Self-Assembled Block Copolymers as a Facile Pathway to Create Functional Nanobiosensor and Nanobiomaterial Surfaces
Abstract
1. Introduction
2. Block Copolymers as Nanoscale Templates
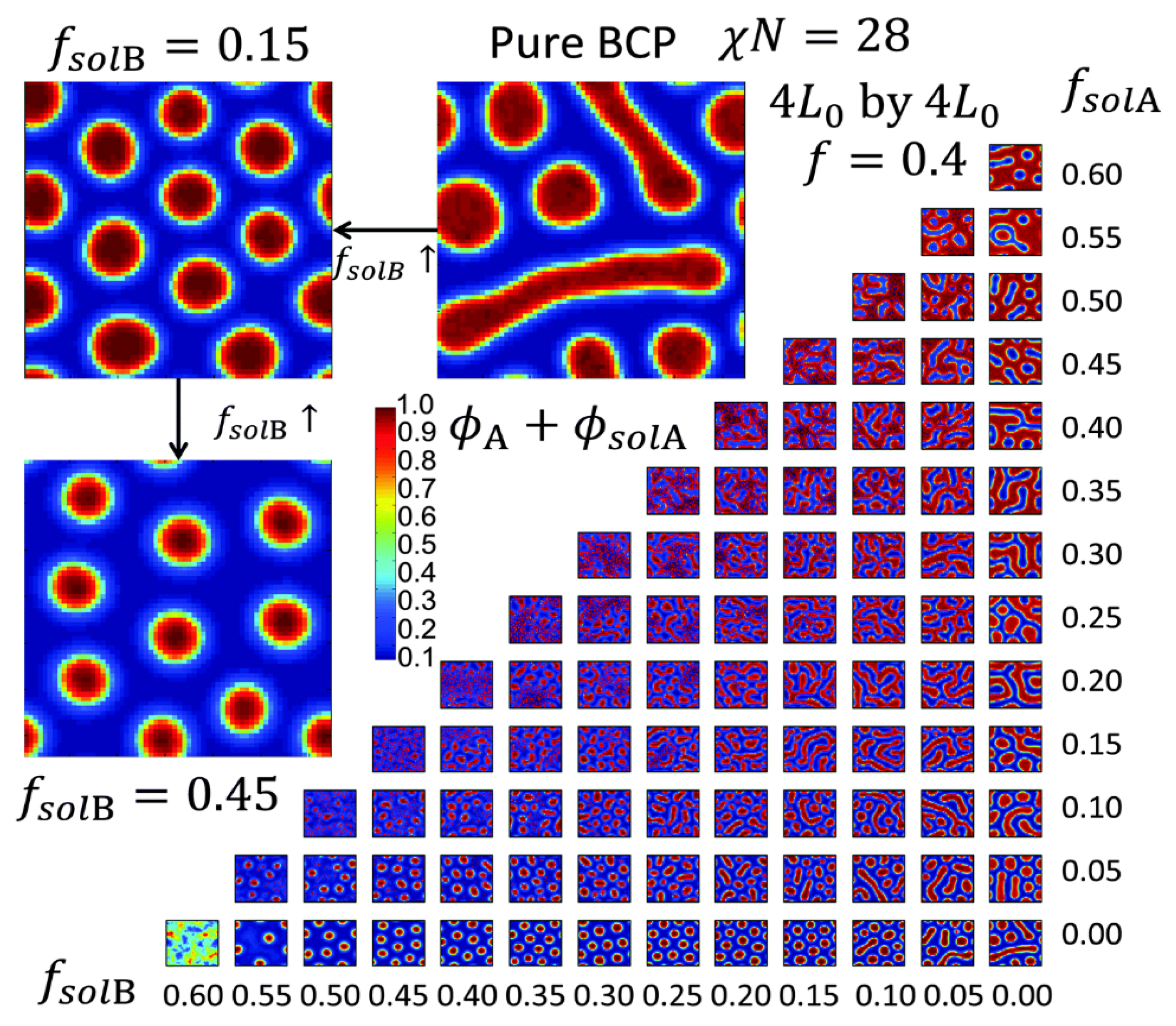
2.1. Block Copolymer Nanostructures in Bulk
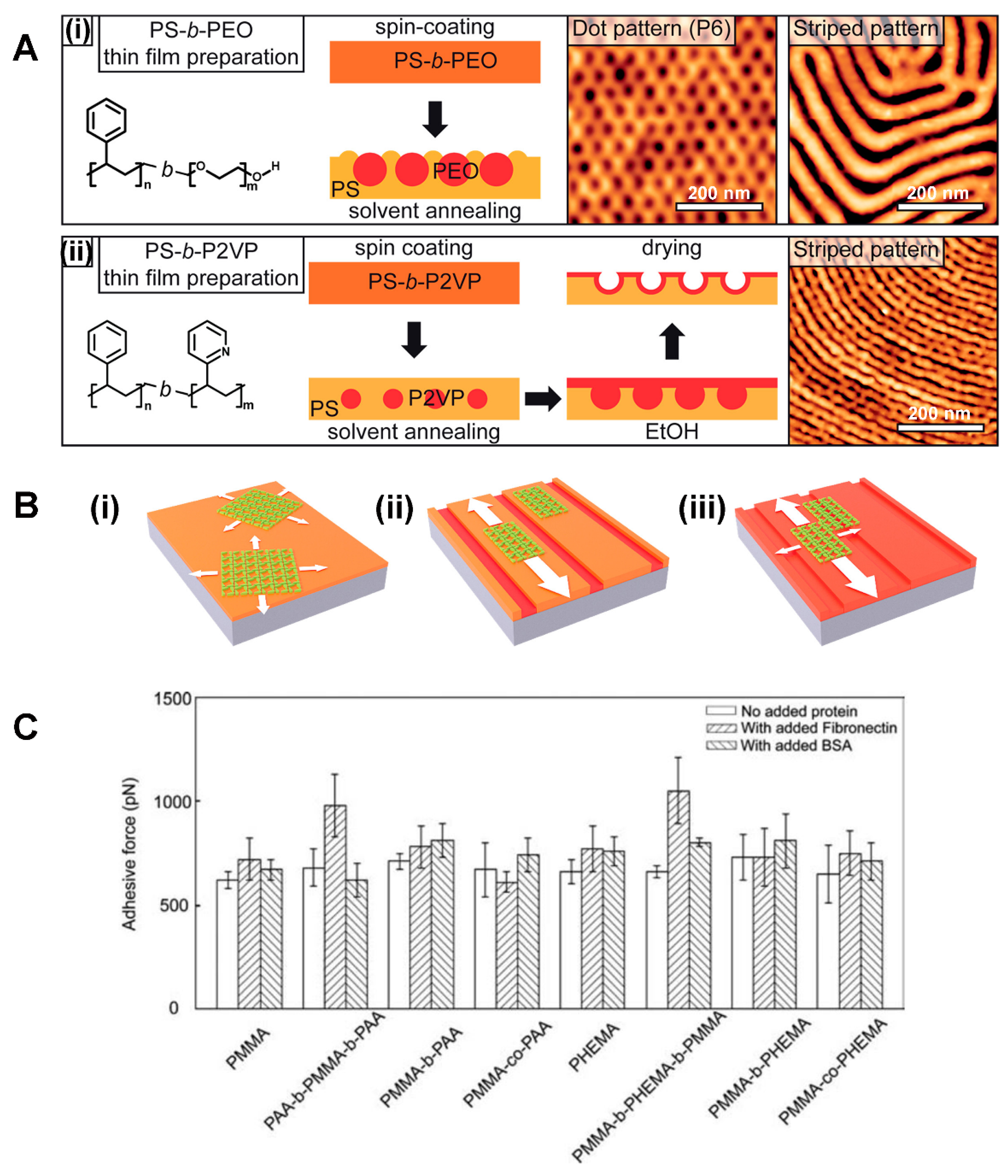
2.2. Block Copolymer Nanostructures in Thin Films
3. Block Copolymer Surfaces Interfacing Biomolecules
3.1. Proteins
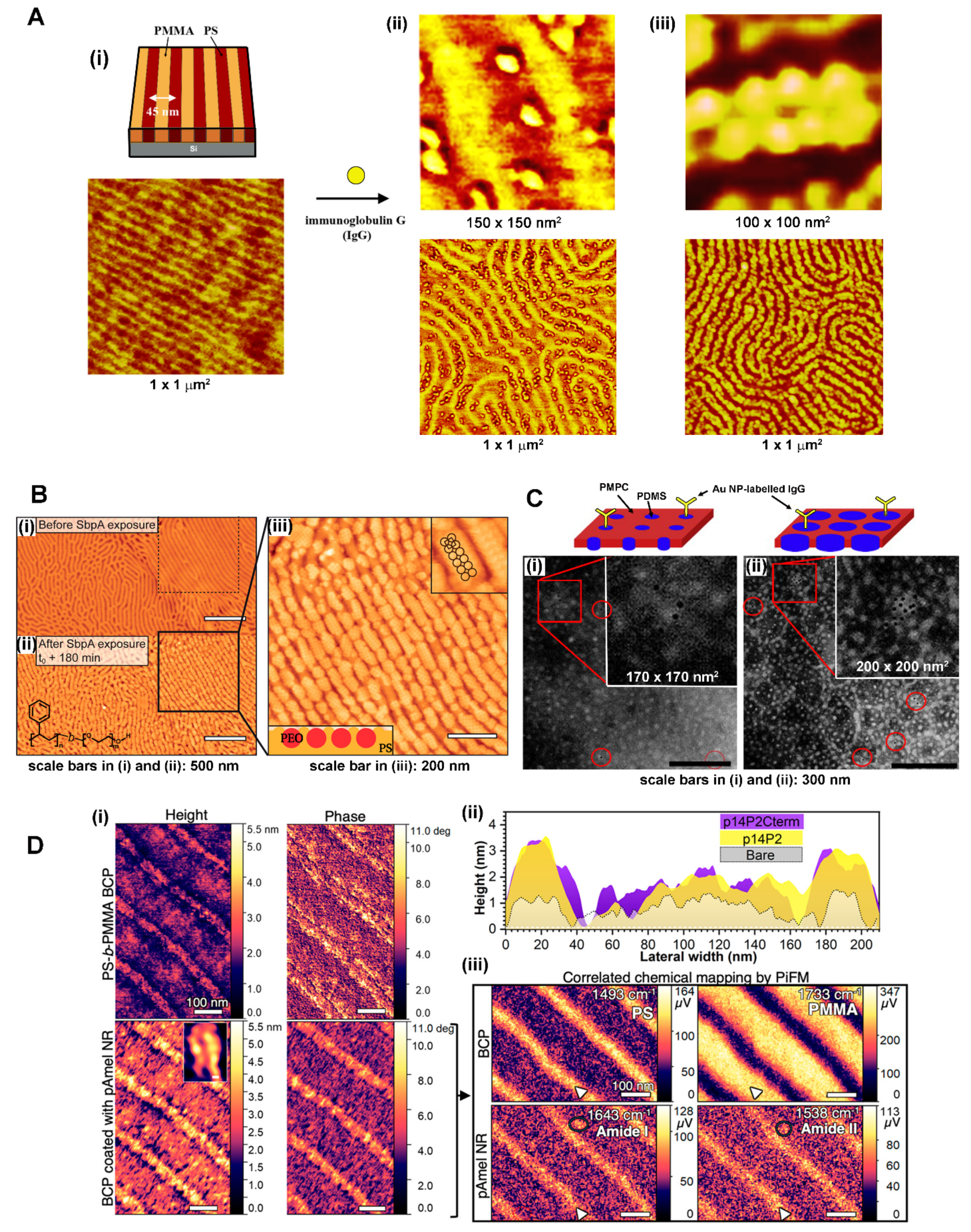
3.1.1. BCP Nanodomains for Proteins: Single-Component Systems
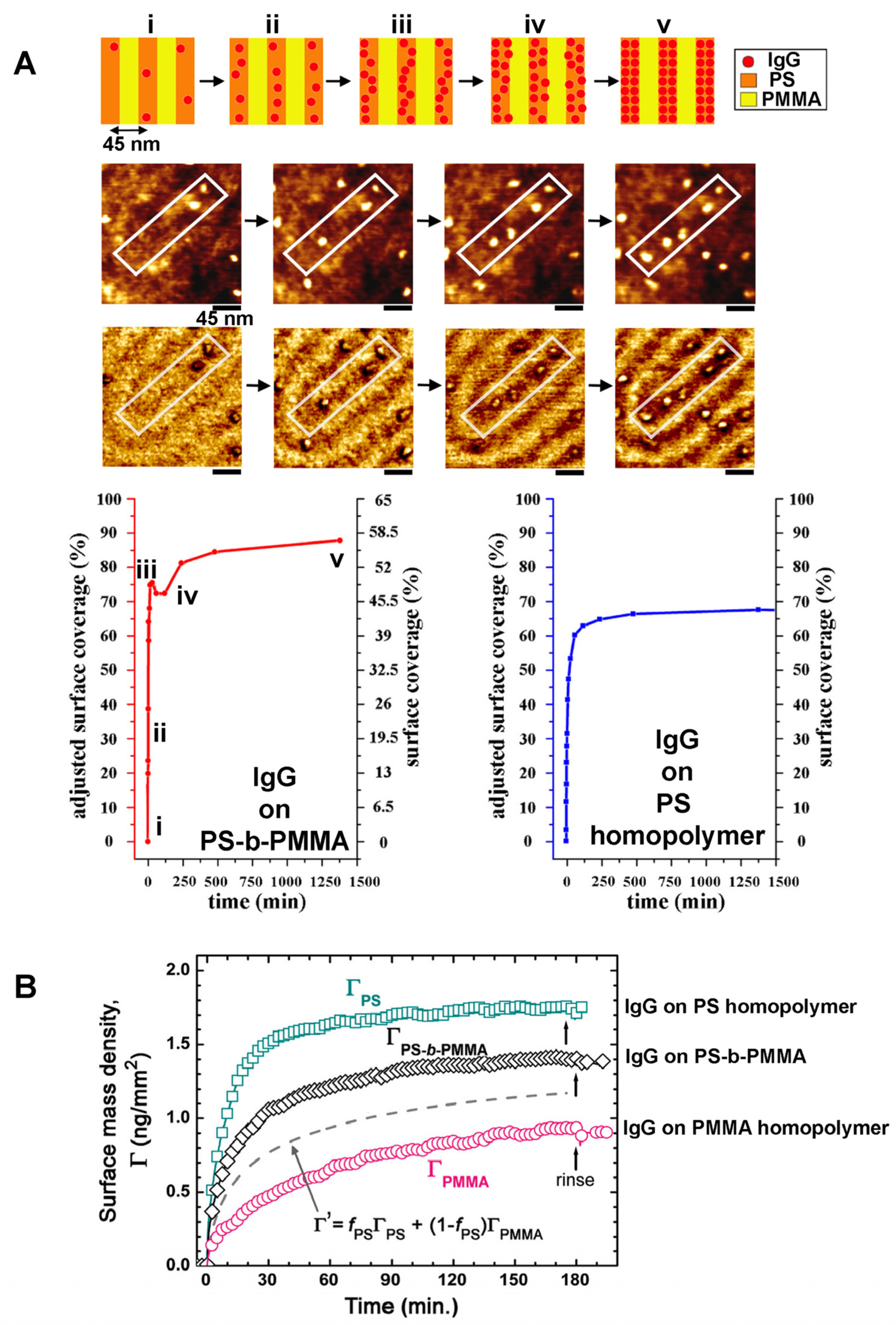
3.1.2. BCP-Guided Protein Assembly on Extended Systems Involving Various BCP Thin Films and Proteins
3.1.3. BCP Nanodomains for Proteins: Multicomponent Systems
3.1.4. Protein Functionality on BCP Thin Films
3.2. Biomineral Nanocrystals
3.3. Cell Adhesive Molecules
3.4. Cells
4. Implications of BCP Nanobiotechnology in Biosensing and Biomaterials
4.1. Implications in Solid-State Protein Arrays
4.2. Implications in Quantitative Bioanalyte Detection
4.3. Implications in Stable Biosensors with High Functionality
4.4. Implications in Tuning Protein Resistance
4.5. Demonstration of Biosensors
5. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-Based Biosensors: Recent Tends, Challenges and Future Perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef] [PubMed]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Fully Integrated Flexible Biosensor for Wearable Continuous Glucose Monitoring. Biosens. Bioelectron. 2022, 196, 113760. [Google Scholar] [CrossRef]
- Seo, Y.; Jeong, S.; Lee, J.; Choi, H.S.; Kim, J.; Lee, H. Innovations in Biomedical Nanoengineering: Nanowell Array Biosensor. Nano Converg. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, L.; Yang, Y.; Du, W.; Ji, W.; Fang, Z.; Hou, X.; Wu, Q.; Zhang, C.; Li, L. Optical Flexible Biosensors: From Detection Principles to Biomedical Applications. Biosens. Bioelectron. 2022, 210, 114328. [Google Scholar] [CrossRef] [PubMed]
- Mahzabeen, F.; Vermesh, O.; Levi, J.; Tan, M.; Alam, I.S.; Chan, C.T.; Gambhir, S.S.; Harris, J.S. Real-Time Point-of-Care Total Protein Measurement with a Miniaturized Optoelectronic Biosensor and Fast Fluorescence-Based Assay. Biosens. Bioelectron. 2021, 180, 112823. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Hua, Z.; Zhang, J.; Guo, P.; Hao, D.; Gao, Y.; Huang, J. Recent Advancements in Flexible and Wearable Sensors for Biomedical and Healthcare Applications. J. Phys. D Appl. Phys. 2022, 55, 134001. [Google Scholar] [CrossRef]
- Soleymani, L.; Li, F. Mechanistic Challenges and Advantages of Biosensor Miniaturization into the Nanoscale. ACS Sens. 2017, 2, 458–467. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavalli, V.K. The Design, Fabrication, and Applications of Flexible Biosensing Devices. Biosens. Bioelectron. 2019, 124–125, 96–114. [Google Scholar] [CrossRef]
- Bhattarai, P.; Hameed, S. Basics of Biosensors and Nanobiosensors. In Nanobiosensors: From Design to Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 1–22. [Google Scholar]
- Mahardika, I.H.; Naorungroj, S.; Khamcharoen, W.; Kin, S.; Rodthongkum, N.; Chailapakul, O.; Shin, K. Point-of-Care Testing (POCT) Devices for DNA Detection: A Comprehensive Review. Adv. NanoBiomed Res. 2023, 3, 2300058. [Google Scholar] [CrossRef]
- Derkus, B. Applying the Miniaturization Technologies for Biosensor Design. Biosens. Bioelectron. 2016, 79, 901–913. [Google Scholar] [CrossRef]
- Stephens, A.D.; Song, Y.; McClellan, B.L.; Su, S.-H.; Xu, S.; Chen, K.; Castro, M.G.; Singer, B.H.; Kurabayashi, K. Miniaturized Microarray-Format Digital ELISA Enabled by Lithographic Protein Patterning. Biosens. Bioelectron. 2023, 237, 115536. [Google Scholar] [CrossRef] [PubMed]
- Sathish, S.; Ishizu, N.; Shen, A.Q. Air Plasma-Enhanced Covalent Functionalization of Poly(methyl methacrylate): High-Throughput Protein Immobilization for Miniaturized Bioassays. ACS Appl. Mater. Interfaces 2019, 11, 46350–46360. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Wang, J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Wang, T.; Yang, H.; Qi, D.; Liu, Z.; Cai, P.; Zhang, H.; Chen, X. Mechano-Based Transductive Sensing for Wearable Healthcare. Small 2018, 14, 1702933. [Google Scholar] [CrossRef]
- Handrea-Dragan, I.M.; Botiz, I.; Tatar, A.-S.; Boca, S. Patterning at the Micro/Nano-Scale: Polymeric Scaffolds for Medical Diagnostic and Cell-Surface Interaction Applications. Colloids Surf. B Biointerfaces 2022, 218, 112730. [Google Scholar] [CrossRef]
- Chiu, D.T.; Jeon, N.L.; Huang, S.; Kane, R.S.; Wargo, C.J.; Choi, I.S.; Ingber, D.E.; Whitesides, G.M. Patterned Deposition of Cells and Proteins onto Surfaces by Using Three-Dimensional Microfluidic Systems. Proc. Natl. Acad. Sci. USA 2000, 97, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning Proteins and Cells Using Soft Lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Easton, C.D.; Styan, K.E.; Leech, P.; Gengenbach, T.R.; Forsythe, J.S.; Hartley, P.G. SU-8 Photolithography on Reactive Plasma Thin-films: Coated Microwells for Peptide Display. Colloids Surf. B Biointerfaces 2013, 108, 313–321. [Google Scholar] [CrossRef]
- Alvarado, R.E.; Nguyen, H.T.; Pepin-Donat, B.; Lombard, C.; Roupioz, Y.; Leroy, L. Optically Assisted Surface Functionalization for Protein Arraying in Aqueous Media. Langmuir 2017, 33, 10511–10516. [Google Scholar] [CrossRef]
- Kargl, R.; Mohan, T.; Köstler, S.; Spirk, S.; Doliška, A.; Stana-Kleinschek, K.; Ribitsch, V. Functional Patterning of Biopolymer Thin Films Using Enzymes and Lithographic Methods. Adv. Funct. Mater. 2013, 23, 308–315. [Google Scholar] [CrossRef]
- Guyomard-Lack, A.; Delorme, N.; Moreau, C.; Bardeau, J.-F.; Cathala, B. Site-Selective Surface Modification Using Enzymatic Soft Lithography. Langmuir 2011, 27, 7629–7634. [Google Scholar] [CrossRef]
- Gdor, E.; Shemesh, S.; Magdassi, S.; Mandler, D. Multienzyme Inkjet Printed 2D Arrays. ACS Appl. Mater. Interfaces 2015, 7, 17985–17992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marelli, B.; Brenckle, M.A.; Mitropoulos, A.N.; Gil, E.-S.; Tsioris, K.; Tao, H.; Kaplan, D.L.; Omenetto, F.G. All-water-based Electron-beam Lithography using Silk as a Resist. Nat. Nanotech. 2014, 9, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Bat, E.; Lee, J.; Lau, U.Y.; Maynard, H.D. Trehalose Glycopolymer Resists Allow Direct Writing of Protein Patterns by Electron-beam Lithography. Nat. Commun. 2015, 6, 6654. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.J.; Paluck, S.J.; Bat, E.; Maynard, H.D. Encapsulated Hydrogels by E-beam Lithography and Their Use in Enzyme Cascade Reactions. Langmuir 2016, 32, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kumar, M.; Calo’, A.; Albisetti, E.; Zheng, X.; Manning, K.B.; Elacqua, E.; Weck, M.; Ulijn, R.V.; Riedo, E. High-Throughput Protein Nanopatterning. Faraday Discuss. 2019, 219, 33–43. [Google Scholar] [CrossRef]
