Starch-Based Functional Films Enhanced with Bacterial Nanocellulose for Smart Packaging: Physicochemical Properties, pH Sensitivity and Colorimetric Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Extraction and Film Preparation
2.1.1. Extraction of Anthocyanins
2.1.2. Film Preparation
2.2. Methods
2.2.1. Determination of the Total Monomeric Anthocyanin Pigment Content
2.2.2. FTIR–ATR Analysis
2.2.3. Swelling Experiments and Weight Loss Calculation
2.2.4. Surface Properties of Starch-Based Films
2.2.5. Colorimetric and Visual Analysis of Starch-Based Films
3. Results and Discussion
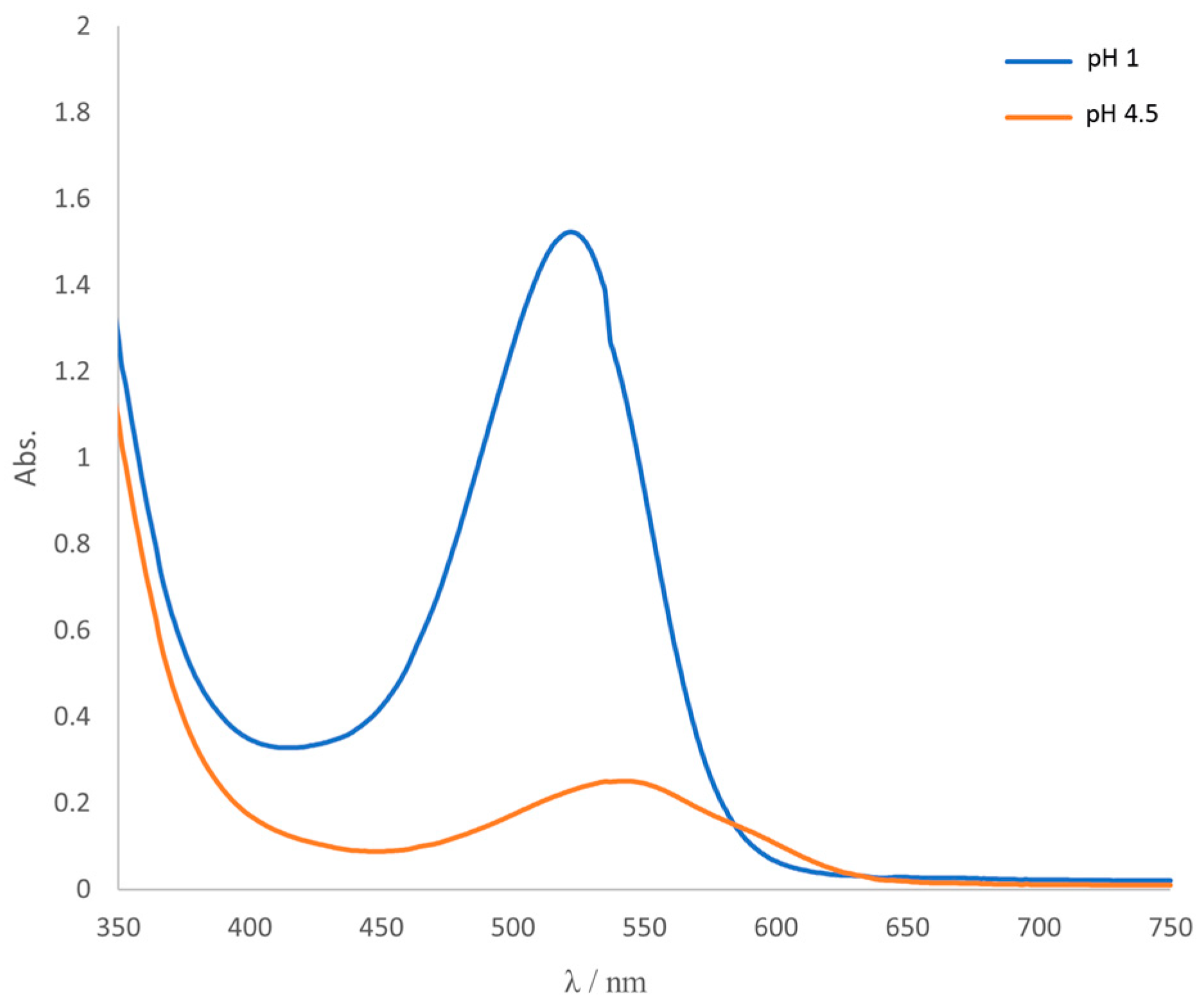
3.1. Determination of the Total Monomeric Anthocyanin Pigment Content
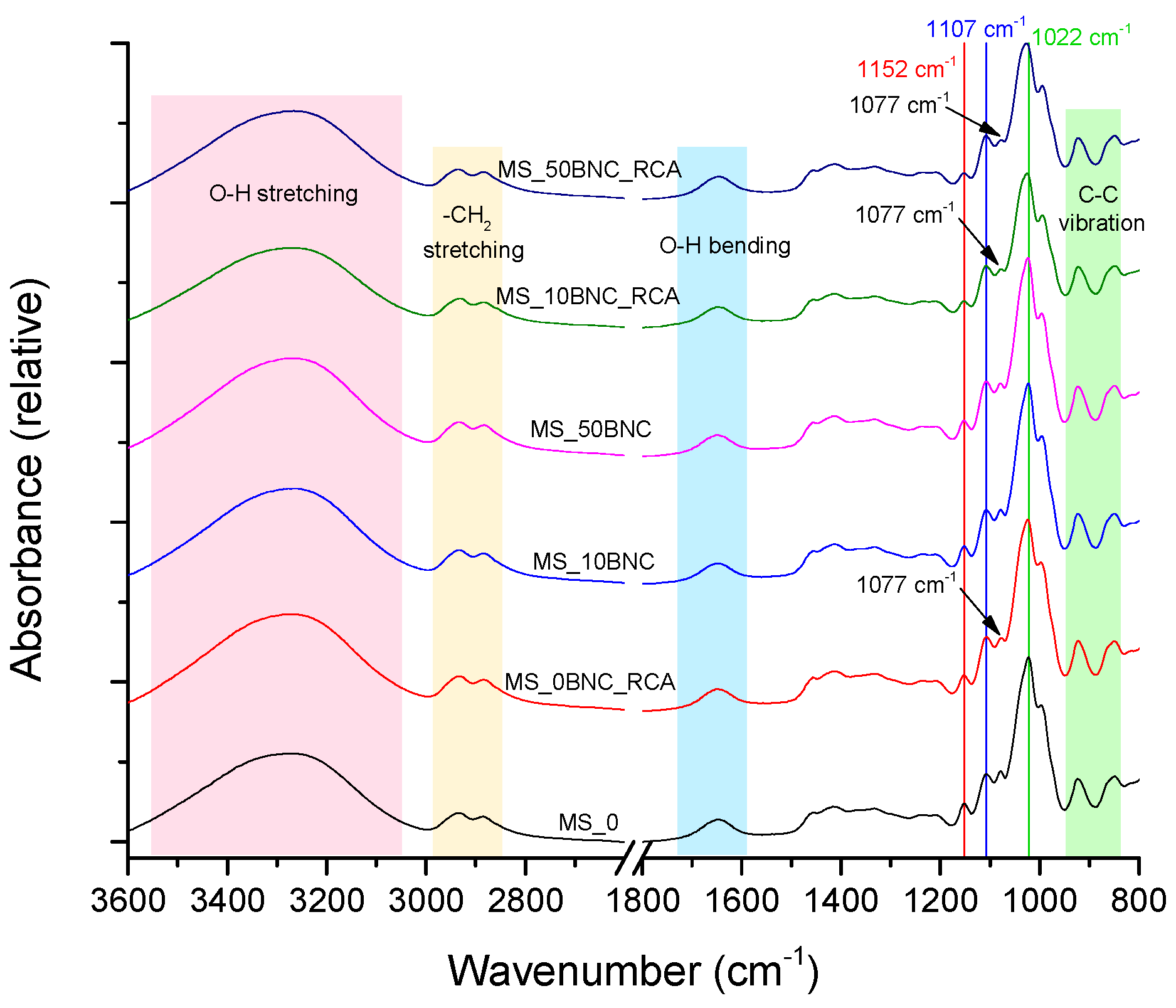
3.2. FTIR–ATR Analysis of Prepared Films
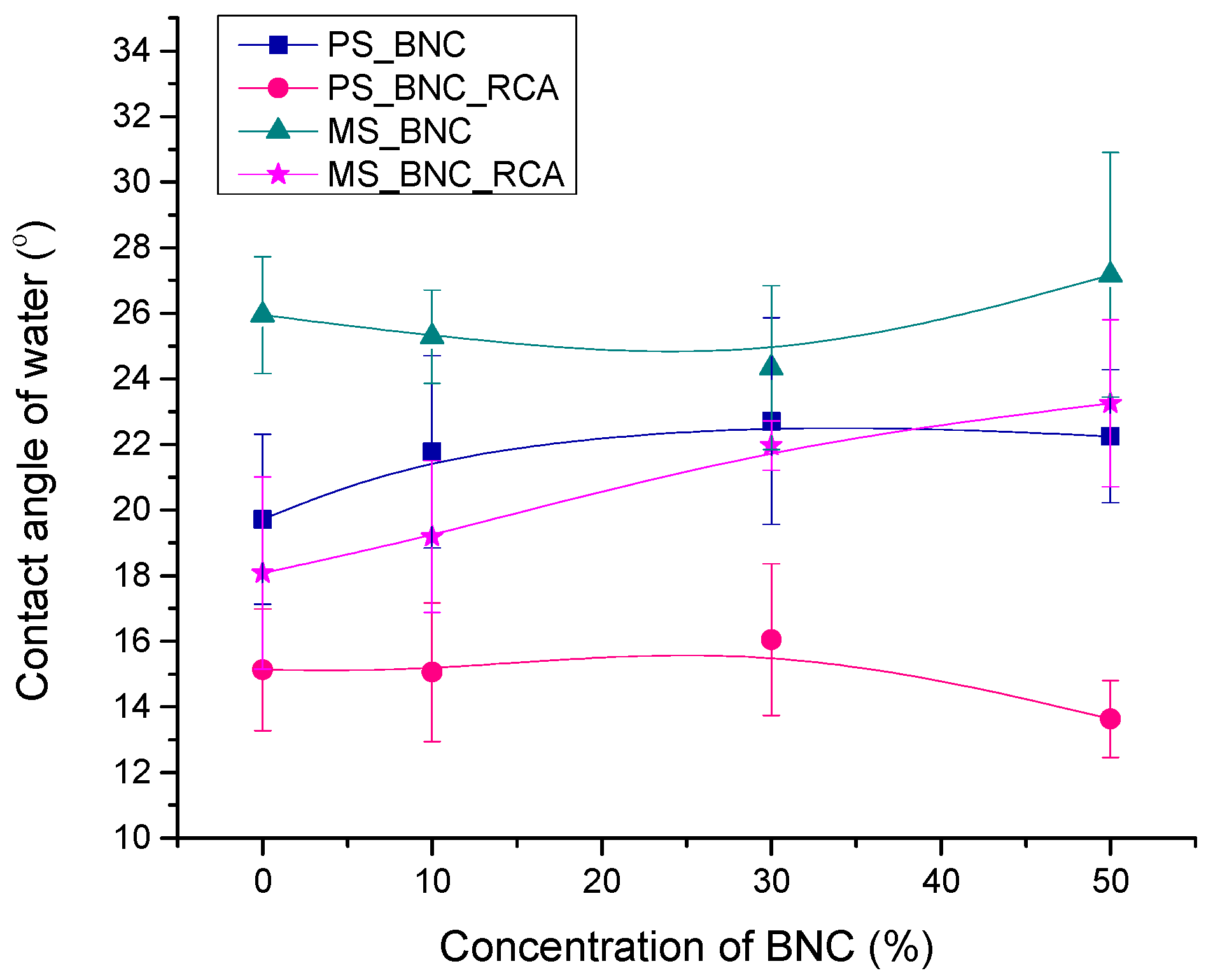
3.3. Water Contact Angle on Films
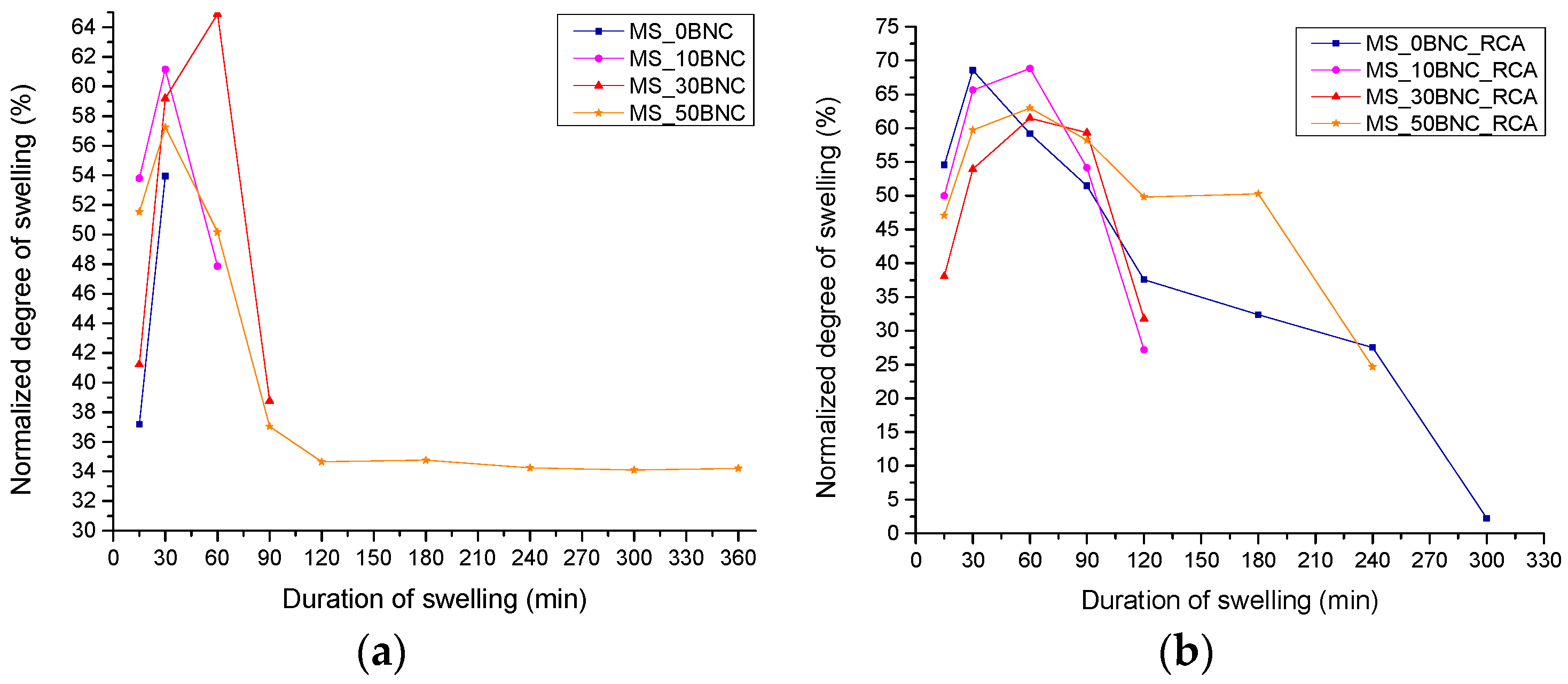
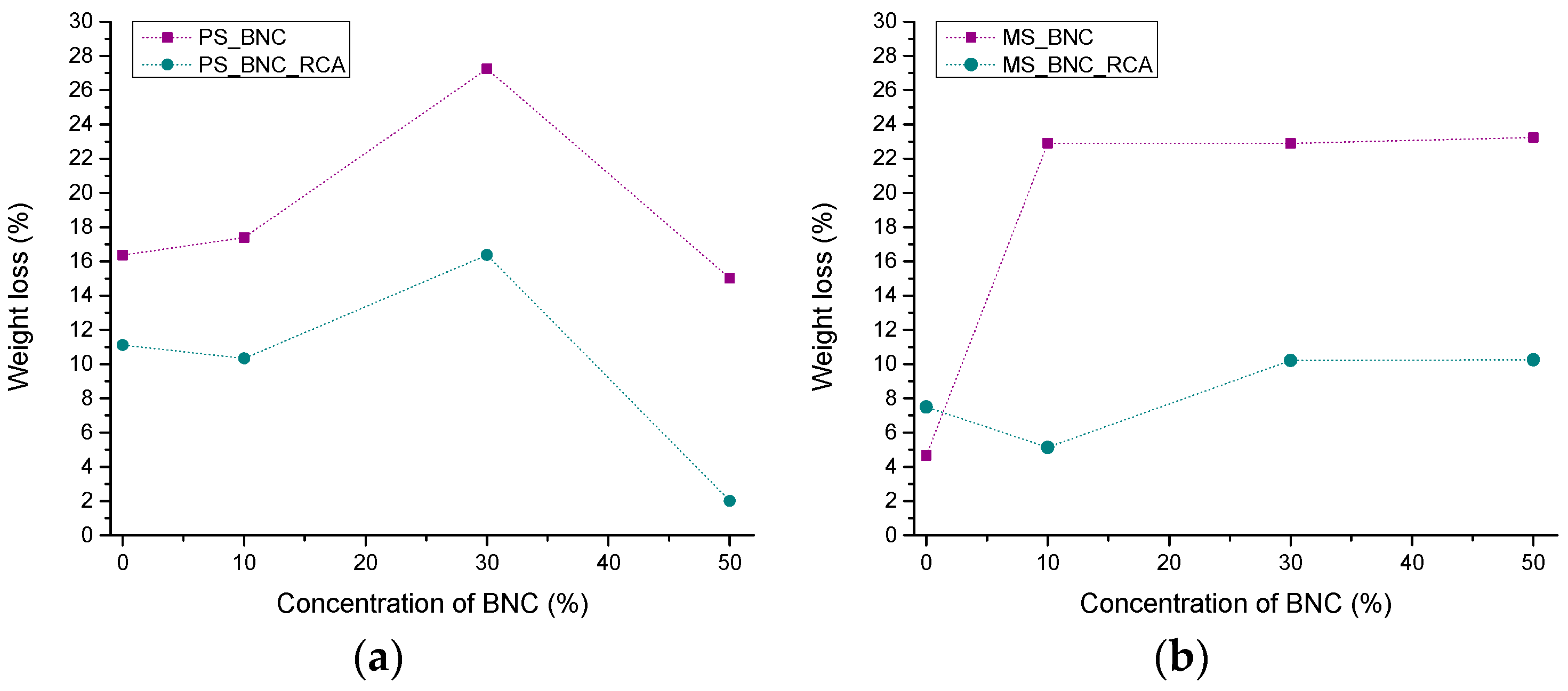
