Biosynthesis of Polyhydroxyalkanoates in Cupriavidus necator B-10646 on Saturated Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. The PHA Producer Strain, Media and Cultivation Technique
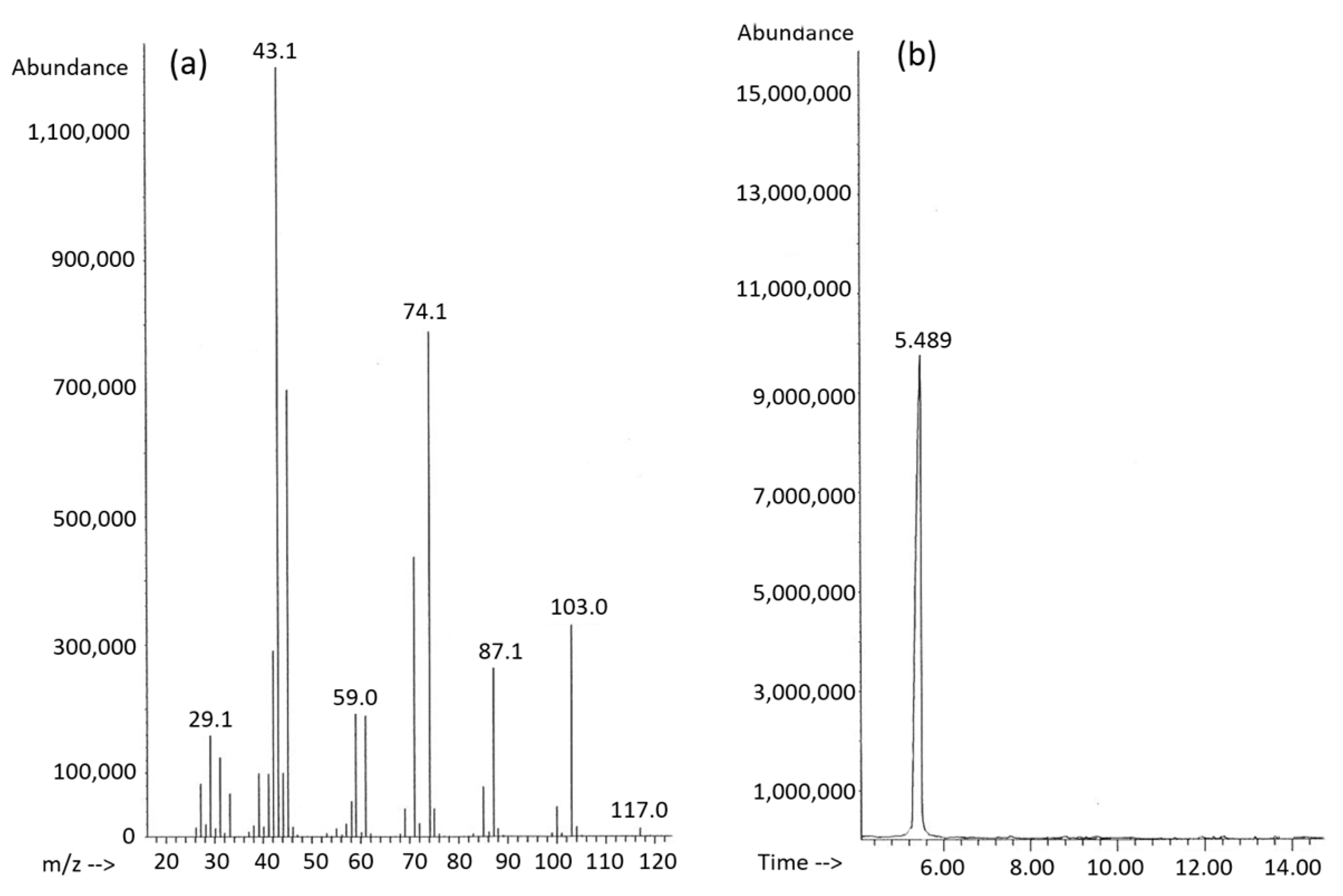
2.2. PHA Analysis
2.3. PHA Properties
2.4. Statistical Analysis
3. Results and Discussion
