [PubMed]
- Ocelić Bulatović, V.; Borković, I.; Kučić Grgić, D.; Jozinović, A. Thermal and Mechanical Properties of Thermoplastic Starch Blends. Kem. Ind. 2018, 67, 21–31. [Google Scholar] [CrossRef]
- Ocelić Bulatović, V.; Govorčin Bajsić, E.; Kučić Grgić, D.; Jozinović, A. Thermal properties of biodegradable PLA/TPS blends. Kem. Ind. 2018, 67, 33–42. [Google Scholar] [CrossRef]
- Li, D.; Luo, C.; Zhou, J.; Dong, L.; Chen, Y.; Liu, G.; Qiao, S. The role of the interface of PLA with thermoplastic starch in the nonisothermal crystallization behavior of PLA in PLA/Thermoplastic Starch/SiO2 Composites. Polymers 2023, 15, 1579. [Google Scholar] [CrossRef] [PubMed]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic starch (TPS)/polylactic acid (PLA) blending methodologies: A review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Liu, D.; Qi, Z.G.; Zhang, Y.; Xu, J.; Guo, B.H. Poly(butylene succinate) (PBS)/ionic liquid plasticized starch blends: Preparation, characterization, and properties. Starch-Stärke 2015, 67, 802–809. [Google Scholar] [CrossRef]
- Suchao-in, K.; Koombhongse, P.; Chirachanchai, S. Starch grafted poly(butylene succinate) via conjugating reaction and its role on enhancing the compatibility. Carbohydr. Polym. 2014, 102, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Jawaid, M.; Lee, C.H. Effect of Modified Tapioca Starch on Mechanical, Thermal, and Morphological Properties of PBS Blends for Food Packaging. Polymers 2018, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Chen, F.; Zhang, H.; Liu, C. High Content of Thermoplastic Starch, Poly(butylenes adipate-co-terephthalate) and Poly(butylene succinate) Ternary Blends with a Good Balance in Strength and Toughness. Polymers 2023, 15, 2040. [Google Scholar] [CrossRef] [PubMed]
- Dammak, M.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with Plasticized Starch for Packaging Applications: Mechanical Properties, Rheological Behaviour and Biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- Castro, J.M.; Montalbán, M.G.; Domene-López, D.; Martín-Gullón, I.; García-Quesada, J.C. Study of the Plasticization Effect of 1-Ethyl-3-methylimidazolium Acetate in TPS/PVA Biodegradable Blends Produced by Melt-Mixing. Polymers 2023, 15, 1788. [Google Scholar] [CrossRef] [PubMed]
- Gajdosova, V.; Strachota, B.; Strachota, A.; Michalkova, D.; Krejcikova, S.; Fulin, P.; Nyc, O.; Brinek, A.; Zemek, M.; Slouf, M. Biodegradable Thermoplastic Starch/Polycaprolactone Blends with Co-Continuous Morphology Suitable for Local Release of Antibiotics. Materials 2022, 15, 1101. [Google Scholar] [CrossRef] [PubMed]
- Bou-Francis, A.; Piercey, M.; Al-Qatami, O.; Mazzanti, G.; Khattab, R.; Ghanem, A. Polycaprolactone Blends for Fracture Fixation in Low Load-bearing Applications. J. Appl. Polym. Sci. 2020, 137, 48940. [Google Scholar] [CrossRef]
- Sagnelli, D.; Cavanagh, R.; Xu, J.; Swainson, S.M.E.; Blennow, A.; Duncan, J.; Taresco, V.; Howdle, S. Starch/Poly (Glycerol-Adipate) Nanocomposite Film as Novel Biocompatible Materials. Coatings 2019, 9, 482. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical review on polylactic acid: Properties, structure, processing, biocomposites, and nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Chabrat, E.; Abdillahi, H.; Rouilly, A.; Rigal, L. Influence of citric acid and water on thermoplastic wheat flour/poly(lactic acid) blends. I: Thermal, mechanical and morphological properties. Ind. Crops Prod. 2012, 37, 238–246. [Google Scholar] [CrossRef]
- Golachowski, A.; Drożdż, W.; Golachowska, M.; Kapelko-Żeberska, M.; Raszewski, B. Production and Properties of Starch Citrates-Current Research. Foods 2020, 18, 1311. [Google Scholar] [CrossRef] [PubMed]
- Srihanam, P.; Srisuwan, Y.; Phromsopha, T.; Manphae, A.; Baimark, Y. Improvement in Phase Compatibility and Mechanical Properties of Poly(L-lactide)-bpoly(ethylene glycol)-b-poly(Llactide)/thermoplastic Starch Blends with Citric Acid. Polymers 2023, 15, 3966. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wahab, M.K.A.; Uylan, D.N.; Ismail, H. Physical and degradation properties of polylactic acid and thermoplastic starch blends–effect of citric acid treatment on starch structures. Bioresources 2017, 12, 3076–3087. [Google Scholar] [CrossRef]
- Kovač, M.; Ravnjak, B.; Šubarić, D.; Vinković, T.; Babić, J.; Ačkar, Đ.; Lončarić, A.; Šarić, A.; Bulatović, V.O.; Jozinović, A. Isolation and Characterization of Starch from Different Potato Cultivars Grown in Croatia. Appl. Sci. 2024, 14, 909. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Buksa, K.; Szumny, A.; Zięba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT-Food Sci. Technol. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Li, K.; Peng, J.; Turng, L.-S.; Huang, H.-X. Dynamic rheological behaviour and morphology of polylactide/poly(butylenes adipate-co-terephthalate) blends with various composition ratios. Adv. Polym. Technol. 2011, 30, 150–157. [Google Scholar] [CrossRef]
- Bota, J.; Vukoje, M.; Brozović, M.; Hrnjak-Murgić, Z. Reduced water permeability of biodegradable PCL nanocomposite coated paperboard packaging. Chem. Biochem. Eng. Q. 2017, 31, 417–424. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Plastics Determination of Tensile Properties Part 1: General principles. International Organisation for Standardisation: Geneva, Switzerland, 2019.