3.4. Swelling Dynamics and Weight Loss of the Films
3.5. Surface Properties of the Films and Adhesion with Polypropylene
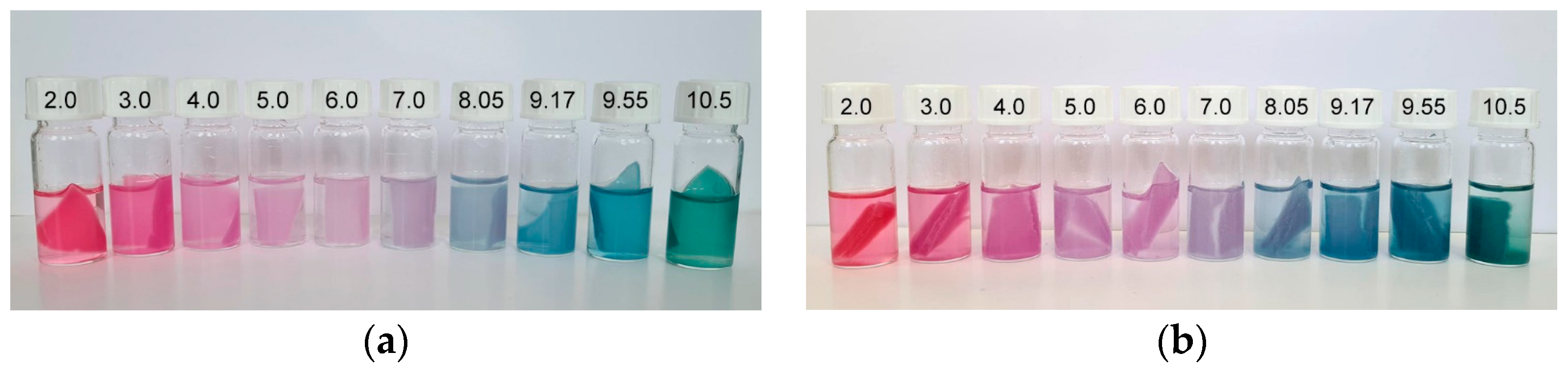
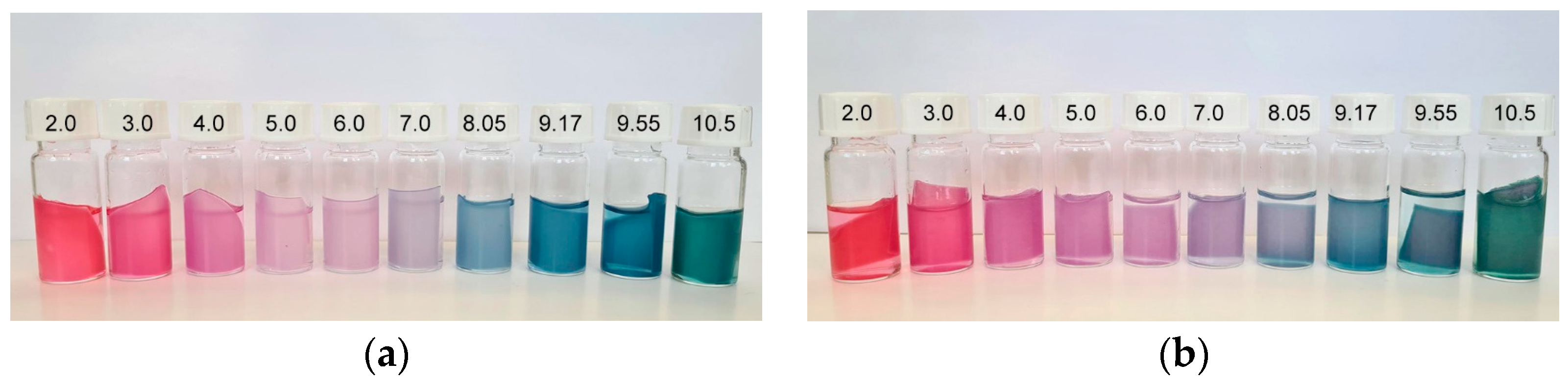
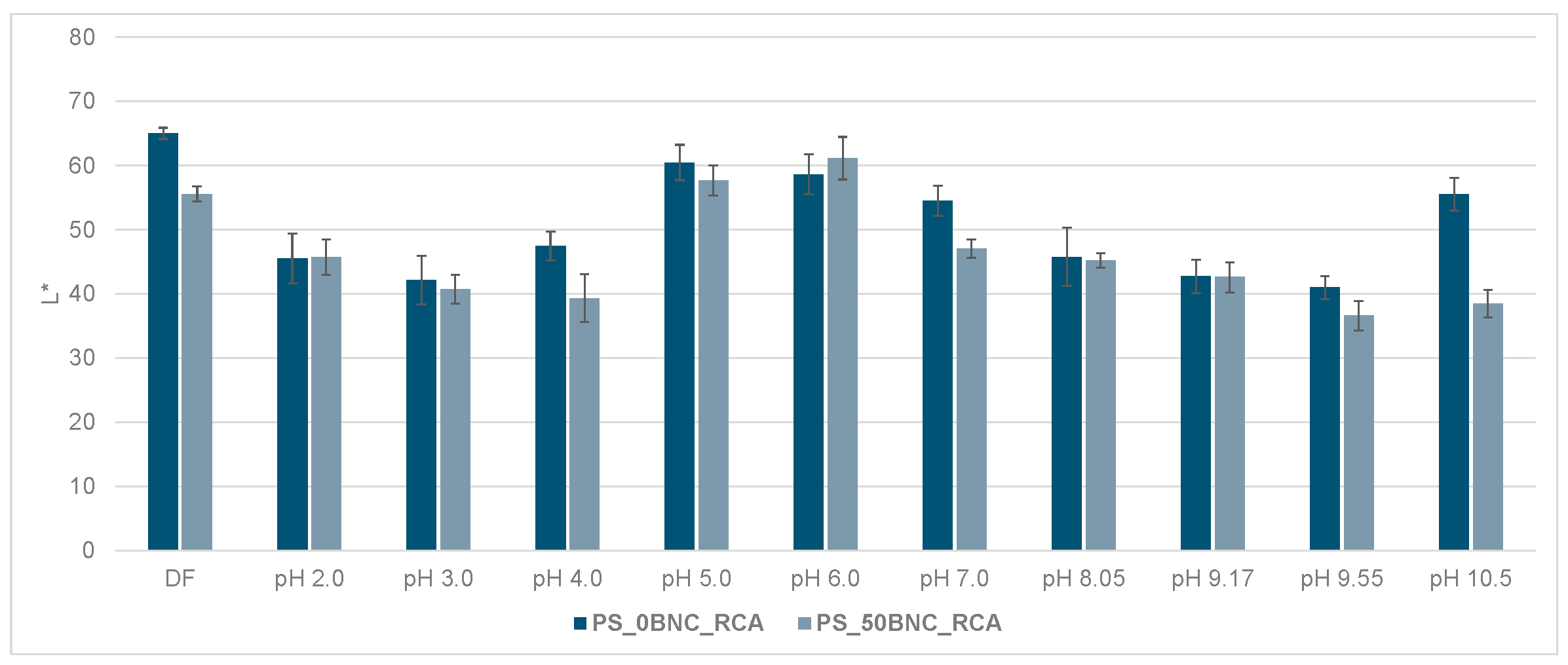
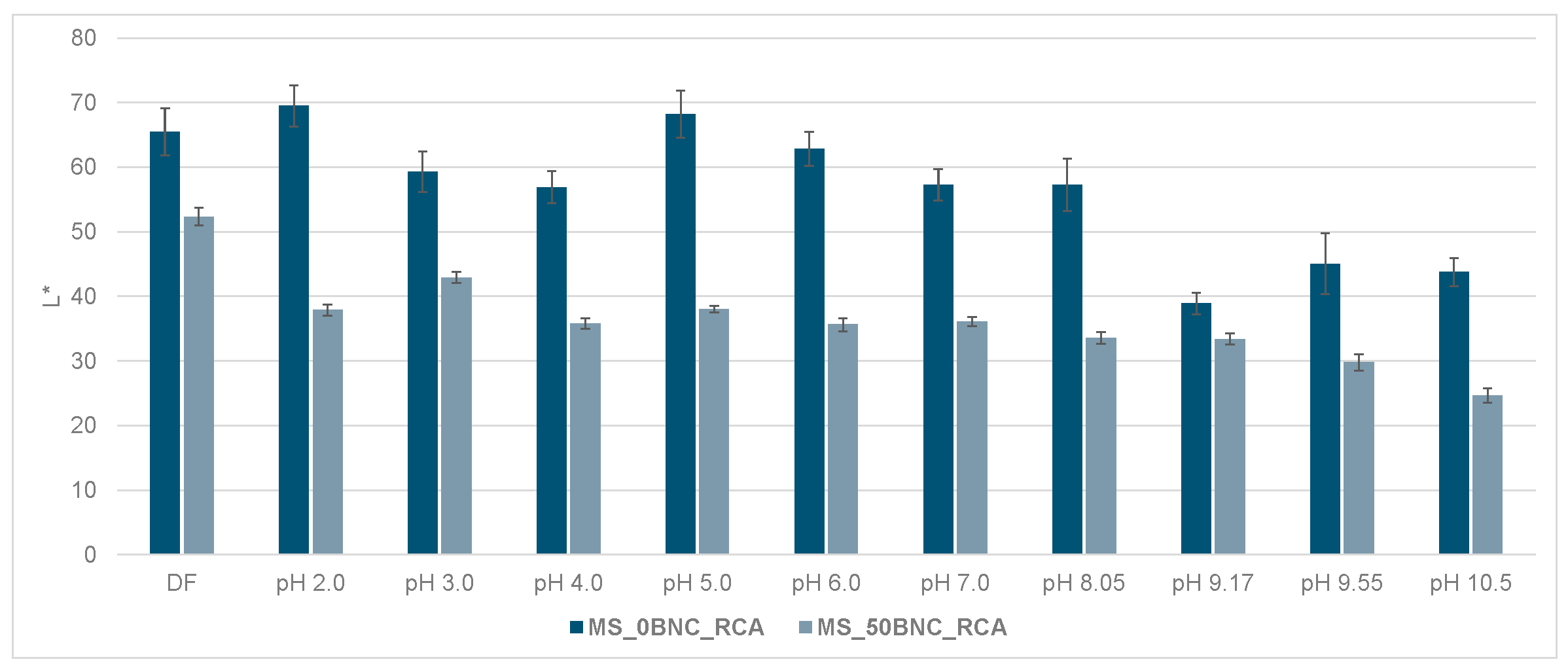
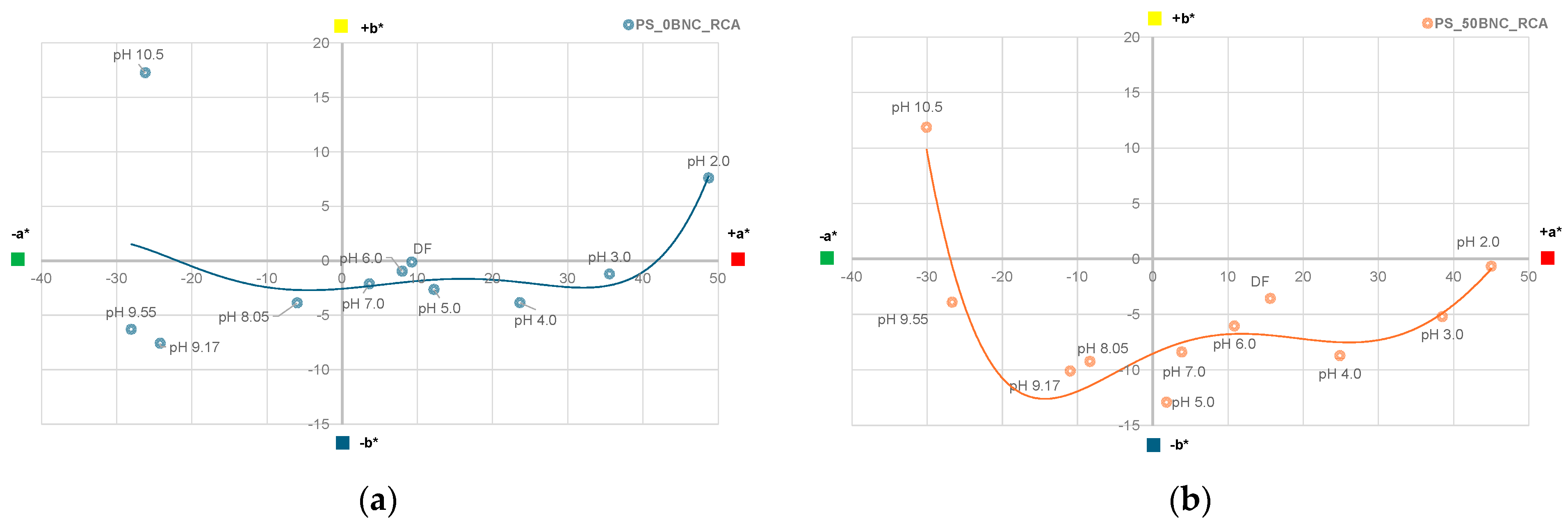
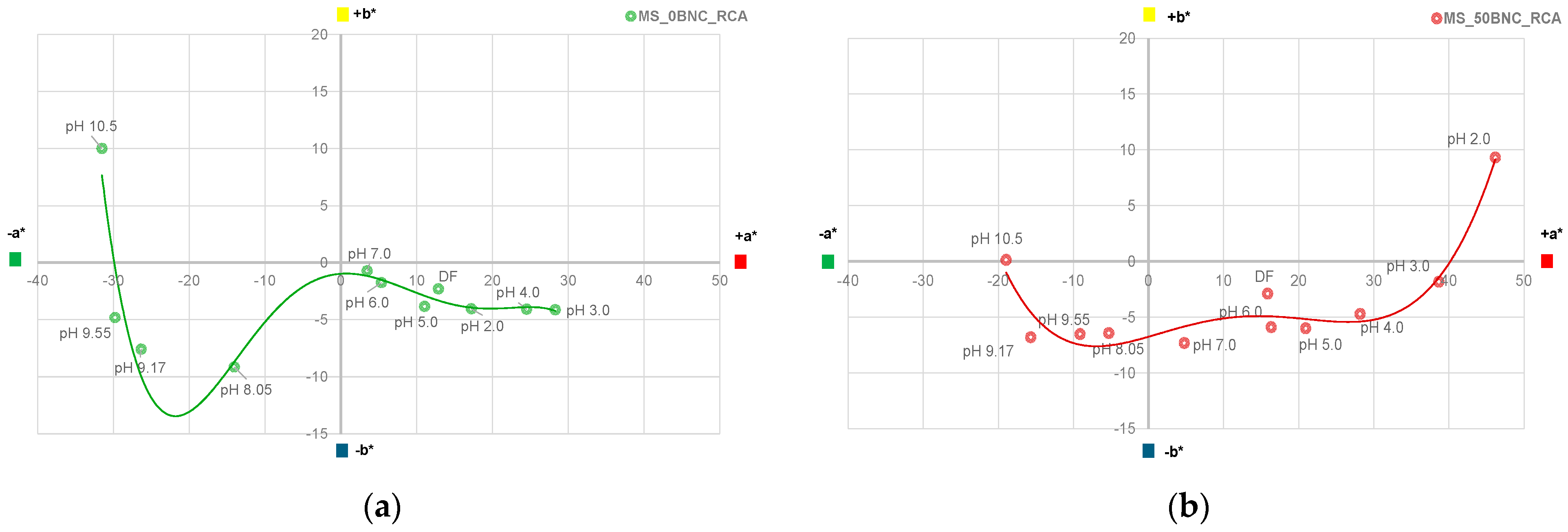
3.6. Colorimetric Properties and Visual Analysis of Starch-Based Films in Varied-pH Environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Cristofoli, N.L.; Lima, A.R.; Tchonkouang, R.D.N.; Quintino, A.C.; Vieira, M.C. Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development. Sustainability 2023, 15, 6153. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA based biocomposites for sustainable products: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T.; Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T. Starch Based Bio-Plastics: The Future of Sustainable Packaging. Open J. Polym. Chem. 2018, 8, 21–33. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Gregor-Svetec, D. Intelligent Packaging. In Nanomaterials for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–247. ISBN 9780323512718. [Google Scholar]
- Thirupathi Vasuki, M.; Kadirvel, V.; Pejavara Narayana, G. Smart packaging—An overview of concepts and applications in various food industries. Food Bioeng. 2023, 2, 25–41. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive Food Packaging: Recent Progress and Technological Prospects. Compr. Rev. Food Sci. Food Saf. 2016, 15, 3–15. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 521914. [Google Scholar] [CrossRef]
- Regulation—1935/2004—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32004R1935 (accessed on 21 July 2024).
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- González, C.M.O.; Schelegueda, L.I.; Ruiz-Henestrosa, V.M.P.; Campos, C.A.; Basanta, M.F.; Gerschenson, L.N. Cassava Starch Films with Anthocyanins and Betalains from Agroindustrial by-Products: Their Use for Intelligent Label Development. Foods 2022, 11, 3361. [Google Scholar] [CrossRef]
- Tahara, Y.; Sekino, T.; Okazaki, J.; Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z.; Kałuża, A. The Influence of Starch Origin on the Properties of Starch Films: Packaging Performance. Materials 2021, 14, 1146. [Google Scholar] [CrossRef]
- Niu, X.; Ma, Q.; Li, S.; Wang, W.; Ma, Y.; Zhao, H.; Sun, J.; Wang, J. Preparation and Characterization of Biodegradable Composited Films Based on Potato Starch/Glycerol/Gelatin. J. Food Qual. 2021, 2021, 6633711. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.L. Starch: Structure, Property, and Determination. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 165–174. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Wang, R.; Liu, P.; Cui, B.; Kang, X.; Yu, B.; Qiu, L.; Sun, C. Effects of pullulanase debranching on the properties of potato starch-lauric acid complex and potato starch-based film. Int. J. Biol. Macromol. 2020, 156, 1330–1336. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of Two pH Responsive Color Changing Bio-Based Films Containing Wasted Fruit Pomace as a Source of Colorants. J. Food Sci. 2019, 84, 2490–2498. [Google Scholar] [CrossRef]
- Hogan, S.A.; Kerry, J.P. Smart Packaging of Meat and Poultry Products. In Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 33–59. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of nanotechnology in food packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Beluhan, S.; Herceg, F.; Pavunc, A.L.; Djaković, S. Preparation and Structural Properties of Bacterial Nanocellulose Obtained from Beetroot Peel Medium. Energies 2022, 15, 9374. [Google Scholar] [CrossRef]
- Perdani, C.G.; Gunawan, S. A short review: Nanocellulose for smart biodegradable packaging in the food industry. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 12032. [Google Scholar] [CrossRef]
- Lavrič, G.; Oberlintner, A.; Filipova, I.; Novak, U.; Likozar, B.; Vrabič-Brodnjak, U. Functional nanocellulose, alginate and chitosan nanocomposites designed as active film packaging materials. Polymers 2021, 13, 2523. [Google Scholar] [CrossRef]
- Lavrič, G.; Medvešček, D.; Skočaj, M. Papermaking properties of bacterial nanocellulose produced from mother of vinegar, a waste product after classical vinegar production. Tappi J. 2020, 19, 197–203. [Google Scholar] [CrossRef]
- Paper, Board, Pulps and Cellulosic Nanomaterials—Determination of Dry Matter Content by Oven-Drying Method—Part 2: Suspension of Cellulosic Nanomaterials 2022. Available online: https://www.iso.org/standard/83615.html (accessed on 21 July 2024).