3.1. Growth and PHA Synthesis by Bacteria C. necator B-10646 on Individual Saturated Fatty Acids
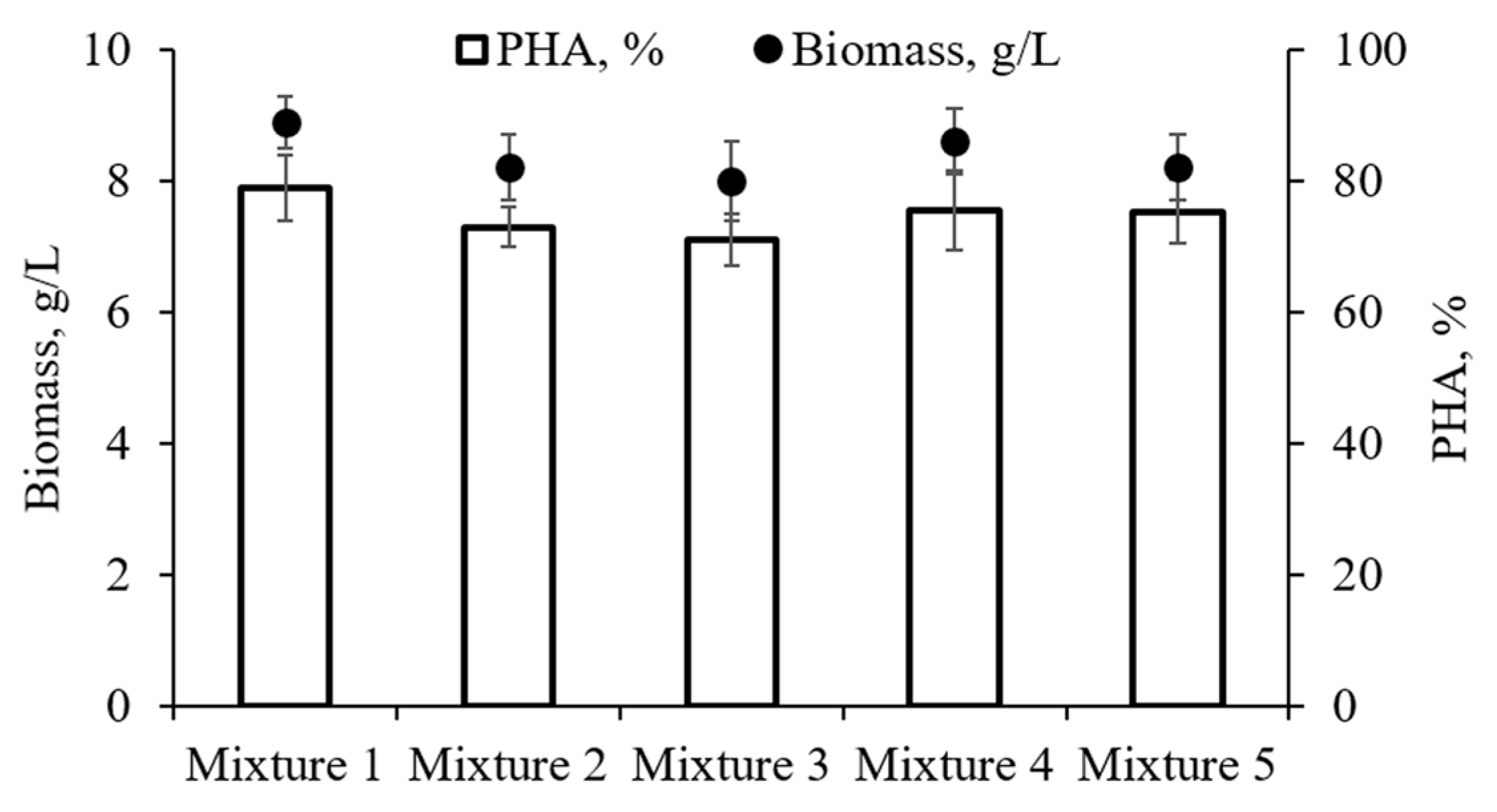
3.2. Growth and PHA Synthesis by Bacteria C. necator B-10646 on Mixtures of Fatty Acids
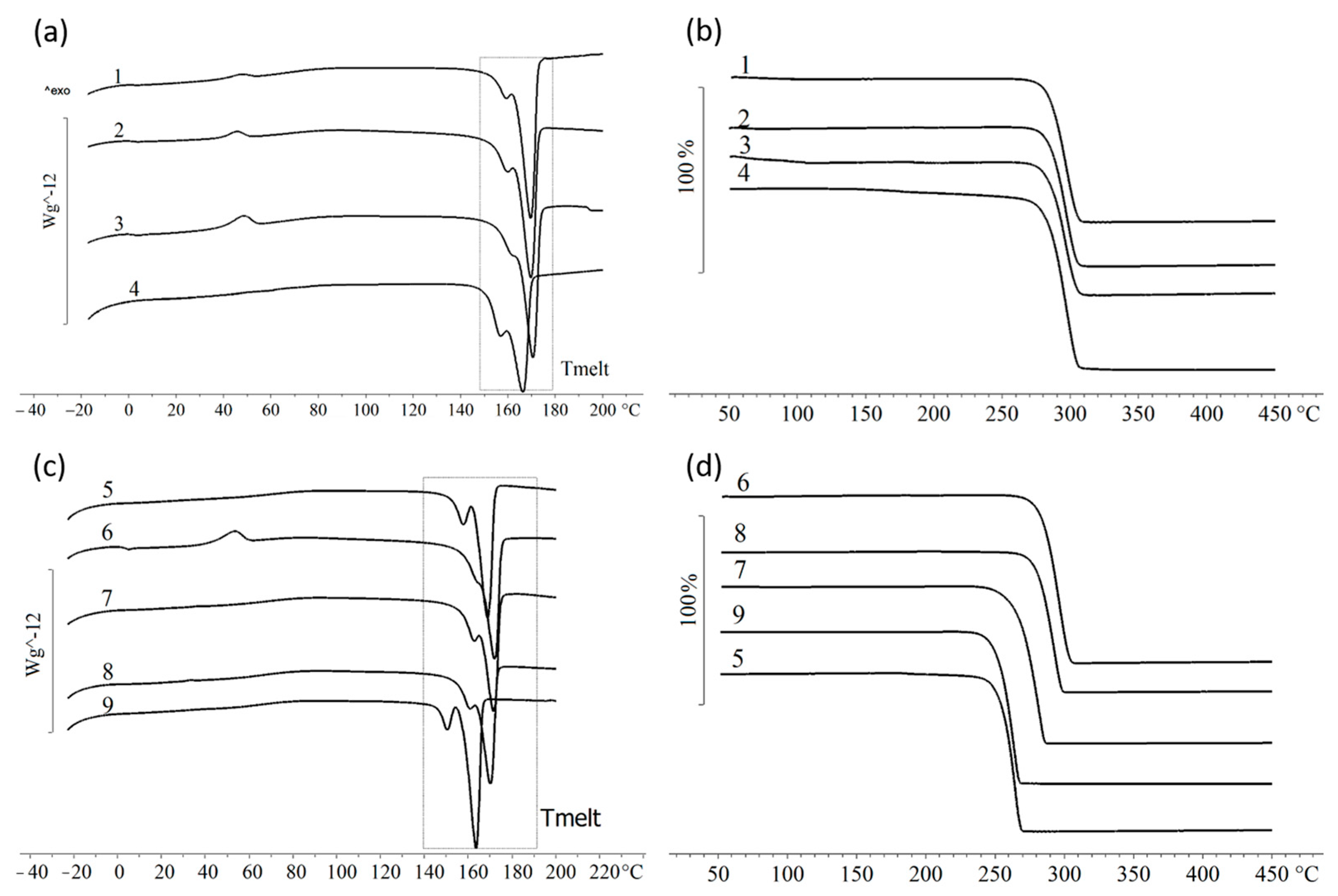
3.3. The Composition and Physicochemical Properties of PHAs Synthesized by C. necator B-10646 on Individual Fatty Acids and Their Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling: Environmental Impact, Societal Issues, Prevention, and Solutions; Letcher, T.M., Ed.; Academic Press: London, UK, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 18 March 2024).