- Chai, J.; Wong, L.S.; Giam, L.; Mirkin, C.A. Single-Molecule Protein Arrays Enabled by Scanning Probe Block Copolymer Lithography. Proc. Natl. Acad. Sci. USA 2011, 108, 19521–19525. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, C.; Zhou, X.; Gao, T.; Liu, D.; Miao, Q.; Zheng, Z. Massively Parallel Patterning of Complex 2D and 3D Functional Polymer Brushes by Polymer Pen Lithography. ACS Appl. Mater. Interfaces 2014, 6, 11955–11964. [Google Scholar] [CrossRef]
- Merino, S.; Retolaza, A.; Trabadelo, V.; Cruz, A.; Heredia, P.; Alduncín, J.A.; Mecerreyes, D.; Fernández-Cuesta, I.; Borrisé, X.; Pérez-Murano, F. Protein Patterning on the Micro- and Nanoscale by Thermal Nanoimprint Lithography on a New Functionalized Copolymer. J. Vac. Sci. Technol. B 2009, 27, 2439–2443. [Google Scholar] [CrossRef]
- Fontelo, R.; Reis, R.L.; Novoa-Carballal, R.; Pashkuleva, I. Preparation, Properties, and Bioapplications of Block Copolymer Nanopatterns. Adv. Healthc. Mater. 2023, 13, 2301810. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Russell, T.P. Pattern Transfer Using Block Copolymers. Philos. Trans. R. Soc. A 2013, 371, 20120306. [Google Scholar] [CrossRef]
- Cho, D.H.; Xie, T.; Truong, J.; Stoner, A.C.; Hahm, J. Recent Advances Towards Single Biomolecule Level Understanding of Protein Adsorption Phenomena Unique to Nanoscale Polymer Surfaces with Chemical Variations. Nano Res. 2020, 13, 1295–1317. [Google Scholar] [CrossRef]
- Hahm, J. Fundamentals of Nanoscale Polymer-Protein Interactions and Potential Contributions to Solid-state Nanobioarrays. Langmuir 2014, 30, 9891–9904. [Google Scholar] [CrossRef]
- Cho, D.H.; Hahm, J.-I. Protein–Polymer Interaction Characteristics Unique to Nanoscale Interfaces: A Perspective on Recent Insights. J. Phys. Chem. B 2021, 125, 6040–6057. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in Top–down and Bottom–up Surface Nanofabrication: Techniques, Applications & Future Prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [PubMed]
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-Polymer Platforms into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Shull, K.R. Mean-Field Theory of Block Copolymers: Bulk Melts, Surfaces, and Thin Films. Macromolecules 1992, 25, 2122–2133. [Google Scholar] [CrossRef]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling Future Nanomanufacturing Through Block Copolymer Self-Assembly: A Review. Nano Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M.; Qiu, F.; Shi, A.-C. Phase Diagram of Diblock Copolymers Confined in Thin Films. J. Phys. Chem. B 2013, 117, 5280–5288. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-C.; Darling, S.B. Block Copolymer Nanostructures for Technology. Polymers 2010, 2, 470–489. [Google Scholar] [CrossRef]
- Segalman, R.A.; McCulloch, B.; Kirmayer, S.; Urban, J.J. Block Copolymers for Organic Optoelectronics. Macromolecules 2009, 42, 9205–9216. [Google Scholar] [CrossRef]
- Darling, S.B. Directing the Self-assembly of Block Copolymers. Prog. Polym. Sci. 2007, 32, 1152–1204. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Park, M.J. Self-Assembly of Block Copolymers with Tailored Functionality: From the Perspective of Intermolecular Interactions. Annu. Rev. Mater. Res. 2020, 50, 521–549. [Google Scholar] [CrossRef]
- Epps, T.H.; O’Reilly, R.K. Block Copolymers: Controlling Nanostructure to Generate Functional Materials–Synthesis, Characterization, and Engineering. Chem. Sci. 2016, 7, 1674–1689. [Google Scholar] [CrossRef] [PubMed]
- Schricker, S.R.; Palacio, M.L.B.; Bhushan, B. Designing Nanostructured Block Copolymer Surfaces to Control Protein Adhesion. Philos. Trans. R. Soc. A 2012, 370, 2348–2380. [Google Scholar] [CrossRef]
- Keddie, D.J. A Guide to the Synthesis of Block Copolymers Using Reversible-Addition Fragmentation Chain Transfer (RAFT) Polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- Dworakowska, S.; Lorandi, F.; Gorczyński, A.; Matyjaszewski, K. Toward Green Atom Transfer Radical Polymerization: Current Status and Future Challenges. Adv. Sci. 2022, 9, 2106076. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wei, Q.; Sheng, W.; Yu, B.; Zhou, F.; Li, B. Driving Polymer Brushes from Synthesis to Functioning. Angew. Chem. Int. Ed. 2023, 62, e202219312. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Jones, G.R.; Tsogtgerel, E.; Nguyen, D.; Keyes, A.; Liu, Y.-S.; Rauf, H.; Ordonez, E.; Puchelle, V.; Basbug Alhan, H.; et al. Linear Block Copolymer Synthesis. Chem. Rev. 2022, 122, 14471–14553. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, P.P.; Moutsios, I.; Manesi, G.-M.; Ivanov, D.A.; Sakellariou, G.; Avgeropoulos, A. Designing High χ Copolymer Materials for Nanotechnology Applications: A Systematic Bulk vs. Thin Films Approach. Prog. Polym. Sci. 2022, 135, 101625. [Google Scholar] [CrossRef]
- Park, S.J.; Bates, F.S.; Dorfman, K.D. Complex Phase Behavior in Binary Blends of AB Diblock Copolymer and ABC Triblock Terpolymer. Macromolecules 2023, 56, 1278–1288. [Google Scholar] [CrossRef]
- Hrubý, M.; Filippov, S.K.; Štěpánek, P. Biomedical Application of Block Copolymers. In Macromolecular Self-Assembly; Billon, L., Borisov, O., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 231–250. [Google Scholar]
- Khandpur, A.K.; Foerster, S.; Bates, F.S.; Hamley, I.W.; Ryan, A.J.; Bras, W.; Almdal, K.; Mortensen, K. Polyisoprene-Polystyrene Diblock Copolymer Phase Diagram Near the Order-Disorder Transition. Macromolecules 1995, 28, 8796–8806. [Google Scholar] [CrossRef]
- Samaddar, P.; Deep, A.; Kim, K.-H. An Engineering Insight into Block Copolymer Self-Assembly: Contemporary Application from Biomedical Research to Nanotechnology. Chem. Eng. J. 2018, 342, 71–89. [Google Scholar] [CrossRef]
- Tang, P.; Qiu, F.; Zhang, H.; Yang, Y. Morphology and Phase Diagram of Complex Block Copolymers: ABC Linear Triblock Copolymers. Phys. Rev. E 2004, 69, 031803. [Google Scholar] [CrossRef] [PubMed]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics-Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Mastroianni, S.E.; Epps, T.H. Interfacial Manipulations: Controlling Nanoscale Assembly in Bulk, Thin film, and Solution Block Copolymer Systems. Langmuir 2013, 29, 3864–3878. [Google Scholar] [CrossRef]