- ISO 17556:2019; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. International Organisation for Standardisation: Geneva, Switzerland, 2019.
- Žagar, E.; Grdadolnik, J. An infrared spectroscopic study of H-bond network in hyperbranched polyester polyol. J. Mol. Struct. 2003, 658, 143–152. [Google Scholar] [CrossRef]
- Glavcheva-Laleva, Z.; Varadinova, L.; Pavlov, D.J. Obtaining of modifiers for reduced friction by esterification of waste glycerol from biodiesel production and sylfat. Chem. Sci. 2015, 3, 1–6. [Google Scholar]
- Babu, A.S.; Parimalavalli, R.; Jagannadham, K.; Rao, J.S. Chemical and structural properties of sweet potato starch treated with organic and inorganic acid. J Food Sci Technol. 2015, 52, 5745–5753. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Parvaiz, M.R. Effect of glycidylmethacrylate (GMA) on the thermal, mechanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef]
- Garlotta, D.A. Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Fukushima, K.; Fina, A.; Geobaldo, F.; Venturello, A.; Camino, G. Properties of poly(lactic acid) nanocomposites based on montmorillonite, sepiolite and zirconium phosphonate. Express Polym. Lett. 2012, 6, 914–926. [Google Scholar] [CrossRef]
- Kalish, J.P.; Aou, K.; Yang, X.; Hsu, S.L. Spectroscopic and thermal analyses of α′ and α crystalline forms of poly(l-lactic acid). Polymer 2011, 52, 814–821. [Google Scholar] [CrossRef]
- Bulatović Ocelić, V.; Kučić Grgić, D.; Mandić, V.; Miloloža, M.; Dybal, J.; Gajdosova, V.; Slouf, M. Biodegradation of LDPE_TPS blends under controlled composting conditions. Polym. Bull. 2023, 80, 3331–3357. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohyd. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; Akhtar, T.; Matsko, N. Mechanical, thermal, rheological and morphological properties of binary and ternary blends of PLA, TPS and PCL. Macromol. Mater. Eng. 2015, 300, 423–435. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Curvelo, A.A.S.; Corrêa, A.C.; Marconcini, J.M.; Glenn, G.M.; Mattoso, L.H.C. Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly (lactic acid). Ind. Crop. Prod. 2012, 37, 61–68. [Google Scholar] [CrossRef]
- Guzmán, M.; Murillo, E. Funcionalización de polietileno de baja densidad con anhídrido maleico enestado fundido. Polímeros 2014, 24, 162–169. [Google Scholar] [CrossRef]
- Piyada, K.; Waranyou, S.; Thawien, W. Mechanical, thermal and structural properties of rice starch films reinforced with rice starch nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Alshehrei, F. Biodegradation of synthetic and natural plastic by microorganisms. Appl. Environ. Microbiol. 2017, 5, 8–19. [Google Scholar]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell Infect. Microbiol. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miloloža, M.; Cvetnić, M.; Kučić Grgić, D.; Ocelić Bulatović, V.; Ukić, Š.; Rogošić, M.; Dionysiou, D.; Kušic, H.; Bolanča, T. Biotreatment strategies for the removal of microplastics from freshwater systems. A review. Environ. Chem. Lett. 2022, 20, 1377–1402. [Google Scholar] [CrossRef]
- Mervan Çakar, M.; Vuković Domanovac, M.; Findrik Blažević, Z. Discovery of plastics-degrading enzymes. Kem. Ind. 2023, 72, 473–485. [Google Scholar]
- Zain, A.H.M.; Ab Wahab, M.K.; Ismail, H. Biodegradation behaviour of thermoplastic starch: The roles of carboxylic acids on cassava starch. J. Polym. Environ. 2018, 26, 691–700. [Google Scholar] [CrossRef]