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching new heights in plastic pollution—Preliminary findings of microplastics on mount everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Onda, D.F.L. Lebensspuren and müllspuren: Drifting plastic bags alter microtopography of seafloor at full ocean depth (10,000 m, Philippine Trench). Cont. Shelf Res. 2022, 250, 104867. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Aguilera, Á.; Viúdez-Moreiras, D.; Pulido-Reyes, G.; González-Toril, E.; Osuna, S.; de Diego-Castilla, G.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci. Total Environ. 2021, 761, 143213. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jia, S.; Ramakrishna, S. Accelerating plastic circularity: A critical assessment of the pathways and processes to circular plastics. Processes 2023, 11, 1457. [Google Scholar] [CrossRef]
- Chen, G.Q.; Chen, X.Y.; Wu, F.Q.; Chen, J.C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Chen, G.Q.; Xiang, H.; Han, J. An updated overview on the regulatory circuits of polyhydroxyalkanoates synthesis. Microb. Biotechnol. 2022, 15, 1446–1470. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—Linking properties, applications, and end-of-life options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html (accessed on 18 March 2024).
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; O’Flaherty, V.; Lens, P.N.L. Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Liang, X.; Wei, M.; Liang, F.; Feng, D.; Xu, C.; Xian, M.; Zou, H. Production and waste treatment of polyesters: Application of bioresources and biotechniques. Crit. Rev. Biotechnol. 2023, 43, 503–520. [Google Scholar] [CrossRef]
- Naitam, M.G.; Tomar, G.S.; Kaushik, R.; Singh, S.; Nain, L. Agro-industrial waste as potential renewable feedstock for biopolymer polyhydroxyalkanoates (PHA) production. Enzym. Eng. 2022, 11, 190–206. Available online: https://www.longdom.org/open-access/agroindustrial-waste-as-potential-renewable-feedstock-for-biopolymer-polyhydroxyalkanoates-pha-production-93784.html#ai (accessed on 2 May 2024).
- Kannah, R.Y.; Kumar, M.D.; Kavitha, S.; Banu, J.R.; Tyagi, V.K.; Rajaguru, P.; Kumar, G. Production and recovery of polyhydroxyalkanoates (PHA) from waste streams—A review. Bioresour. Technol. 2022, 366, 128203. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R.P.; Kumar, S.; Singh, P. Production of polyhydroxyalkanoates from renewable resources: A review on prospects, challenges and applications. Arch. Microbiol. 2023, 205, 172. [Google Scholar] [CrossRef]
- Che, L.; Jin, W.; Zhou, X.; Han, W.; Chen, Y.; Chen, C.; Jiang, G. Current status and future perspectives on the biological production of polyhydroxyalkanoates. Asia-Pac. J. Chem. Eng. 2023, 18, e2899. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Machado, C.M.B.; Brehmer, M.S.; de Oliveira, P.Z.; Binod, P.; Sindhu, R.; Soccol, C.R. Polyhydroxyalkanoates production in biorefineries: A review on current status, challenges and opportunities. Bioresour. Technol. 2023, 393, 130078. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Parlato, M.C.; Valenti, F.; Porto, S.M. Covering plastic films in greenhouses system: A GIS-based model to improve post use suistainable management. J. Environ. Manag. 2020, 263, 110389. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koller, M. Polyhydroxyalkanoate (PHA) bio-polyesters–circular materials for sustainable development and growth. Chem. Biochem. Eng. Q. 2022, 36, 273–293. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.; Yu, H.E.; Cho, I.J.; Kang, M.; Lee, S.Y. Sustainable production and degradation of plastics using microbes. Nat. Microbiol. 2023, 8, 2253–2276. [Google Scholar] [CrossRef] [PubMed]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Adsorptive removal of saturated and unsaturated fatty acids using ion-exchange resins. Ind. Eng. Chem. Res. 2012, 51, 6869–6876. [Google Scholar] [CrossRef]