- Matsen, M.W.; Bates, F.S. Unifying Weak- and Strong-segregation Block Copolymer Theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Matsen, M.W.; Schick, M. Microphase Separation in Starblock Copolymer Melts. Macromolecules 1994, 27, 6761–6767. [Google Scholar] [CrossRef]
- Park, J.; Winey, K.I. Double Gyroid Morphologies in Precise Ion-Containing Multiblock Copolymers Synthesized via Step-Growth Polymerization. JACS Au 2022, 2, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling Nanotechnology with Self Assembled Block Copolymer Patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef]
- Lohmüller, T.; Aydin, D.; Schwieder, M.; Morhard, C.; Louban, I.; Pacholski, C.; Spatz, J.P. Nanopatterning by Block Copolymer Micelle Nanolithography and Bioinspired Applications. Biointerphases 2011, 6, MR1–MR12. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, D.R.; Doerk, G.; Aryal, B.R.; Pang, C.; Davis, R.C.; Harb, J.N.; Woolley, A.T. Block Copolymer Self-Assembly to Pattern Gold Nanodots for Site-Specific Placement of DNA Origami and Attachment of Nanomaterials. Nanoscale 2023, 15, 2188–2196. [Google Scholar] [CrossRef]
- Singh, A.N.; Thakre, R.D.; More, J.C.; Sharma, P.K.; Agrawal, Y.K. Block Copolymer Nanostructures and Their Applications: A Review. Polym. Plast. Technol. Eng. 2015, 54, 1077–1095. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, J.Y.; Kim, S.O. Directed Self-Assembly of Block Copolymers for Universal Nanopatterning. Soft Matter 2013, 9, 2780–2786. [Google Scholar] [CrossRef]
- Yang, G.G.; Choi, H.J.; Han, K.H.; Kim, J.H.; Lee, C.W.; Jung, E.I.; Jin, H.M.; Kim, S.O. Block Copolymer Nanopatterning for Nonsemiconductor Device Applications. ACS Appl. Mater. Interfaces 2022, 14, 12011–12037. [Google Scholar] [CrossRef]
- Killops, K.L.; Gupta, N.; Dimitriou, M.D.; Lynd, N.A.; Jung, H.; Tran, H.; Bang, J.; Campos, L.M. Nanopatterning Biomolecules by Block Copolymer Self-Assembly. ACS Macro Lett. 2012, 1, 758–763. [Google Scholar] [CrossRef]
- Oleske, K.W.; Barteau, K.P.; Turker, M.Z.; Beaucage, P.A.; Estroff, L.A.; Wiesner, U. Block Copolymer Directed Nanostructured Surfaces as Templates for Confined Surface Reactions. Macromolecules 2017, 50, 542–549. [Google Scholar] [CrossRef]
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed Self-Assembly of Block Copolymers: A Tutorial Review of Strategies for Enabling Nanotechnology with Soft Matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Hahm, J. Nanoscale Protein Patterning using Self-Assembled Diblock Copolymers. Langmuir 2005, 21, 6652–6655. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Xie, T.; Mulcahey, P.J.; Kelleher, N.P.; Hahm, J.-I. Distinctive Adsorption Mechanism and Kinetics of Immunoglobulin G on a Nanoscale Polymer Surface. Langmuir 2022, 38, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.A.; Bang, J.; Hawker, C.J.; Kim, D.H.; Knoll, W. Modulation of Protein–Surface Interactions on Nanopatterned Polymer Films. Biomacromolecules 2009, 10, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chattoraj, J.; Mulcahey, P.J.; Kelleher, N.P.; Del Gado, E.; Hahm, J.-I. Revealing the Principal Attributes of Protein Adsorption on Block Copolymer Surfaces with Direct Experimental Evidence at the Single Protein Level. Nanoscale 2018, 10, 9063–9076. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Parajuli, O.; Dorfman, A.; Kipp, D.; Hahm, J. Activity Study of Self-Assembled Proteins on Nanoscale Diblock Copolymer Templates. Langmuir 2007, 23, 7416–7422. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Ronaldson, K.; Bailey, N.A.; Lynd, N.A.; Killops, K.L.; Vunjak-Novakovic, G.; Campos, L.M. Hierarchically Ordered Nanopatterns for Spatial Control of Biomolecules. ACS Nano 2014, 8, 11846–11853. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Matsuno, R.; Takai, M.; Ishihara, K. Cell Adhesion on Phase-separated Surface of Block Copolymer Composed of Poly(2-methacryloyloxyethyl phosphorylcholine) and Poly(dimethylsiloxane). Biomaterials 2009, 30, 5330–5340. [Google Scholar] [CrossRef]
- Stel, B.; Gunkel, I.; Gu, X.; Russell, T.P.; De Yoreo, J.J.; Lingenfelder, M. Contrasting Chemistry of Block Copolymer Films Controls the Dynamics of Protein Self-Assembly at the Nanoscale. ACS Nano 2019, 13, 4018–4027. [Google Scholar] [CrossRef]
- Akkineni, S.; Doerk, G.S.; Shi, C.; Jin, B.; Zhang, S.; Habelitz, S.; De Yoreo, J.J. Biomimetic Mineral Synthesis by Nanopatterned Supramolecular-Block Copolymer Templates. Nano Lett. 2023, 23, 4290–4297. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ravensbergen, K.; Alabanza, A.; Soldin, D.; Hahm, J.-I. Distinct Adsorption Configurations and Self-Assembly Characteristics of Fibrinogen on Chemically Uniform and Alternating Surfaces including Block Copolymer Nanodomains. ACS Nano 2014, 8, 5257–5269. [Google Scholar] [CrossRef]
- Xie, T.; Vora, A.; Mulcahey, P.J.; Nanescu, S.E.; Singh, M.; Choi, D.S.; Huang, J.K.; Liu, C.-C.; Sanders, D.P.; Hahm, J. Surface Assembly Configurations and Packing Preferences of Fibrinogen Mediated by the Periodicity and Alignment Control of Block Copolymer Nanodomains. ACS Nano 2016, 10, 7705–7720. [Google Scholar] [CrossRef]
- Matsusaki, M.; Omichi, M.; Kadowaki, K.; Kim, B.H.; Kim, S.O.; Maruyama, I.; Akashi, M. Protein Nanoarrays on a Highly-Oriented Lamellar Surface. Chem. Commun. 2010, 46, 1911–1913. [Google Scholar] [CrossRef]
- Song, S.; Xie, T.; Ravensbergen, K.; Hahm, J.-I. Ascertaining Effects of Nanoscale Polymeric Interfaces on Competitive Protein Adsorption at the Individual Protein Level. Nanoscale 2016, 8, 3496–3509. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhu, J.; Liang, H. Heterogeneous Patterns on Block Copolymer Thin Film via Solvent Annealing: Effect on Protein Adsorption. J. Chem. Phys. 2015, 142, 101908. [Google Scholar] [CrossRef]
- Shen, L.; Garland, A.; Wang, Y.; Li, Z.; Bielawski, C.W.; Guo, A.; Zhu, X.-Y. Two Dimensional Nanoarrays of Individual Protein Molecules. Small 2012, 8, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.L.B.; Schricker, S.R.; Bhushan, B. Block Copolymer Arrangement and Composition Effects on Protein Conformation Using Atomic Force Microscope-Based Antigen–Antibody Adhesion. J. Biomed. Mater. Res. 2012, 100A, 978–988. [Google Scholar] [CrossRef]