- Weir, N.A.; Buchanan, F.J.; Orr, J.F.; Dickson, G.R. Degradation of poly-L-lactide. Part 1: In vitro and in vivo physiological temperature degradation. Proc. Inst. Mech. Eng. H J. Eng. Med. 2004, 218, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Olewnik-Kruszkowska, E.; Burkowska-But, A.; Tarach, I.; Walczak, M.; Jakubowska, E. Biodegradation of polylactide-based composites with an addition of a compatibilizing agent in different environments. Inter. Biodeterior. Biodegrad. 2020, 147, 104840. [Google Scholar] [CrossRef]
- Afiq, M.M.; Azura, A.R. Effect of sago starch loadings on soil decomposition of Natural Rubber Latex (NRL) composite films mechanical properties. Inter. Biodeterior. Biodegrad. 2013, 85, 139–149. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.T.; Selke, S.E.; Tsuji, H. (Eds.) Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; Wiley-Blackwell, John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohyd. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Koelling, K.W.; Chalmers, J.J. Characterization of the degradation of polylactic acid polymer in a solid substrate environment. Biotechnol. Progr. 1998, 14, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Palai, B.; Mohanty, S.; Nayak, S.K. A Comparison on biodegradation behaviour of polylactic acid (PLA) based blown films by incorporating thermoplasticized starch (TPS) and poly (butylene succinate-co-adipate) (PBSA) Biopolymer in Soil. J. Polym. Environ. 2021, 29, 2772–2788. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]












| Sample | Tg (°C) | Tc (°C) | ΔHc (Jg−1) | Tm (°C) | ΔHm (Jg−1) | χc (%) |
|---|---|---|---|---|---|---|
| PLA | 58.5 | 112.4 | 20.9 | 152.1 | 20.2 | 21.7 |
| TPS_SL/PLA 60/40 | 56.7 | 114.4 | 13.0 | 148.4 | 12.5 | 33.6 |
| TPS_SL/PLA 50/50 | 56.6 | 116.1 | 14.4 | 148.9 | 14.1 | 30.2 |
| TPS_SL/PLA 40/60 | 54.9 | 110.8 | 21.3 | 153.3 | 20.2 | 36.2 |
| TPS_SL_5CA/PLA 60/40 | 56.2 | 111.3 | 13.6 | 154.2 | 13.5 | 36.3 |
| TPS_SL_5CA/PLA 50/50 | 55.3 | 110.2 | 16.4 | 153.1 | 15.3 | 32.9 |
| TPS_SL_5CA/PLA 40/60 | 55.1 | 110.4 | 20.0 | 153.7 | 19.2 | 34.4 |
| TPS_SL_10CA/PLA 60/40 | 55.9 | 104.4 | 13.2 | 153.3 | 11.7 | 31.4 |
| TPS_SL_10CA/PLA 50/50 | 55.2 | 104.6 | 15.8 | 152.7 | 14.1 | 30.3 |
| TPS_SL_10CA/PLA 40/60 | 54.5 | 109.9 | 17.6 | 153.6 | 18.8 | 33.6 |
| TPS_SL_20CA/PLA 60/40 | 54.6 | 110.5 | 13.5 | 153.8 | 12.4 | 33.4 |
| TPS_SL_20CA/PLA 50/50 | 53.8 | 110.1 | 17.1 | 153.3 | 16.4 | 35.2 |
| TPS_SL_20CA/PLA 40/60 | 55.4 | 109.7 | 20.1 | 155.3 | 19.5 | 34.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jozinović, A.; Kovač, M.; Ocelić Bulatović, V.; Kučić Grgić, D.; Miloloža, M.; Šubarić, D.; Ačkar, Đ. Biopolymeric Blends of Thermoplastic Starch and Polylactide as Sustainable Packaging Materials. Polymers 2024, 16, 1268. https://doi.org/10.3390/polym16091268
Jozinović A, Kovač M, Ocelić Bulatović V, Kučić Grgić D, Miloloža M, Šubarić D, Ačkar Đ. Biopolymeric Blends of Thermoplastic Starch and Polylactide as Sustainable Packaging Materials. Polymers. 2024; 16(9):1268. https://doi.org/10.3390/polym16091268
Chicago/Turabian StyleJozinović, Antun, Mario Kovač, Vesna Ocelić Bulatović, Dajana Kučić Grgić, Martina Miloloža, Drago Šubarić, and Đurđica Ačkar. 2024. "Biopolymeric Blends of Thermoplastic Starch and Polylactide as Sustainable Packaging Materials" Polymers 16, no. 9: 1268. https://doi.org/10.3390/polym16091268
APA StyleJozinović, A., Kovač, M., Ocelić Bulatović, V., Kučić Grgić, D., Miloloža, M., Šubarić, D., & Ačkar, Đ. (2024). Biopolymeric Blends of Thermoplastic Starch and Polylactide as Sustainable Packaging Materials. Polymers, 16(9), 1268. https://doi.org/10.3390/polym16091268