- AMEC. Management of Wastes from Atlantic Seafood Processing Operations; AMEC Earth and Environment Limited: Dartmouth, NS, Canada, 2003. [Google Scholar]
- Bong, C.P.C.; Alam, M.N.H.Z.; Samsudin, S.A.; Jamaluddin, J.; Adrus, N.; Yusof, A.H.M.; Muis, Z.A.; Hashim, H.; Salleh, M.M.; Abdullah, A.R.; et al. A review on the potential of polyhydroxyalkanoates production from oil-based substrates. J. Environ. Manag. 2021, 298, 113461. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J. Biotechnol. 2015, 214, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Saad, V.; Gutschmann, B.; Grimm, T.; Widmer, T.; Neubauer, P.; Riedel, S.L. Low-quality animal by-product streams for the production of PHA-biopolymers: Fats, fat/protein-emulsions and materials with high ash content as low-cost feedstocks. Biotechnol. Lett. 2021, 43, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Gutschmann, B.; Maldonado Simões, M.; Schiewe, T.; Schröter, E.S.; Münzberg, M.; Neubauer, P.; Bockisch, A.; Riedel, S.L. Continuous feeding strategy for polyhydroxyalkanoate production from solid waste animal fat at laboratory-and pilot-scale. Microb. Biotechnol. 2023, 16, 295–306. [Google Scholar] [CrossRef]
- Thuoc, D.V.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Thuoc, D.V.; Anh, V.T. Bioconversion of crude fish Oil into poly-3-hydroxybutyrate by Ralstonia sp. M91. Appl. Biochem. Microbiol. 2021, 57, 219–225. [Google Scholar] [CrossRef]
- Loan, T.T.; Trang, D.T.Q.; Huy, P.Q.; Ninh, P.X.; Thuoc, D.V. A fermentation process for the production of poly (3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnol. Rep. 2022, 33, e00700. [Google Scholar] [CrossRef]
- Zhila, N.O.; Kiselev, E.G.; Volkov, V.V.; Mezenova, O.Y.; Sapozhnikova, K.Y.; Shishatskaya, E.I.; Volova, T.G. Properties of Degradable Polyhydroxyalkanoates Synthesized from New Waste Fish Oils (WFOs). Int. J. Mol. Sci. 2023, 24, 14919. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Tsuge, T.; Doi, Y. Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym. Degrad. Stab. 2003, 80, 183–194. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, B.S. Production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha from soybean oil. New Biotechnol. 2011, 28, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, S.; Singh, D. Microbial polyhydroxyalkanoates from extreme niches: Bioprospection status, opportunities and challenges. Int. J. Biol. Macromol. 2020, 147, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Sapozhnikova, K.; Zhila, N. Cupriavidus necator B-10646 growth and polyhydroxyalkanoates production on different plant oils. Int. J. Biol. Macromol. 2020, 164, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kahar, P.; Tsuge, T.; Taguchi, K.; Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stab. 2004, 83, 79–86. [Google Scholar] [CrossRef]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Synthesis and properties of polyhydroxyalkanoates on waste fish oil from the production of canned sprats. Processes 2023, 11, 2113. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K. Poly(hydroxyalkanoate) biosynthesis from crude Alaskan pollock (Theragra chalcogramma) oil. J. Polym. Environ. 2008, 16, 221–229. [Google Scholar] [CrossRef]