- George, P.A.; Doran, M.R.; Croll, T.I.; Munro, T.P.; Cooper-White, J.J. Nanoscale Presentation of Cell Adhesive Molecules via Block Copolymer Self-Assembly. Biomaterials 2009, 30, 4732–4737. [Google Scholar] [CrossRef]
- Liu, D.; Che Abdullah, C.A.; Sear, R.P.; Keddie, J.L. Cell Adhesion on Nanopatterned Fibronectin Substrates. Soft Matter 2010, 6, 5408–5416. [Google Scholar] [CrossRef]
- Hiraguchi, Y.; Nagahashi, K.; Shibayama, T.; Hayashi, T.; Yano, T.-A.; Kushiro, K.; Takai, M. Effect of the Distribution of Adsorbed Proteins on Cellular Adhesion Behaviors Using Surfaces of Nanoscale Phase-Reversed Amphiphilic Block Copolymers. Acta Biomater. 2014, 10, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Arranja, A.; Chen, H.; Mytnyk, S.; Wang, Y.; Oldenhof, S.; van Esch, J.H.; Mendes, E. A Nano-Fibrous Platform of Copolymer Patterned Surfaces for Controlled Cell Alignment. RSC Adv. 2018, 8, 21777–21785. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, T.; Keddie, J.L. Protein Nanopatterning on Self-Organized Poly(styrene-b-isoprene) Thin Film Templates. Langmuir 2009, 25, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Osypova, A.; Magnin, D.; Sibret, P.; Aqil, A.; Jérôme, C.; Dupont-Gillain, C.; Pradier, C.M.; Demoustier-Champagne, S.; Landoulsi, J. Dual Stimuli-Responsive Coating Designed Through Layer-By-Layer Assembly of PAA-b-PNIPAM Block Copolymers for the Control of Protein Adsorption. Soft Matter 2015, 11, 8154–8164. [Google Scholar] [CrossRef] [PubMed]
- Reynhout, I.C.; Delaittre, G.; Kim, H.-C.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Nanoscale Organization of Proteins via Block Copolymer Lithography and Non-Covalent Bioconjugation. J. Mater. Chem. B 2013, 1, 3026–3030. [Google Scholar] [CrossRef] [PubMed]
- Presley, A.D.; Chang, J.J.; Xu, T. Directed Co-Assembly of Heme Proteins with Amphiphilic Block Copolymers Toward Functional Biomolecular Materials. Soft Matter 2011, 7, 172–179. [Google Scholar] [CrossRef]
- Horrocks, M.S.; Kollmetz, T.; O’Reilly, P.; Nowak, D.; Malmström, J. Quantitative Analysis of Biomolecule Release from Polystyrene-Block-Polyethylene Oxide Thin Films. Soft Matter 2022, 18, 4513–4526. [Google Scholar] [CrossRef] [PubMed]
- George, P.A.; Quinn, K.; Cooper-White, J.J. Hierarchical Scaffolds via Combined Macro- and Micro-phase Separation. Biomaterials 2010, 31, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lilge, I.; Schönherr, H. Block Copolymer Brushes for Completely Decoupled Control of Determinants of Cell–Surface Interactions. Angew. Chem. Int. Ed. 2016, 55, 13114–13117. [Google Scholar] [CrossRef]
- Malmström, J.; Wason, A.; Roache, F.; Yewdall, N.A.; Radjainia, M.; Wei, S.; Higgins, M.J.; Williams, D.E.; Gerrard, J.A.; Travas-Sejdic, J. Protein Nanorings Organized by Poly(styrene-block-ethylene oxide) Self-Assembled Thin Films. Nanoscale 2015, 7, 19940–19948. [Google Scholar] [CrossRef]
- Parajuli, O.; Gupta, A.; Kumar, N.; Hahm, J. Evaluation of Enzymatic Activity on Nanoscale PS-b-PMMA Diblock Copolymer Domains. J. Phys. Chem. B 2007, 111, 14022–14027. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, F.; De Rosa, C.; Malafronte, A.; Di Girolamo, R.; Santillo, C.; Gerelli, Y.; Fragneto, G.; Barker, R.; Pavone, V.; Maglio, O.; et al. Nano-in-Nano Approach for Enzyme Immobilization Based on Block Copolymers. ACS Appl. Mater. Interfaces 2017, 9, 29318–29327. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Hirschfeld-Warneken, V.C.; Lohmüller, T.; Heil, P.; Blümmel, J.; Cavalcanti-Adam, E.A.; López-García, M.; Walther, P.; Kessler, H.; Geiger, B.; et al. Induction of Cell Polarization and Migration by a Gradient of Nanoscale Variations in Adhesive Ligand Spacing. Nano Lett. 2008, 8, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of Integrin Function by Nanopatterned Adhesive Interfaces. Chem. Phys. Chem. 2004, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, K.; Tan, E.; Ho, L.; Bundick, S.; Baker, S.M.; Niemz, A. Deposition of DNA-Functionalized Gold Nanospheres into Nanoporous Surfaces. Langmuir 2006, 22, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.C.; Pound, E.; Woolley, A.T.; Linford, M.R.; Harb, J.N.; Davis, R.C. Chemical Alignment of DNA Origami to Block Copolymer Patterned Arrays of 5 nm Gold Nanoparticles. Nano Lett. 2011, 11, 1981–1987. [Google Scholar] [CrossRef]
- Fontelo, R.; Soares da Costa, D.; Reis, R.L.; Novoa-Carballal, R.; Pashkuleva, I. Bactericidal Nanopatterns Generated by Block Copolymer Self-Assembly. Acta Biomater. 2020, 112, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Fontelo, R.; da Costa, D.S.; Reis, R.L.; Novoa-Carballal, R.; Pashkuleva, I. Block Copolymer Nanopatterns Affect Cell Spreading: Stem Versus Cancer Bone Cells. Colloids Surf. B Biointerfaces 2022, 219, 112774. [Google Scholar] [CrossRef] [PubMed]
- Khor, H.L.; Kuan, Y.; Kukula, H.; Tamada, K.; Knoll, W.; Moeller, M.; Hutmacher, D.W. Response of Cells on Surface-Induced Nanopatterns: Fibroblasts and Mesenchymal Progenitor Cells. Biomacromolecules 2007, 8, 1530–1540. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, J.W.; Kwark, Y.-J.; Kim, S.H.; Lee, K.Y. The Height of Cell-Adhesive Nanoposts Generated by Block Copolymer/Surfactant Complex Systems Influences the Preosteoblast Phenotype. Colloids Surf. B Biointerfaces 2014, 123, 679–684. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Jia, Y.; Xu, M.; Wang, Y.; Wang, J.; Wen, T.; Wang, L. Micro-and-Nanometer Topological Gradient of Block Copolymer Fibrous Scaffolds Towards Region-Specific Cell Regulation. J. Colloid Interface Sci. 2022, 606, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Peng, W.; Lin, M.; Wang, F.; Zhang, Y. Gold Nanoparticle-Enhanced Detection of DNA Hybridization by a Block Copolymer-Templating Fiber-Optic Localized Surface Plasmon Resonance Biosensor. Nanomaterials 2021, 11, 616. [Google Scholar] [CrossRef]
- Guo, T.; Gao, J.; Qin, X.; Zhang, X.; Xue, H. A Novel Glucose Biosensor Based on Hierarchically Porous Block Copolymer Film. Polymers 2018, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Sigolaeva, L.V.; Günther, U.; Pergushov, D.V.; Gladyr, S.Y.; Kurochkin, I.N.; Schacher, F.H. Sequential pH-Dependent Adsorption of Ionic Amphiphilic Diblock Copolymer Micelles and Choline Oxidase Onto Conductive Substrates: Toward the Design of Biosensors. Macromol. Biosci. 2014, 14, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Bas, S.Z.; Cummins, C.; Selkirk, A.; Borah, D.; Ozmen, M.; Morris, M.A. A Novel Electrochemical Sensor Based on Metal Ion Infiltrated Block Copolymer Thin Films for Sensitive and Selective Determination of Dopamine. ACS Appl. Nano Mater. 2019, 2, 7311–7318. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Chen, J.Z.Y. Morphologies and Phase Diagrams of ABC Star Triblock Copolymers Confined in a Spherical Cavity. Soft Matter 2013, 9, 4843–4854. [Google Scholar] [CrossRef]