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Chen, M.; Yan, T.; Huang, J.; Zhou, Y.; Hu, Y. Fabrication of halochromic smart films by immobilizing red cabbage anthocyanins into chitosan/oxidized-chitin nanocrystals composites for real-time hairtail and shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 179, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, R.M.M.; Hamed, D.A.; Gomaa, O.M. Red cabbage extract immobilized in bacterial cellulose film as an eco-friendly sensor to monitor microbial contamination and gamma irradiation of stored cucumbers. World J. Microbiol. Biotechnol. 2024, 40, 258. [Google Scholar] [CrossRef] [PubMed]
- Strauch, R.C.; Mengist, M.F.; Pan, K.; Yousef, G.G.; Iorizzo, M.; Brown, A.F.; Lila, M.A. Variation in anthocyanin profiles of 27 genotypes of red cabbage over two growing seasons. Food Chem. 2019, 301, 125289. [Google Scholar] [CrossRef] [PubMed]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Muhammad, A.; Roslan, A.; Sanusi, S.N.; Shahimi, M.Q.; Nazari, N.Z. Mechanical properties of bioplastic form cellulose nanocrystal (CNC) mangosteen peel using glycerol as plasticizer. J. Phys. Conf. Ser. 2019, 1349, 12099. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Pounds, K.; Jairam, S.; Bao, H.; Meng, S.; Zhang, L.; Godinez, S.; Savin, D.A.; Pelletier, W.; Correll, M.J.; Tong, Z. Glycerol-Based Dendrimer Nanocomposite Film as a Tunable pH-Sensor for Food Packaging. ACS Appl. Mater. Interfaces 2021, 13, 23268–23281. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Improved thermal processing for food texture modification. In Modifying Food Texture; Volume 1: Novel Ingredients and Processing Techniques; Woodhead Publishing: Sawston, UK, 2015; pp. 115–131. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Methods for the Calculation of Surface Free Energy of Solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Israelachvili, J. (Ed.) Intermolecular and Surface Forces, 3rd ed.; Academic Press: Waltham, MA, USA, 2011. [Google Scholar] [CrossRef]
- ICC.1:2004-10. Available online: https://www.color.org/icc1v42.pdf (accessed on 21 July 2024).
- Fairchild, M.D. Color Appearance Models; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Torrenegra, M.E.; Solano, R. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Biodegradable Films Based on Modified Colombian Starches of Cassava and Yam. Artic. Int. J. ChemTech Res. 2018, 11, 184–192. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z.; Bednarczyk, E.; Tryznowski, M.; Kobiela, T. A Comparative Investigation of the Surface Properties of Corn-Starch-Microfibrillated Cellulose Composite Films. Materials 2023, 16, 3320. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Akhila, P.P.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Sudheer, K.P.; Sinha, S.K.; et al. Effect of energetic neutrals on the kithul starch retrogradation; Potential utilization for improving mechanical and barrier properties of films. Food Chem. 2023, 398, 133881. [Google Scholar] [CrossRef] [PubMed]
- Pantić, M.; Nowak, M.; Lavrič, G.; Knez, Ž.; Novak, Z.; Zizovic, I. Enhancing the properties and morphology of starch aerogels with nanocellulose. Food Hydrocoll. 2024, 156, 110345. [Google Scholar] [CrossRef]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Li, Z.; Lv, H.; Li, T.; Si, G.; Wang, Q.; Lv, J.; Hu, X. Reagent-free simultaneous determination of glucose and cholesterol in whole blood by FTIR–ATR. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 192–197. [Google Scholar] [CrossRef]
- Lim, H.J.; Tang, S.Y.; Chan, K.W.; Manickam, S.; Yu, L.J.; Tan, K.W. A starch/gelatin-based Halochromic film with black currant anthocyanin and Nanocellulose-stabilized cinnamon essential oil Pickering emulsion: Towards real-time Salmon freshness assessment. Int. J. Biol. Macromol. 2024, 274, 133329. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Xu, Y.; Jia, C.; Zhang, B.; Niu, M.; Zhao, S. Structural changes of rice starch and activity inhibition of starch digestive enzymes by anthocyanins retarded starch digestibility. Carbohydr. Polym. 2021, 261, 117841. [Google Scholar] [CrossRef] [PubMed]
- García, N.L.; Famá, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
- Nuwamanya, E.; Baguma, Y.; Wembabazi, E.; Rubaihayo, P. A comparative study of the physicochemical properties of starches from root, tuber and cereal crops. Afr. J. Biotechnol. 2011, 10, 12018–12030. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Hasjim, J.; Gidley, M.J. Physicochemical and Structural Properties of Maize and Potato Starches as a Function of Granule Size. J. Agric. Food Chem. 2011, 59, 10151–10161. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Li, C.; Wang, Z.; Xu, F.; Chen, D.; Liu, J. Preparation of cost-effective and hydrophobic freshness indicating labels based on passion fruit peel powder and stearic acid. Food Biosci. 2023, 53, 102758. [Google Scholar] [CrossRef]
- Zhang, H.C.; Yu, C.N.; Li, X.Z.; Wang, L.F.; Huang, J.; Tong, J.; Lin, Y.; Min, Y.; Liang, Y. Recent Developments of Nanocellulose and its Applications in Polymeric Composites. ES Food Agrofor. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films- A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Arias, C.I.; Fuente, L.; Do, L.; Siqueira, V.; Tadini, C.C.; Maniglia, B.C.; La Fuente, C.I.A.; Do, L.; Siqueira, V.; et al. Carbohydrate Nanomaterials Addition to Starch-Based Packaging: A Review about Fundamentals and Application. Starch-Stärke 2021, 73, 2100057. [Google Scholar] [CrossRef]
- Tomašegović, T.; Mahović Poljaček, S.; Strižić Jakovljević, M.; Urbas, R. Effect of the Common Solvents on UV-Modified Photopolymer and EPDM Flexographic Printing Plates and Printed Ink Films. Coatings 2020, 10, 136. [Google Scholar] [CrossRef]
- Arghavani, S.; Mohseni-Shahri, F.S.; Moeinpour, F. Anthocyanin-loaded bacterial cellulose nanofiber as a green sensor for monitoring the selective naked eye and visual detection of Al(III) Ions. Anal. Sci. Adv. 2023, 4, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Dataphysics. Optical Contact Angle Measuring and Contour Analysis: Why Measuring with at Least Three Test Liquids Is Recommended for Surface Energy Analysis, Application Note. Available online: www.dataphysics-instruments.com (accessed on 21 July 2024).