- Akiyama, M.; Taima, Y.; Doi, Y. Production of poly (3-hydroxyalkanoates) by a bacterium of the genus Alcaligenes utilizing long-chain fatty acids. Appl. Microbiol. Biotechnol. 1992, 37, 698–701. [Google Scholar] [CrossRef]
- Chen, B.Y.; Shiau, T.J.; Wei, Y.H.; Chen, W.M. Feasibility study on polyhydroxybutyrate production of dye-decolorizing bacteria using dye and amine-bearing cultures. J. Taiwan Inst. Chem. Eng. 2012, 43, 241–245. [Google Scholar] [CrossRef]
- Chen, B.Y.; Hung, J.Y.; Shiau, T.J.; Wei, Y.H. Exploring two-stage fermentation strategy of polyhydroxyalkanoate production using Aeromonas hydrophila. Biochem. Eng. J. 2013, 78, 80–84. [Google Scholar] [CrossRef]
- Valappil, S.P.; Peiris, D.; Langley, G.J.; Herniman, J.M.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoate (PHA) biosynthesis from structurally unrelated carbon sources by a newly characterized Bacillus spp. J. Biotechnol. 2007, 127, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Povolo, S.; Basaglia, M.; Fontana, F.; Morelli, A.; Casella, S. Poly(hydroxyalkanoate) production by Cupriavidus necator from fatty waste can be enhanced by phaZ1 inactivation. Chem. Biochem. Eng. Q. 2015, 29, 67–74. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T. Effects of carbon substrates on biodegradable polymer composition and stability produced by Delftia tsuruhatensis Bet002 isolated from palm oil mill effluent. Polym. Degrad. Stab. 2012, 97, 1224–1231. [Google Scholar] [CrossRef]
- Chee, J.Y.; Tan, Y.; Samian, M.R.; Sudesh, K. Isolation and characterization of a Burkholderia sp. USM (JCM15050) capable of producing polyhydroxyalkanoate (PHA) from triglycerides, fatty acids and glycerols. J. Polym. Environ. 2010, 18, 584–592. [Google Scholar] [CrossRef]
- Khan, N.; Jamil, N. Biosynthesis of poly-3-hydroxybutyrate by Rhodococcus pyridinivorans using unrelated carbon sources. Adv. Life Sci. 2021, 8, 128–132. [Google Scholar]
- Chen, G.; Zhang, G.; Park, S.; Lee, S. Industrial scale production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 2001, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.G.; Povolo, S.; Favaro, L.; Fontana, F.; Basaglia, M.; Casella, S. Engineering Delftia acidovorans DSM39 to produce polyhydroxyalkanoates from slaughterhouse waste. Int. J. Biol. Macromol. 2014, 71, 21–27. [Google Scholar] [CrossRef]
- Morlino, M.S.; García, R.S.; Savio, F.; Zampieri, G.; Morosinotto, T.; Treu, L.; Campanaro, S. Cupriavidus necator as a platform for PHA production: An overview of strains, metabolism, and modeling approaches. Biotechnol. Adv. 2023, 69, 108264. [Google Scholar] [CrossRef]
- Grigull, V.H.; Domingos da Silva, D.; Formolo Garcia, M.C.; Furlan, S.A.; Testa Pezzin, A.P.; Lima dos Santos Schneider, A.; Falcão Aragão, G. Production and characterization of poly (3-hydroxybutyrate) from oleic acid by Ralstonia eutropha. Food Technol. Biotechnol. 2008, 46, 223–228. [Google Scholar]