- Sun, M.; Wang, P.; Qiu, F.; Tang, P.; Zhang, H.; Yang, Y. Morphology and Phase Diagram of ABC Linear Triblock Copolymers: Parallel Real-Space Self-Consistent-Field-Theory Simulation. Phys. Rev. E 2008, 77, 016701. [Google Scholar] [CrossRef] [PubMed]
- Epps, T.H.; Cochran, E.W.; Hardy, C.M.; Bailey, T.S.; Waletzko, R.S.; Bates, F.S. Network Phases in ABC Triblock Copolymers. Macromolecules 2004, 37, 7085–7088. [Google Scholar] [CrossRef]
- Tang, P.; Qiu, F.; Zhang, H.; Yang, Y. Morphology and Phase Diagram of Complex Block Copolymers: ABC Star Triblock Copolymers. J. Phys. Chem. B 2004, 108, 8434–8438. [Google Scholar] [CrossRef]
- Tyler, C.A.; Qin, J.; Bates, F.S.; Morse, D.C. SCFT Study of Nonfrustrated ABC Triblock Copolymer Melts. Macromolecules 2007, 40, 4654–4668. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Z.-G. Morphology of ABC Triblock Copolymers. Macromolecules 1995, 28, 7215–7223. [Google Scholar] [CrossRef]
- Matsen, M.W. Effect of Architecture on the Phase Behavior of AB-Type Block Copolymer Melts. Macromolecules 2012, 45, 2161–2165. [Google Scholar] [CrossRef]
- Lodge, T.P. Block Copolymers: Long-Term Growth with Added Value. Macromolecules 2020, 53, 2–4. [Google Scholar] [CrossRef]
- Song, S.; Milchak, M.; Zhou, H.B.; Lee, T.; Hanscom, M.; Hahm, J.I. Nanoscale Protein Arrays of Rich Morphologies via Self-Assembly on Chemically Treated Diblock Copolymer Surfaces. Nanotechnology 2013, 24, 095601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Yan, Q.; Nealey, P.F.; de Pablo, J.J. Monte Carlo Simulations of Diblock Copolymer Thin Films Confined Between Two Homogeneous Surfaces. J. Chem. Phys. 2000, 112, 450–464. [Google Scholar] [CrossRef]
- Morkved, T.L.; Lopes, W.A.; Hahm, J.; Sibener, S.J.; Jaeger, H.M. Silicon Nitride Membrane Substrates for the Investigation of Local Structure in Polymer Thin Films. Polymer 1998, 39, 3871–3875. [Google Scholar] [CrossRef]
- Fasolka, M.J.; Mayes, A.M. Block Copolymer Thin Films: Physics and Applications. Annu. Rev. Mater. Res. 2001, 31, 323–355. [Google Scholar] [CrossRef]
- Segalman, R.A. Patterning with Block Copolymer Thin Films. Mater. Sci. Eng. R Rep. 2005, 48, 191–226. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Tritschler, U.; Pearce, S.; Gwyther, J.; Whittell, G.R.; Manners, I. 50th Anniversary Perspective: Functional Nanoparticles from the Solution Self-Assembly of Block Copolymers. Macromolecules 2017, 50, 3439–3463. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block Copolymer Solution Self-Assembly: Recent Advances, Emerging Trends, and Applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanostructure Fabrication Using Block Copolymers. Nanotechnol. 2003, 14, R39. [Google Scholar] [CrossRef]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Bates, F.S. On the Origins of Morphological Complexity in Block Copolymer Surfactants. Science 2003, 300, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Eisenberg, A. A Model of Micellization for Block Copolymers in Solutions. Macromolecules 1993, 26, 7353–7360. [Google Scholar] [CrossRef]
- Jin, C.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nanopatterning via Solvent Vapor Annealing of Block Copolymer Thin Films. Chem. Mater. 2017, 29, 176–188. [Google Scholar] [CrossRef]
- Kumar, N.; Parajuli, O.; Hahm, J. Two-Dimensionally Self-Arranged Protein Nanoarrays on Diblock Copolymer Templates. J. Phys. Chem. B 2007, 111, 4581–4587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, K.; Eisenberg, A. Ion-Induced Morphological Changes in “Crew-Cut” Aggregates of Amphiphilic Block Copolymers. Science 1996, 272, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eisenberg, A. Multiple Morphologies and Characteristics of “Crew-Cut” Micelle-like Aggregates of Polystyrene-b-poly(acrylic acid) Diblock Copolymers in Aqueous Solutions. J. Am. Chem. Soc. 1996, 118, 3168–3181. [Google Scholar] [CrossRef]
- Bhargava, P.; Zheng, J.X.; Li, P.; Quirk, R.P.; Harris, F.W.; Cheng, S.Z.D. Self-Assembled Polystyrene-block-poly(ethylene oxide) Micelle Morphologies in Solution. Macromolecules 2006, 39, 4880–4888. [Google Scholar] [CrossRef]
- Lin, Y.; Böker, A.; He, J.; Sill, K.; Xiang, H.; Abetz, C.; Li, X.; Wang, J.; Emrick, T.; Long, S.; et al. Self-Directed Self-assembly of Nanoparticle/Copolymer mixtures. Nature 2005, 434, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, S.H.; Russell, T.P. Controlling Orientation and Functionalization in Thin Films of Block Copolymers. Macromol. Rapid Commun. 2009, 30, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Milchak, M.; Zhou, H.; Lee, T.; Hanscom, M.; Hahm, J.-I. Elucidation of Novel Nanostructures by Time-Lapse Monitoring of Polystyrene-block-Polyvinylpyridine under Chemical Treatment. Langmuir 2012, 28, 8384–8391. [Google Scholar] [CrossRef] [PubMed]
- Hannon, A.F.; Bai, W.; Alexander-Katz, A.; Ross, C.A. Simulation Methods for Solvent Vapor Annealing of Block Copolymer Thin Films. Soft Matter 2015, 11, 3794–3805. [Google Scholar] [CrossRef] [PubMed]
- Lopes, W.A.; Jaeger, H.M. Hierarchical Self-assembly of Metal Nanostructures on Diblock Copolymer Scaffolds. Nature 2001, 414, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Darling, S.B.; Yufa, N.A.; Cisse, A.L.; Bader, S.D.; Sibener, S.J. Self-Organization of FePt Nanoparticles on Photochemically Modified Diblock Copolymer Templates. Adv. Mater. 2005, 17, 2446–2450. [Google Scholar] [CrossRef]
- Brassat, K.; Lindner, J.K.N. Nanoscale Block Copolymer Self-Assembly and Microscale Polymer Film Dewetting: Progress in Understanding the Role of Interfacial Energies in the Formation of Hierarchical Nanostructures. Adv. Mater. Interfaces 2020, 7, 1901565. [Google Scholar] [CrossRef]
- Lau, K.H.A.; Bang, J.; Kim, D.H.; Knoll, W. Self-Assembly of Protein Nanoarrays on Block Copolymer Templates. Adv. Funct. Mater. 2008, 18, 3148–3157. [Google Scholar] [CrossRef]
- Malmström, J.; Travas-Sejdic, J. Block Copolymers for Protein Ordering. J. Appl. Polym. Sci. 2014, 131, 40360. [Google Scholar] [CrossRef]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption Through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1700995. [Google Scholar] [CrossRef]