- El-Naggar, N.E.A.; El-Malkey, S.E.; Abu-Saied, M.A.; Mohammed, A.B.A. Exploration of a novel and efficient source for production of bacterial nanocellulose, bioprocess optimization and characterization. Sci. Rep. 2022, 12, 18533. [Google Scholar] [CrossRef] [PubMed]
- Dima, S.O.; Panaitescu, D.M.; Orban, C.; Ghiurea, M.; Doncea, S.M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.A.; Trica, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Nanocellulose Reinforced Starch Polymer Composites: A Review of Preparation, Properties and Application. In Proceedings of the 5th International Conference on Applied Sciences and Engineering (ICASEA, 2018), Brinchang, Malaysia, 7–8 April 2018. [Google Scholar]
- Tomé, L.C.; Fernandes, S.C.M.; Perez, D.S.; Sadocco, P.; Silvestre, A.J.D.; Neto, C.P.; Marrucho, I.M.; Freire, C.S.R. The role of nanocellulose fibers, starch and chitosan on multipolysaccharide based films. Cellulose 2013, 20, 1807–1818. [Google Scholar] [CrossRef]
- Kirwan, M.J.; Plant, S.; Strawbridge, J.W. Plastics in Food Packaging. In Food and Beverage Packaging Technology, 2nd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 157–212. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2023, 6, 117. [Google Scholar] [CrossRef]
- Han, J.H.; Zhang, Y.; Buffo, R. Surface chemistry of food, packaging and biopolymer materials. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 45–59. [Google Scholar] [CrossRef]
- Aydemir, C.; Altay, B.N.; Akyol, M. Surface analysis of polymer films for wettability and ink adhesion. Color Res. Appl. 2021, 46, 489–499. [Google Scholar] [CrossRef]
- Vo, T.V.; Dang, T.H.; Chen, B.H. Synthesis of Intelligent pH Indicative Films from Chitosan/Poly(vinyl alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Munmai, A.; Somsook, E. The Determination of the pKa of Red Cabbage Anthocyanin by the Spectrophotometric Method and Nonlinear Curve Fitting. Chem. Educ. 2011, 16, 1–3. [Google Scholar]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Vonnie, J.M.; Rovina, K.; Yin, K.W.; Nur’Aqilah, M.N. Synthesis and Physicochemical Characterization of Polymer Film-Based Anthocyanin and Starch. Biosensors 2022, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased ternary films of thermoplastic starch, bacterial nanocellulose and gallic acid for active food packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Sousa, D.; Basílio, N.; Oliveira, J.; de Freitas, V.; Pina, F. A New Insight into the Degradation of Anthocyanins: Reversible versus the Irreversible Chemical Processes. J. Agric. Food Chem. 2022, 70, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.V.; Morais, A.I.S.; Trigueiro, P.; de Souza, J.S.N.; Damacena, D.H.L.; Brandão-Lima, L.C.; Bezerra, R.D.S.; Fonseca, M.G.; Silva-Filho, E.C.; Osajima, J.A. Organic–Inorganic Hybrid Pigments Based on Bentonite: Strategies to Stabilize the Quinoidal Base Form of Anthocyanin. Int. J. Mol. Sci. 2023, 24, 2417. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Yokota, M.; Yamada, H.; Tatsuzawa, F.; Doi, M. Identification of Chalcones and their Contribution to Yellow Coloration in Dahlia (Dahlia variabilis) Ray Florets. Hortic. J. 2021, 90, 450–459. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Wang, Q.; Lin, G.; Niu, B.; Guo, R.; Yan, H.; Wang, H. Sodium alginate/chitosan-based intelligent bilayer film with antimicrobial activity for pork preservation and freshness monitoring. Food Control 2023, 148, 109615. [Google Scholar] [CrossRef]



| Sample | BNC Conc. (%) | Addition of RCA |
|---|---|---|
| PS_0BNC/MS_0BNC | 0 | / |
| PS_10BNC/MS_10BNC | 10 | / |
| PS_30BNC/MS_30BNC | 30 | / |
| PS_50BNC/MS_50BNC | 50 | / |
| PS_0BNC_RCA/MS_0BNC_RCA | 0 | RCA |
| PS_10BNC_RCA/MS_10BNC_RCA | 10 | RCA |
| PS_30BNC_RCA/MS_30BNC_RCA | 30 | RCA |
| PS_50BNC_RCA/MS_50BNC_RCA | 50 | RCA |
| Absorbance | pH 1.0 | pH 4.5 |
|---|---|---|
| A520 | 1.521 | 0.225 |
| A700 | 0.023 | 0.011 |
| Film Sample | γ12 (mJ/m2) | (mJ/m2) | (mJ/m2) |
|---|---|---|---|
| PS_0BNC | 9.71 | 117.52 | −18.48 |
| PS_10BNC | 10.12 | 113.47 | −15.25 |
| PS_30BNC | 10.02 | 112.03 | −13.61 |
| PS_50BNC | 9.37 | 116.71 | −16.99 |
| PS_0BNC_RCA | 10.29 | 117.08 | −19.19 |
| PS_10BNC_RCA | 10.16 | 117.47 | −19.33 |
| PS_30BNC_RCA | 11.05 | 115.33 | −18.97 |
| PS_50BNC_RCA | 10.02 | 118.25 | −19.83 |
| MS_0BNC | 9.33 | 115.65 | −15.85 |
| MS_10BNC | 9.03 | 115.53 | −15.13 |
| MS_30BNC | 9.15 | 114.89 | −14.73 |
| MS_50BNC | 9.08 | 115.14 | −14.84 |
| MS_0BNC_RCA | 9.89 | 116.70 | −18.04 |
| MS_10BNC_RCA | 10.13 | 117.02 | −18.82 |
| MS_30BNC_RCA | 9.86 | 116.19 | −17.451 |
| MS_50BNC_RCA | 9.66 | 115.91 | −16.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahović Poljaček, S.; Tomašegović, T.; Strižić Jakovljević, M.; Jamnicki Hanzer, S.; Murković Steinberg, I.; Žuvić, I.; Leskovac, M.; Lavrič, G.; Kavčič, U.; Karlovits, I. Starch-Based Functional Films Enhanced with Bacterial Nanocellulose for Smart Packaging: Physicochemical Properties, pH Sensitivity and Colorimetric Response. Polymers 2024, 16, 2259. https://doi.org/10.3390/polym16162259
Mahović Poljaček S, Tomašegović T, Strižić Jakovljević M, Jamnicki Hanzer S, Murković Steinberg I, Žuvić I, Leskovac M, Lavrič G, Kavčič U, Karlovits I. Starch-Based Functional Films Enhanced with Bacterial Nanocellulose for Smart Packaging: Physicochemical Properties, pH Sensitivity and Colorimetric Response. Polymers. 2024; 16(16):2259. https://doi.org/10.3390/polym16162259
Chicago/Turabian StyleMahović Poljaček, Sanja, Tamara Tomašegović, Maja Strižić Jakovljević, Sonja Jamnicki Hanzer, Ivana Murković Steinberg, Iva Žuvić, Mirela Leskovac, Gregor Lavrič, Urška Kavčič, and Igor Karlovits. 2024. "Starch-Based Functional Films Enhanced with Bacterial Nanocellulose for Smart Packaging: Physicochemical Properties, pH Sensitivity and Colorimetric Response" Polymers 16, no. 16: 2259. https://doi.org/10.3390/polym16162259
APA StyleMahović Poljaček, S., Tomašegović, T., Strižić Jakovljević, M., Jamnicki Hanzer, S., Murković Steinberg, I., Žuvić, I., Leskovac, M., Lavrič, G., Kavčič, U., & Karlovits, I. (2024). Starch-Based Functional Films Enhanced with Bacterial Nanocellulose for Smart Packaging: Physicochemical Properties, pH Sensitivity and Colorimetric Response. Polymers, 16(16), 2259. https://doi.org/10.3390/polym16162259