- Schneider, A.L.S.; Silva, D.D.; Garcia, M.C.F.; Grigull, V.H.; Mazur, L.P.; Furlan, S.A.; Pezzin, A.P.T. Biodegradation of poly (3-hydroxybutyrate) produced from Cupriavidus necator with different concentrations of oleic acid as nutritional supplement. J. Polym. Environ. 2010, 18, 401–406. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Kiselev, E.; Vinogradova, O.; Nikolaeva, E.; Chistyakov, A.; Sukovatiy, A.; Shishatskaya, E. A glucose-utilizing strain, Cupriavidus euthrophus B-10646: Growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS ONE 2014, 9, e87551. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Kiselev, E.; Zhila, N.; Shishatskaya, E. Synthesis of PHAs by Hydrogen Bacteria in a Pilot Production Process. Biomacromolecules 2019, 20, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.O.; Kalacheva, G.S.; Kiselev, E.G.; Volova, T.G. Synthesis of polyhydroxyalkanoates from oleic acid by Cupriavidus necator B-10646. J. Sib. Fed. Univ. Biol. 2020, 13, 208–217. [Google Scholar] [CrossRef]
- Zhila, N.O.; Kalacheva, G.S.; Fokht, V.V.; Bubnova, S.S.; Volova, T.G. Biosynthesis of Poly (3-Hydroxybutyrate-co-3-Hydroxyvalerate) by Cupriavidus necator B-10646 from Mixtures of Oleic Acid and 3-Hydroxyvalerate Precursors. J. Sib. Fed. Univ. Biol. 2020, 13, 331–341. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I. Cupriavidus eutrophus Bacterial Strain VKPM B-10646-A Producer of Polyhydroxyalkanoates and a Method of Their Production (Cupriavidus eutrophus Shtamm Bakterii VKPM B-10646-Produtsent Poligidroksialkanoatov i Sposob Ikh Polucheniya). RU2439143C1, 10 January 2012. (In Russian). [Google Scholar]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Mikrobiol. 1961, 38, 209–222. [Google Scholar] [CrossRef]
- Braunegg, G.; Sonnleitner, B.Y.; Lafferty, R.M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Budde, C.F.; Riedel, S.L.; Hübner, F.; Risch, S.; Popović, M.K.; Rha, C.; Sinskey, A.J. Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl. Microbiol. Biotechnol. 2011, 89, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Kek, Y.K.; Lee, W.H.; Sudesh, K. Efficient bioconversion of palm acid oil and palm kernel acid oil to poly (3-hydroxybutyrate) by Cupriavidus necator. Can. J. Chem. 2008, 86, 533–539. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly (3-hydroxybutyrate-3-hydroxyvalerate) from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.N.S.; Mohd Annuar, M.S.; Simarani, K. Microbial biosynthesis and in vivo depolymerization of intracellular medium-chain-length poly-3-hydroxyalkanoates as potential route to platform chemicals. Biotechnol. Appl. Biochem. 2018, 65, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.L.; Yu, V.; Wai, L.; Yu, H.F. Production of medium-chain-length polyhydroxyalkanoates by Pseudomonas aeruginosa with fatty acids and alternative carbon sources. Appl. Biochem. Biotechnol. 2006, 132, 933–941. [Google Scholar] [CrossRef]
- Chung, A.L.; Jin, H.L.; Huang, L.J.; Ye, H.M.; Chen, J.C.; Wu, Q.; Chen, G.Q. Biosynthesis and characterization of poly (3-hydroxydodecanoate) by β-oxidation inhibited mutant of Pseudomonas entomophila L48. Biomacromolecules 2011, 12, 3559–3566. [Google Scholar] [CrossRef]
- Ouyang, S.P.; Liu, Q.; Fang, L.; Chen, G.Q. Construction of PHA-operon-defined knockout mutants of Pseudomonas putida KT2442 and their applications in poly(hydroxyalkanoate) production. Macromol. Biosci. 2007, 7, 227–233. [Google Scholar] [CrossRef]
- Ouyang, S.P.; Luo, R.C.; Chen, S.S.; Liu, Q.; Chung, A.; Wu, Q.; Chen, G.Q. Production of polyhydroxyalkanoates with high 3-hydroxydodecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Biomacromolecules 2007, 8, 2504–2511. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, G.; Zhou, X.R.; Chen, G.Q. Biosynthesis of poly (3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metabol. Eng. 2011, 13, 11–17. [Google Scholar] [CrossRef]