- Hahm, J. Polymeric Surface-Mediated, High-Density Nano-Assembly of Functional Protein Arrays. J. Biomed. Nanotechnol. 2011, 7, 731–742. [Google Scholar] [CrossRef]
- Bhushan, B.; Schricker, S.R. A Review of Block Copolymer-Based Biomaterials That Control Protein and Cell Interactions. J. Biomed. Mater. Res. 2014, 102, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Parajuli, O.; Gupta, A.; Hahm, J. Elucidation of Protein Adsorption Behavior on Polymeric Surfaces: Towards High Density, High Payload, Protein Templates. Langmuir 2008, 24, 2688–2694. [Google Scholar] [CrossRef]
- Leung, B.O.; Hitchcock, A.P.; Cornelius, R.M.; Brash, J.L.; Scholl, A.; Doran, A. Using X-PEEM to Study Biomaterials: Protein and Peptide Adsorption to a Polystyrene–Poly(methyl methacrylate)-b-Polyacrylic Acid Blend. J. Electron. Spectrosc. Relat. Phenom. 2012, 185, 406–416. [Google Scholar] [CrossRef]
- Keller, T.F.; Schönfelder, J.; Reichert, J.; Tuccitto, N.; Licciardello, A.; Messina, G.M.L.; Marletta, G.; Jandt, K.D. How the Surface Nanostructure of Polyethylene Affects Protein Assembly and Orientation. ACS Nano 2011, 5, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.W.; Hirsh, J.; Marder, V.J.; Clowes, A.W.; George, J.N. Hemostasis and Thrombosis: Basic Principles and Clinical Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Zuyderhoff, E.M.; Dupont-Gillain, C.C. Nano-organized Collagen Layers Obtained by Adsorption on Phase-Separated Polymer Thin Films. Langmuir 2012, 28, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Prieto-Simon, B.; Voelcker, N.H. Porous Polymeric Membranes: Fabrication Techniques and Biomedical Applications. J. Mater. Chem. B 2021, 9, 2129–2154. [Google Scholar] [CrossRef] [PubMed]
- Puiggalí-Jou, A.; del Valle, L.J.; Alemán, C. Biomimetic Hybrid Membranes: Incorporation of Transport Proteins/Peptides into Polymer Supports. Soft Matter 2019, 15, 2722–2736. [Google Scholar] [CrossRef]
- Droumaguet, B.L.; Grande, D. Diblock and Triblock Copolymers as Nanostructured Precursors to Functional Nanoporous Materials: From Design to Application. ACS Appl. Mater. Interfaces 2023, 15, 58023–58040. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; Schotter, J.; Kästle, G.A.; Emley, N.; Shibauchi, T.; Krusin-Elbaum, L.; Guarini, K.; Black, C.T.; Tuominen, M.T.; Russell, T.P. Ultrahigh-Density Nanowire Arrays Grown in Self-Assembled Diblock Copolymer Templates. Science 2000, 290, 2126–2129. [Google Scholar] [CrossRef]
- Olayo-Valles, R.; Lund, M.S.; Leighton, C.; Hillmyer, M.A. Large Area Nanolithographic Templates by Selective Etching of Chemically Stained Block Copolymer Thin Films. J. Mater. Chem. 2004, 14, 2729–2731. [Google Scholar] [CrossRef]
- Zalusky, A.S.; Olayo-Valles, R.; Wolf, J.H.; Hillmyer, M.A. Ordered Nanoporous Polymers from Polystyrene–Polylactide Block Copolymers. J. Am. Chem. Soc. 2002, 124, 12761–12773. [Google Scholar] [CrossRef] [PubMed]
- Leiston-Belanger, J.M.; Russell, T.P.; Drockenmuller, E.; Hawker, C.J. A Thermal and Manufacturable Approach to Stabilized Diblock Copolymer Templates. Macromolecules 2005, 38, 7676–7683. [Google Scholar] [CrossRef]
- Bamford, C.H.; Cooper, S.L.; Tsuruta, T. The Vroman Effect; VSP BV: Utrecht, The Netherlands, 1992. [Google Scholar]
- Hirsh, S.L.; McKenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M.M.M. The Vroman Effect: Competitive Protein Exchange with Dynamic Multilayer Protein Aggregates. Colloids Surf. B Biointerfaces 2013, 103, 395–404. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Lim, S.-M.; Albertorio, F.; Kim, G.; Gurau, M.C.; Yang, R.D.; Holden, M.A.; Cremer, P.S. The Vroman Effect: A Molecular Level Description of Fibrinogen Displacement. J. Am. Chem. Soc. 2003, 125, 12782–12786. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Siedlecki, C.A.; Vogler, E.A. Mixology of Protein Solutions and the Vroman Effect. Langmuir 2004, 20, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.L.B.; Schricker, S.R.; Bhushan, B. Bioadhesion of Various Proteins on Random, Diblock and Triblock Copolymer Surfaces and the Effect of pH Conditions. J. R. Soc. Interface 2011, 8, 630–640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slack, S.M.; Horbett, T.A. The Vroman Effect: A Critical Review. In Proteins at Interfaces II: Fundamentals and Applications; Horbett, T.A., Brash, J.L., Eds.; ACS: Washington, DC, USA, 1995; pp. 112–128. [Google Scholar]
- Horbett, T.A. The Role of Adsorbed Proteins in Tissue Response to Biomaterials. In Biomaterials Science-An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: New York, NY, USA, 2004; pp. 237–246. [Google Scholar]
- Wan, J.; Thomas, M.S.; Guthrie, S.; Vullev, V.I. Surface-Bound Proteins with Preserved Functionality. Ann. Biomed. Eng. 2009, 37, 1190–1205. [Google Scholar] [CrossRef]
- Cha, T.W.; Guo, A.; Zhu, X.-Y. Enzymatic Activity on a Chip: The Critical Role of Protein Orientation. Proteomics 2005, 5, 416–419. [Google Scholar] [CrossRef]
- Nudelman, F.; Sommerdijk, N.A.J.M. Biomineralization as an Inspiration for Materials Chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef]
- Davis, S.A.; Dujardin, E.; Mann, S. Biomolecular Inorganic Materials Chemistry. Curr. Opin. Solid State Mater. Sci. 2003, 7, 273–281. [Google Scholar] [CrossRef]
- Deng, X.; Hasan, A.; Elsharkawy, S.; Tejeda-Montes, E.; Tarakina, N.V.; Greco, G.; Nikulina, E.; Stormonth-Darling, J.M.; Convery, N.; Rodriguez-Cabello, J.C.; et al. Topographically Guided Hierarchical Mineralization. Mater. Today Bio 2021, 11, 100119. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-Z.; Ge, J. New Observations of the Hierarchical Structure of Human Enamel, from Nanoscale to Microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef]
- Beniash, E.; Stifler, C.A.; Sun, C.-Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The Hidden Structure of Human Enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [PubMed]
- Politakos, N. Block Copolymers in 3D/4D Printing: Advances and Applications as Biomaterials. Polymers 2023, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of Calcium Phosphate Nanoparticles in Biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Plummer, S.T.; Wang, Q.; Bohn, P.W.; Stockton, R.; Schwartz, M.A. Electrochemically Derived Gradients of the Extracellular Matrix Protein Fibronectin on Gold. Langmuir 2003, 19, 7528–7536. [Google Scholar] [CrossRef]
- Kang, C.E.; Gemeinhart, E.J.; Gemeinhart, R.A. Cellular Alignment by Grafted Adhesion Peptide Surface Density Gradients. J. Biomed. Mater. Res. 2004, 71A, 403–411. [Google Scholar] [CrossRef]
- Dertinger, S.K.W.; Jiang, X.; Li, Z.; Murthy, V.N.; Whitesides, G.M. Gradients of Substrate-bound Laminin Orient Axonal Specification of Neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 12542–12547. [Google Scholar] [CrossRef]
- Maheshwari, G.; Brown, G.; Lauffenburger, D.A.; Wells, A.; Griffith, L.G. Cell Adhesion and Motility Depend on Nanoscale RGD Clustering. J. Cell Sci. 2000, 113, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix and the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional Polymer Surfaces for Controlling Cell Behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic Overview of Soft Materials as a Novel Frontier for MRI Contrast Agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Katayama, R.; Lien Nguyen, T.; Oki, Y.; Tsujimoto, A.; Yusa, S.-i.; Shiraishi, K.; Matsumoto, A. Different Antifouling Effects of Random and Block Copolymers Comprising 2-methacryloyloxyethyl Phosphorylcholine and Dodecyl Methacrylate. Eur. Polym. J. 2020, 136, 109932. [Google Scholar] [CrossRef]
- Angenendt, P.; Glökler, J.; Konthur, Z.; Lehrach, H.; Cahill, D.J. 3D Protein Microarrays: Performing Multiplex Immunoassays on a Single Chip. Anal. Chem. 2003, 75, 4368–4372. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.J. Protein and Antibody Arrays and Their Medical Applications. J. Immunol. Methods 2001, 250, 81–91. [Google Scholar] [CrossRef]
- Gong, P.; Grainger, D.W. Microarrays: Methods and Protocols, 2nd ed.; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Kersten, B.; Wanker, E.E.; Hoheisel, J.D.; Angenendt, P. Multiplexed Approaches in Protein Microarray Technology. Expert Rev. Proteom. 2005, 2, 499–510. [Google Scholar] [CrossRef]
- MacBeath, G. Protein Microarrays and Proteomics. Nat. Genet. 2002, 32, 526–532. [Google Scholar] [CrossRef]
- MacBeath, G.; Schreiber, S.L. Printing Proteins as Microarrays for High-Throughput Function Determination. Science 2000, 289, 1760–1763. [Google Scholar] [CrossRef]
- Mendoza, L.G.; McQuary, P.; Mongan, A.; Gangadharan, R.; Brignac, S.; Eggers, M. High-Throughput Microarray-Based Enzyme-Linked Immunosorbent Assay (ELISA). BioTechniques 1999, 27, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Pavlickova, P.; Schneider, E.M.; Hug, H. Advances in Recombinant Antibody Microarrays. Clin. Chim. Acta 2004, 343, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, A.; Rouse, R.; Hardiman, G. Protein Microarrays: Challenges and Promises. Pharmacogenomics 2002, 3, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Templin, M.F.; Stoll, D.; Schrenk, M.; Traub, P.C.; Vohringer, C.F.; Joos, T.O. Protein Microarray Technology. Trends Biotechnol. 2002, 20, 160–166. [Google Scholar] [CrossRef]
- Xu, Q.; Lam, K.S. Protein and Chemical Microarrays-Powerful Tools for Proteomics. J. Biomed. Biotechnol. 2003, 2003, 257–266. [Google Scholar] [CrossRef]
- Seo, J.; Shin, J.-Y.; Leijten, J.; Jeon, O.; Camci-Unal, G.; Dikina, A.D.; Brinegar, K.; Ghaemmaghami, A.M.; Alsberg, E.; Khademhosseini, A. High-Throughput Approaches for Screening and Analysis of Cell Behaviors. Biomaterials 2018, 153, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fohlerová, Z.; Pekárek, J.; Basova, E.; Neužil, P. Recent Advances in Lab-on-a-Chip Technologies for Viral Diagnosis. Biosens. Bioelectron. 2020, 153, 112041. [Google Scholar] [CrossRef]
- Brambilla, D.; Chiari, M.; Gori, A.; Cretich, M. Towards Precision Medicine: The Role and Potential of Protein and Peptide Microarrays. Analyst 2019, 144, 5353–5367. [Google Scholar] [CrossRef]
- Bowser, B.L.; Robinson, R.A.S. Enhanced Multiplexing Technology for Proteomics. Annu. Rev. Anal. Chem. 2023, 16, 379–400. [Google Scholar] [CrossRef]
- Zhang, H.; Miller, B.L. Immunosensor-Based Label-free and Multiplex Detection of Influenza Viruses: State of the Art. Biosens. Bioelectron. 2019, 141, 111476. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A Focused Review on Three-Dimensional Bioprinting Technology for Artificial Organ Fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef] [PubMed]
- Brittain, W.J.; Brandsetter, T.; Prucker, O.; Rühe, J. The Surface Science of Microarray Generation–A Critical Inventory. ACS Appl. Mater. Interfaces 2019, 11, 39397–39409. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented Immobilization of Antibodies onto Sensing Platforms-A Critical Review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Clancy, K.F.A.; Dery, S.; Laforte, V.; Shetty, P.; Juncker, D.; Nicolau, D.V. Protein Microarray Spots are Modulated by Patterning Method, Surface Chemistry and Processing Conditions. Biosens. Bioelectron. 2019, 130, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Phizicky, E.; Bastiaens, P.I.H.; Zhu, H.; Snyder, M.; Fields, S. Protein Analysis on a Proteomic Scale. Nature 2003, 422, 208–215. [Google Scholar] [CrossRef]
- Anderson, K.S.; Ramachandran, N.; Wong, J.; Raphael, J.V.; Hainsworth, E.; Demirkan, G.; Cramer, D.; Aronzon, D.; Hodi, F.S.; Harris, L.; et al. Application of Protein Microarrays for Multiplexed Detection of Antibodies to Tumor Antigens in Breast Cancer. J. Proteome Res. 2008, 7, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Spatz, J.P.; Mössmer, S.; Hartmann, C.; Möller, M.; Herzog, T.; Krieger, M.; Boyen, H.-G.; Ziemann, P.; Kabius, B. Ordered Deposition of Inorganic Clusters from Micellar Block Copolymer Films. Langmuir 2000, 16, 407–415. [Google Scholar] [CrossRef]
- Leung, B.O.; Hitchcock, A.P.; Cornelius, R.; Brash, J.L.; Scholl, A.; Doran, A. X-ray Spectromicroscopy Study of Protein Adsorption to a Polystyrene–Polylactide Blend. Biomacromolecules 2009, 10, 1838–1845. [Google Scholar] [CrossRef]
- Li, L.; Hitchcock, A.P.; Cornelius, R.; Brash, J.L.; Scholl, A.; Doran, A. X-ray Microscopy Studies of Protein Adsorption on a Phase Segregated Polystyrene/Polymethylmethacrylate Surface. 2. Effect of pH on Site Preference. J. Phys. Chem. B 2008, 112, 2150–2158. [Google Scholar] [CrossRef]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent Trends in Peptide and Protein-Based Hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Weinman, C.J.; Gunari, N.; Krishnan, S.; Dong, R.; Paik, M.Y.; Sohn, K.E.; Walker, G.C.; Kramer, E.J.; Fischer, D.A.; Ober, C.K. Protein Adsorption Resistance of Anti-Biofouling Block Copolymers Containing Amphiphilic Side Chains. Soft Matter 2010, 6, 3237–3243. [Google Scholar] [CrossRef]
- Wu, H.-X.; Zhang, X.-H.; Huang, L.; Ma, L.-F.; Liu, C.-J. Diblock Polymer Brush (PHEAA-b-PFMA): Microphase Separation Behavior and Anti-Protein Adsorption Performance. Langmuir 2018, 34, 11101–11109. [Google Scholar] [CrossRef] [PubMed]



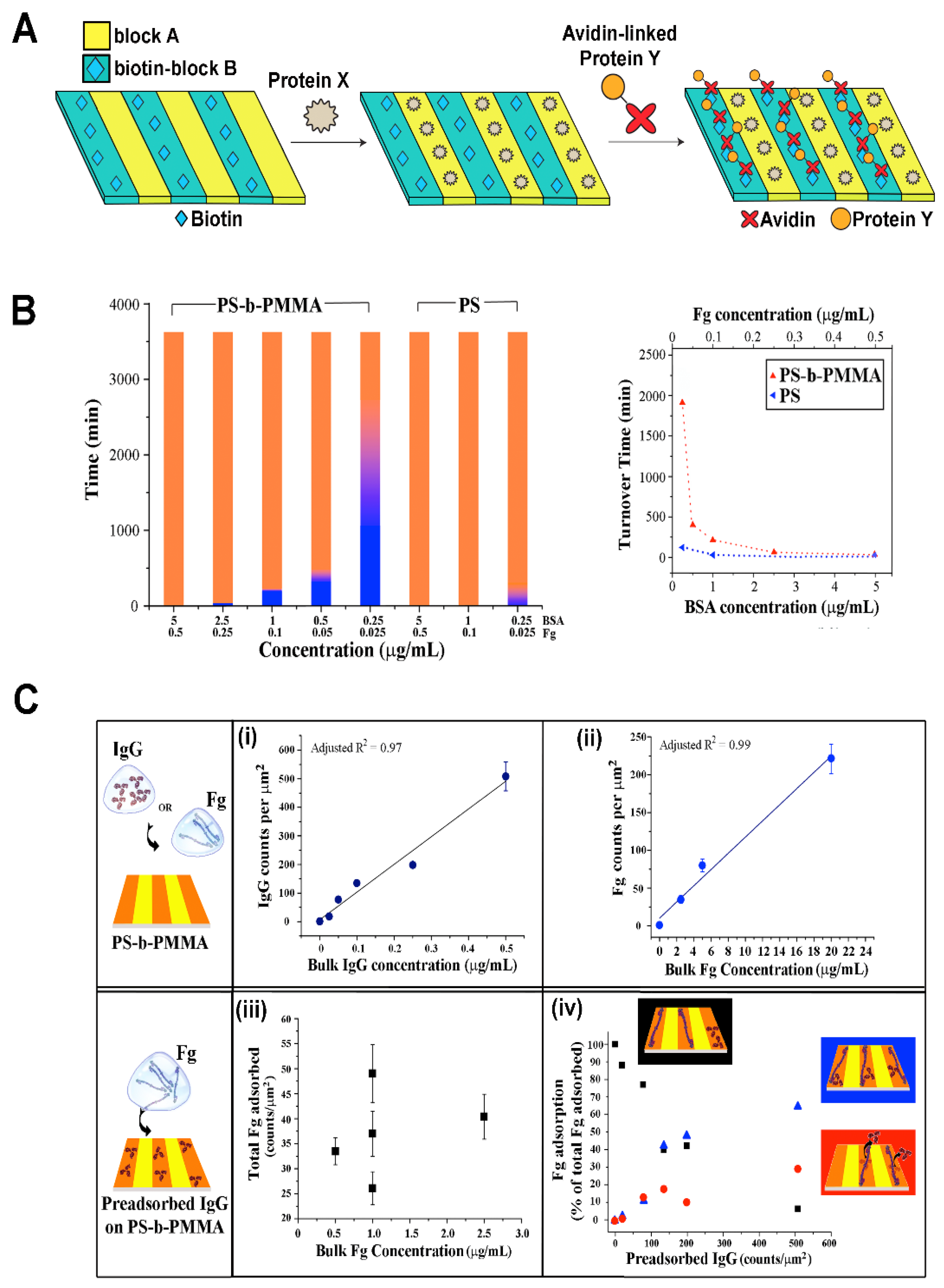
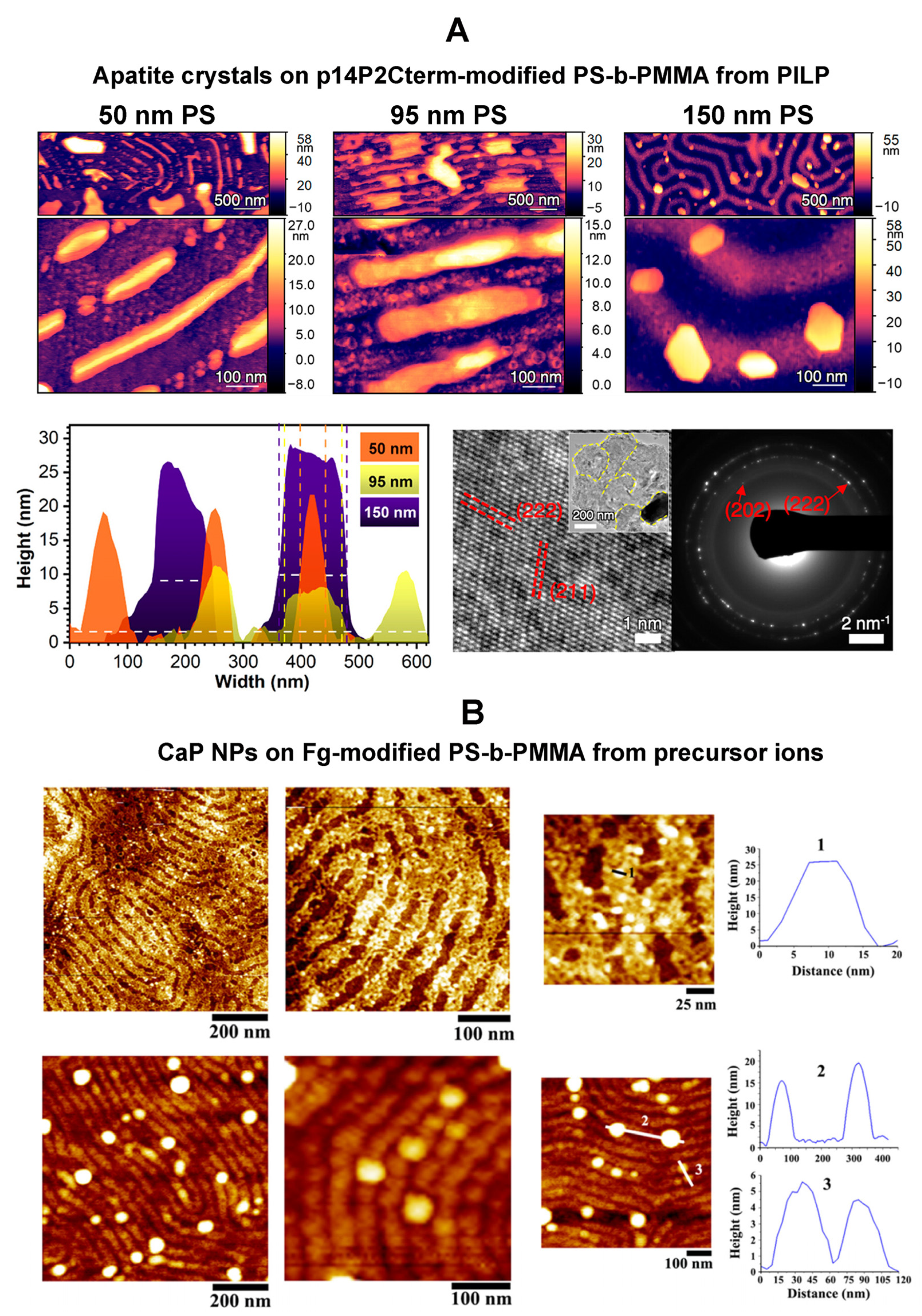
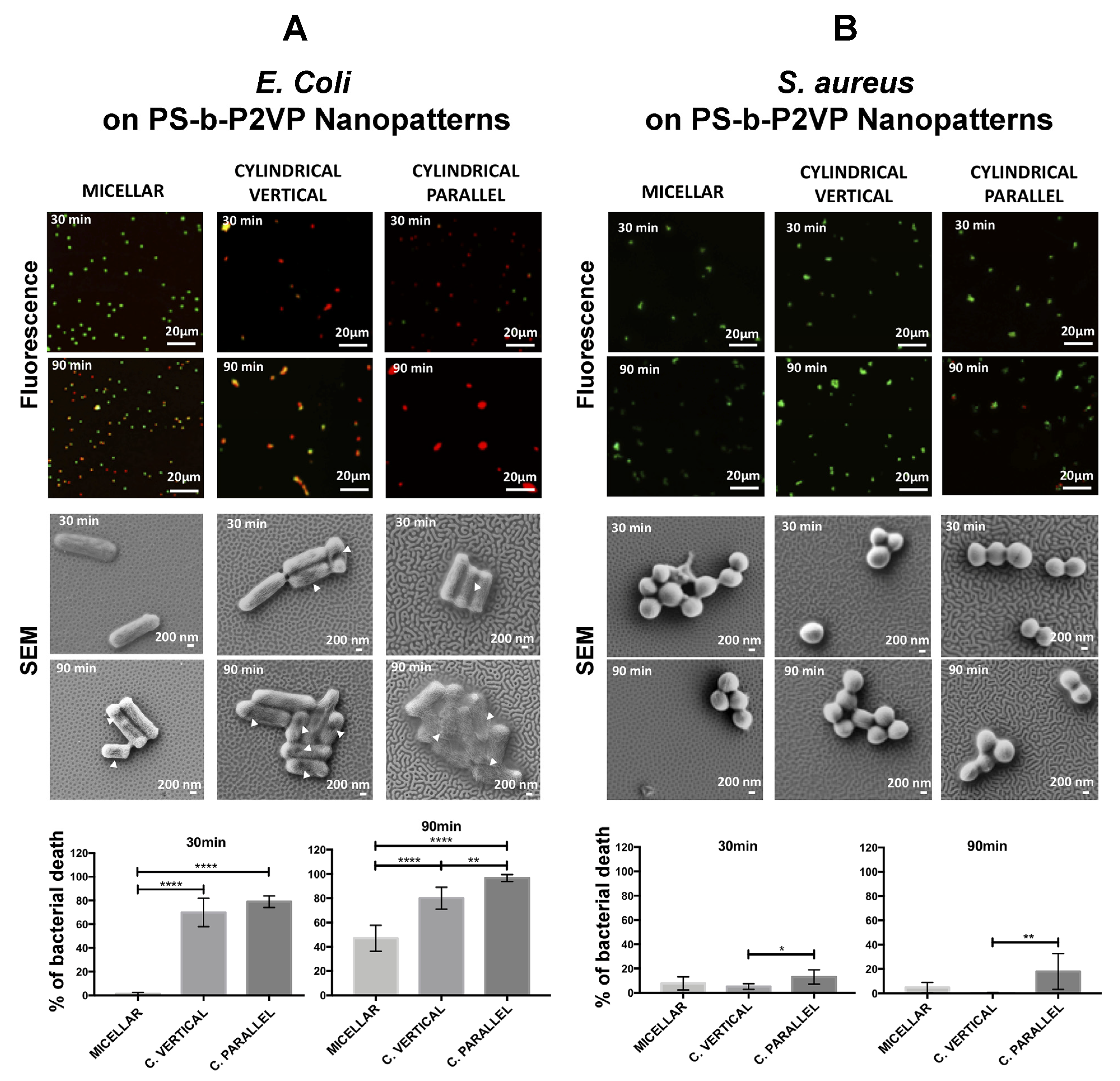

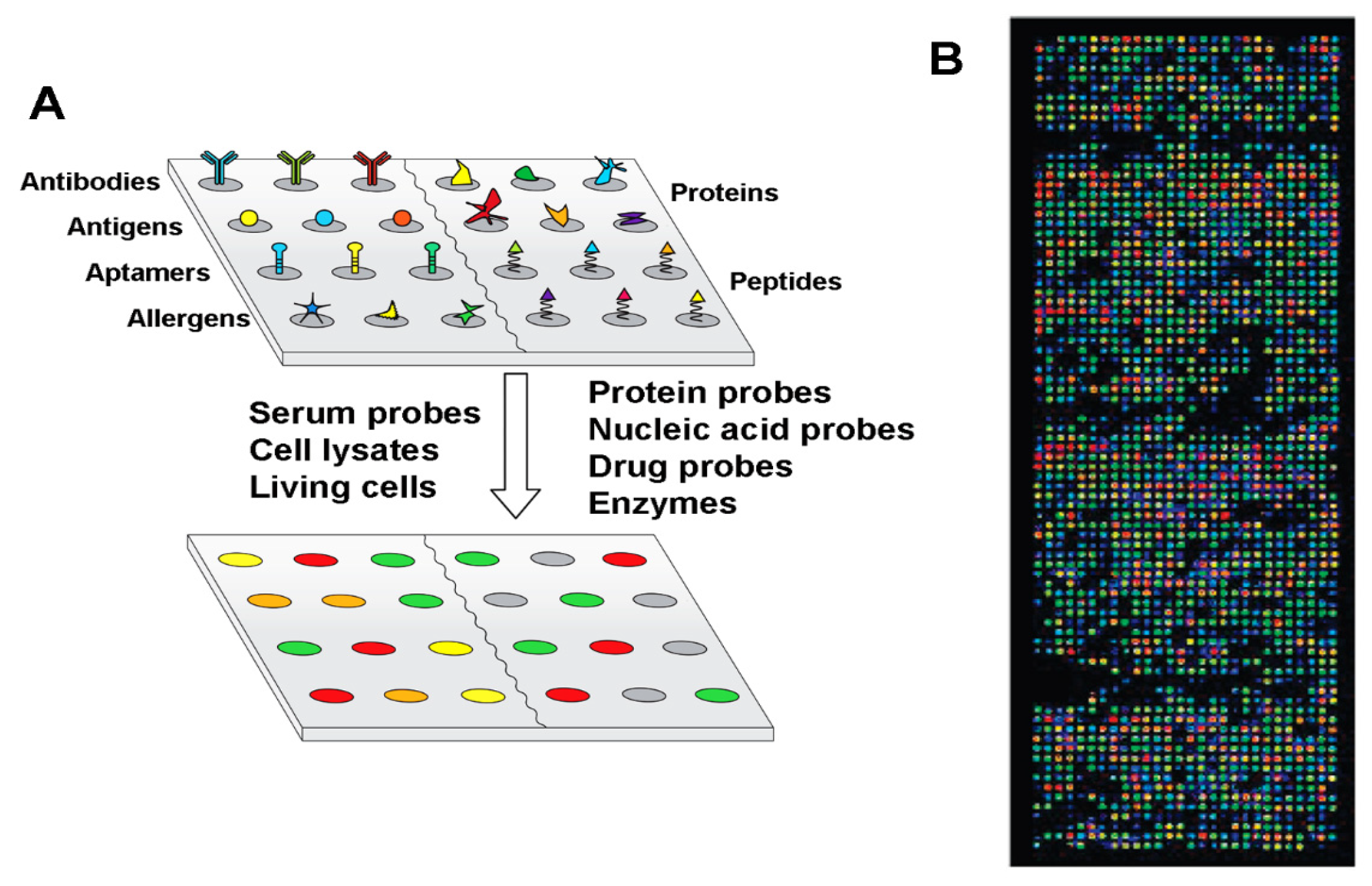
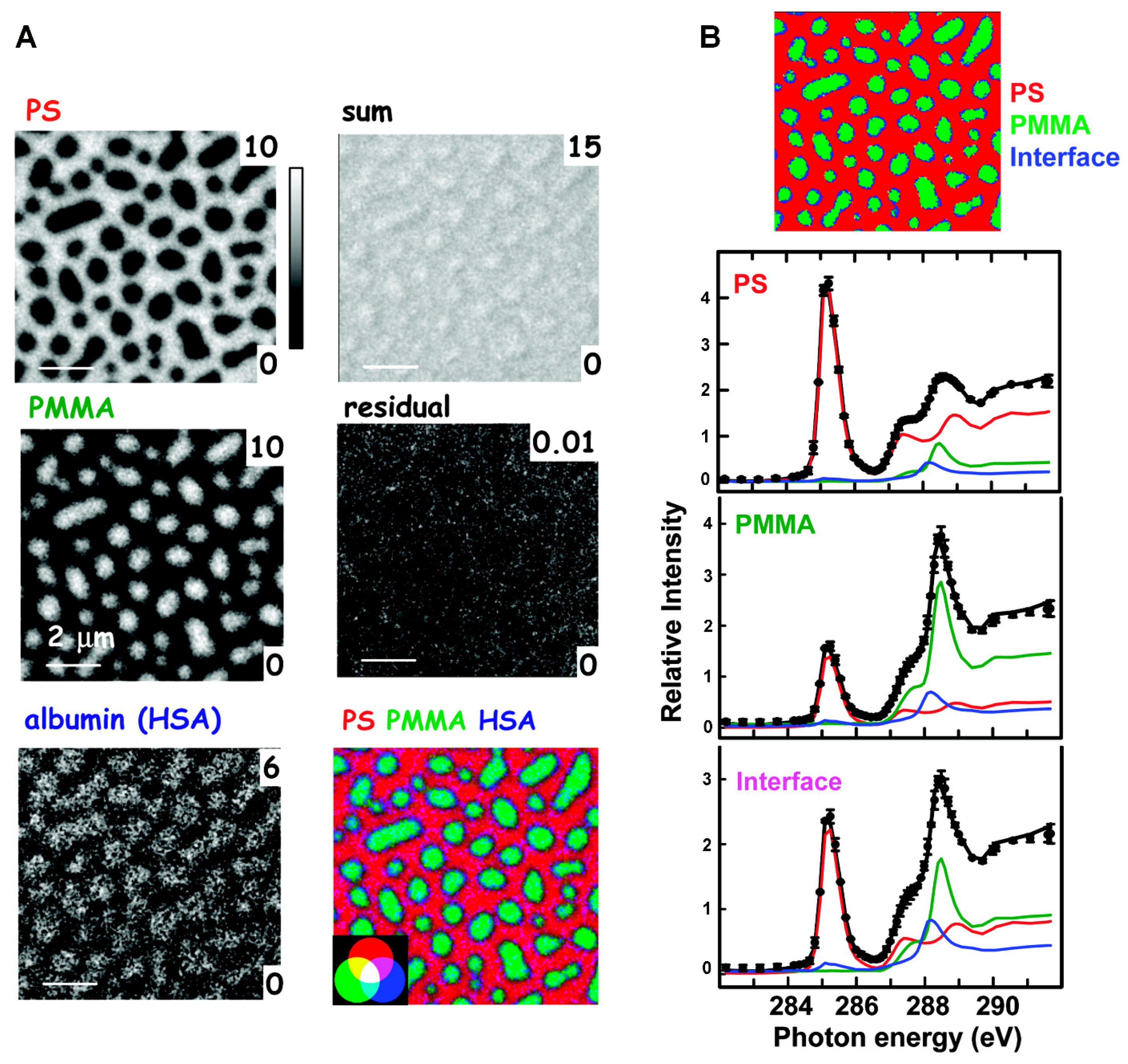

| Biomolecule Name (Abbreviation) | BCP Nanotemplate Used with Biomolecules | Section Covered | Ref. |
|---|---|---|---|
| Proteins and Peptides | |||
| Immunoglobulin G (IgG) | Polystyrene-block-polymethylmethacrylate (PS-b-PMMA) | 3.1.1. 3.1.2. 3.1.3. 3.1.4. | [75] [76,77] [78] [79] |
| Poly(styrene-co-4-bromostyrene)-block-polyethylene oxide (P(S-co-BrS)-b-PEO) | 3.1.3. | [80] | |
| Poly(2-methacryloyloxyethyl phosphorylcholine)-block-poly(dimethylsiloxane) (PMPC-b-PDMS) | 3.1.1. | [81] | |
| S-layer protein (SbpA) | Polystyrene-block-polyethylene oxide (PS-b-PEO) | 3.1.1.&3.1.2. | [82] |
| Polystyrene-block-poly(2-vinylpyridine) (PS-b-P2VP) | 3.1.2. | [82] | |
| Amelogenin (Amel) | PS-b-PMMA | 3.1.1. 3.2. | [83] |
| Fibrinogen (Fg) | PS-b-PMMA | 3.1.1. 3.1.2. 3.1.3. 3.1.4. 3.2. | [84,85] [86] [78,87] [85] [85] |
| Polystyrene-block-poly(2-hydroxyethyl methacrylate) (PS-b-PHEMA) | 3.1.2. 3.1.4. | [88,89] [89] | |
| γ-globulin | PS-b-PMMA | 3.1.2. | [86] |
| Fibronectin (FN) | PS-b-PMMA | 3.1.2. | [86] |
| PMPC-b-PDMS | 3.1.2. | [81] | |
| Polymethylmethacrylate-block-polyacrylic acid (PMMA-b-PAA) Polymethylmethacrylate-block-poly(2-hydroxyethyl methacrylate) (PMMA-b-PHEMA) Polyacrylic acid-block-polymethylmethacrylate-block-polyacrylic acid (PAA-b-PMMA-b-PAA) Polymethylmethacrylate-block-poly(2-hydroxyethyl methacrylate)-block-polymethylmethacrylate (PMMA-b-PHEMA-b-PMMA) | 3.1.2. | [90] | |
| PS-b-PEO | 3.1.2. | [91] | |
| Polystyrene-block-polyisoprene (PS-b-PI) | 3.3. | [92] | |
| PMPC-block-poly(3-methacryloyloxy propyltris(trimethylsilyloxy) silane) (PMPTSSi) | 3.3. | [93] | |
| Thrombomodulin (TM) | PS-b-PMMA | 3.1.2. | [86] |
| Type I collagen (Col I) | PS-b-PMMA | 3.1.2. | [86] |
| Collagen fibrils | PS-b-PEO | 3.4. | [94] |
| Human/bovine serum albumin (HSA/BSA) | PS-b-PMMA | 3.1.3. | [78,87] |
| PS-b-PI | 3.1.2. | [95] | |
| Ovalbumin (OVA) | Poly(acrylic acid)-block-poly(N-isopropyl acrylamide) (PAA-b-PNIPAM) | 3.1.2. | [96] |
| Streptavidin (SAv) | Polyethylene glycol-block-polystyrene (PEG-b-PS) | 3.1.2. | [97] |
| Myoglobin (Mb) | Polystyrene-block-poly(2-hydroxyethyl methacrylate) (PS-b-PHEMA) | 3.1.2.&3.1.4. | [89] |
| PS-b-PEO | 3.1.2. | [98] | |
| Lysozyme (LZM) | PS-b-PHEMA | 3.1.2.&3.1.4. | [89] |
| PS-b-PEO | 3.1.2. | [99] | |
| Green fluorescent protein (GFP) | PS-b-PEO | 3.1.2. | [91] |
| Arginine-Glycine-Aspartate (RGD) peptide motifs | PS-b-PEO | 3.1.2. 3.3. | [91] [91,100] |
| Polyacrylamide/bis-acrylamide-block-poly(acrylic acid) (PAAm/bisAAm-b-PAA) | 3.3. 3.4. | [101] | |
| TAT peptide | PS-b-PEO | 3.1.2. | [99] |
| Coiled-coil α-helix bundle (heme-binding motif) | PS-b-PEO | 3.1.2. | [98] |
| Lsmα protein | PS-b-PEO | 3.1.2. | [102] |
| Horseradish peroxidase (HRP) | PS-b-PMMA | 3.1.4. | [79,103] |
| Polystyrene-block-polyethylene oxide/polystyrene-block-poly(l-lactide) (PS-b-PEO/ PS-b-PLLA) | 3.1.2. 3.1.4. | [104] | |
| avß3 integrin receptor of c(-RGDfK-) | Polystyrene-block-poly(2-vinylpyridine) (PS-b-P2VP) | 3.1.2. 3.3. | [105,106] |
| Tyrosinase | PS-b-PMMA | 3.1.4. | [79] |
| Nucleic Acids | |||
| DNA origami | PS-b-PMMA | 3.1.2. | [68,107] |
| PS-b-P2VP | 3.1.2. | [108] | |
| Cells | |||
| Chinese Hamster ovary cells (CHO) | PS-b-PI | 3.3. | [92] |
| MC3T3-osteoblasts | PS-b-P2VP | 3.3. | [105,106] |
| B16-melanocytes | PS-b-P2VP | 3.3. | [106] |
| REF52-fibroblasts | PS-b-P2VP | 3.3. | [106] |
| 3T3 and NIH-3T3 fibroblasts | PS-b-P2VP | 3.3. | [106] |
| PS-b-PEO | 3.3. 3.4. | [91,100] [94] | |
| Polyacrylamide/bis-acrylamide-block-poly(acrylic acid) (PAAm/bisAAm-b-PAA) | 3.4. | [101] | |
| L929 fibroblasts | PMPC-block-poly(3-methacryloyloxy propyltris(trimethylsilyloxy) silane) (PMPC-b-PMPTSSi) | 3.3. | [93] |
| PMPC-b-PDMS-PMPC | 3.4. | [81] | |
| Escherichia coli (E.coli) | PS-b-P2VP | 3.4. | [109] |
| Staphylococcus aureus (S.aureus) | PS-b-P2VP | 3.4. | [109] |
| Bone marrow mesenchymal stem cells (BMMSC), Mesenchymal precursor cells | PS-b-P2VP | 3.4. | [110] |
| PS-b-P2VP Polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) | 3.4. | [111] | |
| Osteosarcoma cells (SaOS-2) | PS-b-P2VP | 3.4. | [110] |
| Dermal fibroblasts | PS-b-P2VP PS-b-P4VP | 3.4. | [111] |
| Mouse preosteoblasts (MC3T3-E1) | Polystyrene-block-poly(ethylene oxide)/dodecylbenzenesulfonic acid (PS-b-PEO/DBSA) | 3.4. | [112] |
| Pancreatic tumor cells, PaTu 8988t | PAAm/bisAAm-b-PAA | 3.4. | [101] |
| Endothelial cells (ECs) | Polystyrene-block-poly(ethylene-co-butylene)-block-polystyrene (SEBS) | 3.4. | [113] |
| Biomineral Nanocrystals | |||
| Calcium phosphate (CaP), Hydroxy-apatite (HAP), Triple CaP (TCP) | PS-b-PMMA | 3.2. | [83,85] |
| Biosensors | |||
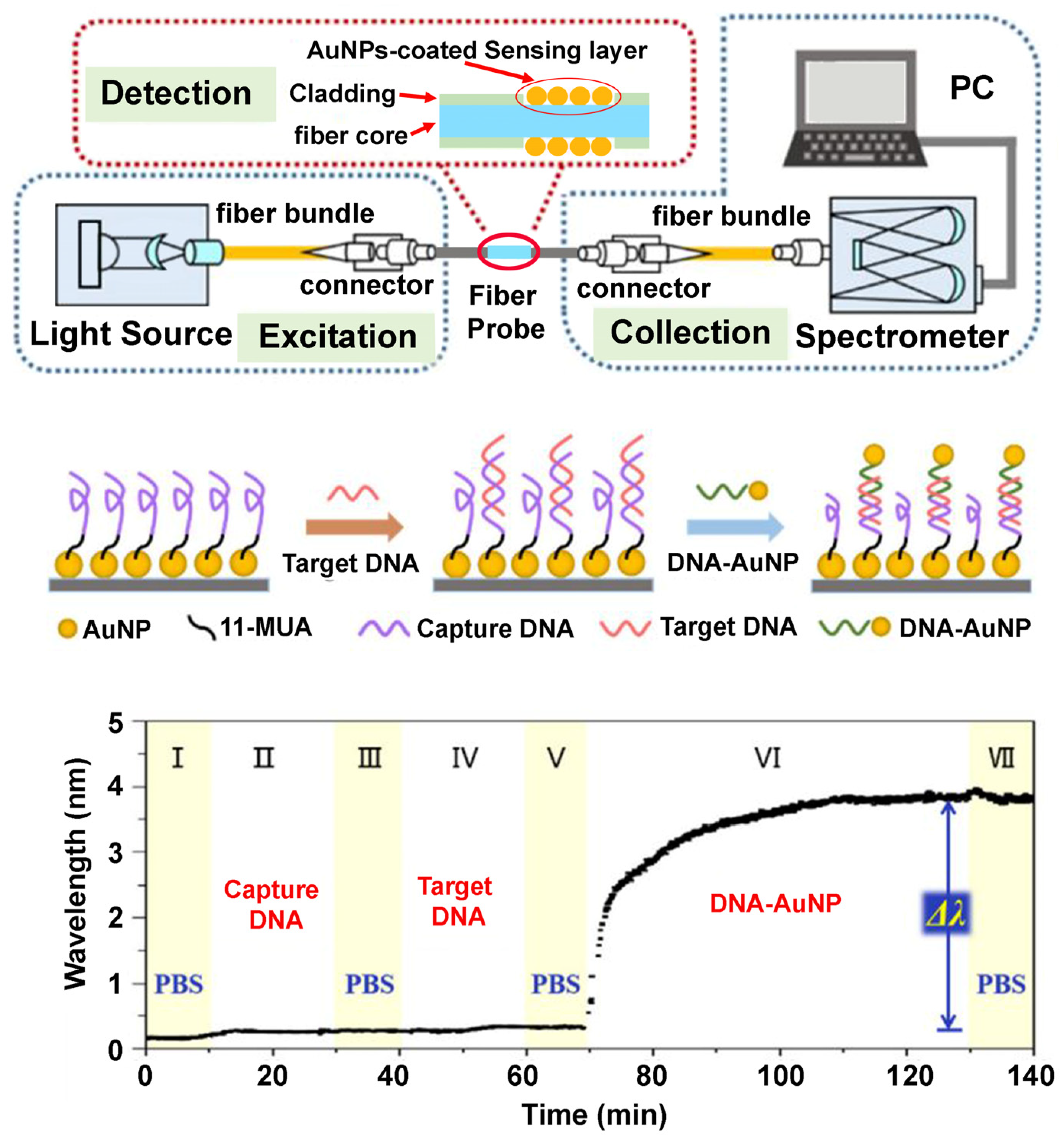
| rop B gene | PS-b-P4VP | 4.4.&4.5. | [114] |
| Glucose oxidase (GOx)/Glucose | PS-b-P4VP | 4.5. | [115] |
| Choline oxidase (ChO)/Choline | Poly(n-butylmethacrylate)-block-poly(N,N-dimethylaminoethyl methacrylate) (PnBMA-b-PDMAEMA) | 4.5. | [116] |
| Dopamine (DA) | PS-b-P4VP | 4.5. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sytu, M.R.C.; Cho, D.H.; Hahm, J.-i. Self-Assembled Block Copolymers as a Facile Pathway to Create Functional Nanobiosensor and Nanobiomaterial Surfaces. Polymers 2024, 16, 1267. https://doi.org/10.3390/polym16091267
Sytu MRC, Cho DH, Hahm J-i. Self-Assembled Block Copolymers as a Facile Pathway to Create Functional Nanobiosensor and Nanobiomaterial Surfaces. Polymers. 2024; 16(9):1267. https://doi.org/10.3390/polym16091267
Chicago/Turabian StyleSytu, Marion Ryan C., David H. Cho, and Jong-in Hahm. 2024. "Self-Assembled Block Copolymers as a Facile Pathway to Create Functional Nanobiosensor and Nanobiomaterial Surfaces" Polymers 16, no. 9: 1267. https://doi.org/10.3390/polym16091267
APA StyleSytu, M. R. C., Cho, D. H., & Hahm, J.-i. (2024). Self-Assembled Block Copolymers as a Facile Pathway to Create Functional Nanobiosensor and Nanobiomaterial Surfaces. Polymers, 16(9), 1267. https://doi.org/10.3390/polym16091267