- Tan, I.K.P.; Kumar, K.S.; Theanmalar, M.; Gan, S.N.; Gordon Iii, B. Saponified palm kernel oil and its major free fatty acids as carbon substrates for the production of polyhydroxyalkanoates in Pseudomonas putida PGA1. Appl. Microbiol. Biotechnol. 1997, 47, 207–211. [Google Scholar] [CrossRef]
- Impallomeni, G.; Guglielmino, S.P.; Carnazza, S.; Ferreri, A.; Ballistreri, A. Tween 20 and its major free fatty acids as carbon substrates for the production of polyhydroxyalkanoates in Pseudomonas aeruginosa ATCC 27853. J. Polym. Environ. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T. Growth kinetics, effect of carbon substrate in biosynthesis of mcl-PHA by Pseudomonas putida Bet001. Braz. J. Microbiol. 2014, 45, 427–438. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, G.; Wu, Q.; Chen, G.Q.; Zhang, R. Production of polyhydroxyalkanoates by Pseudomonas nitroreducens. Antonie Leeuwenhoek 1999, 75, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hong, K.; Chen, G.Q.; Wu, Q.; Zhang, R.Q.; Huang, W. Production of polyesters consisting of medium chain length 3-hydroxyalkanoic acids by Pseudomonas mendocina 0806 from various carbon sources. Antonie Leeuwenhoek 2000, 77, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Wong, H.H.; Choi, J.I.; Lee, S.H.; Lee, S.C.; Han, C.S. Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol. Bioeng. 2000, 68, 466–470. [Google Scholar] [CrossRef]
- Lee, S.Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996, 14, 431–438. [Google Scholar] [CrossRef]
- Lu, J.; Brigham, C.J.; Rha, C.; Sinskey, A.J. Characterization of an extracellular lipase and its chaperone from Ralstonia eutropha H16. Appl. Microbiol. Biotechnol. 2013, 97, 2443–2454. [Google Scholar] [CrossRef]
- Brigham, C.J.; Budde, C.F.; Holder, J.W.; Zeng, Q.; Mahan, A.E.; Rha, C.; Sinskey, A.J. Elucidation of β-oxidation pathways in Ralstonia eutropha H16 by examination of global gene expression. J. Bacteriol. 2010, 192, 5454–5464. [Google Scholar] [CrossRef]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The effect of food preservatives on pH homeostasis in Escherichia coli. Microbiology 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.L.; Lu, J.; Stahl, U.; Brigham, C.J. Lipid and fatty acid metabolism in Ralstonia eutropha: Relevance for the biotechnological production of value-added products. Appl. Microbiol. Biotechnol. 2014, 98, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Riedel, S.L.; Bader, J.; Brigham, C.J.; Budde, C.F.; Yusof, Z.A.M.; Rha, C.; Sinskey, A.J. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol. Bioeng. 2012, 109, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Pavoncello, V.; Barras, F.; Bouveret, E. Degradation of exogenous fatty acids in Escherichia coli. Biomolecules 2022, 12, 1019. [Google Scholar] [CrossRef]
- Black, P.N.; DiRusso, C.C. Transmembrane movement of exogenous long-chain fatty acids: Proteins, enzymes, and vectorial esterification. Microbiol. Mol. Biol. Rev. 2023, 67, 454–472. [Google Scholar] [CrossRef]




| Strain | X, g/L | P(3HB), % | Mw, kDa | Đ | Tmelt, °C | Tdegr, °C | Cx, % | Reference |
|---|---|---|---|---|---|---|---|---|
| Lauric acid | ||||||||
| Alcaligenes sp. AK 201 | ~2.4 | 40% | 304 | 1.9 | 102 | - | - | [44] |
| Aeromonas hydrophila | 2.7–4.3 | 19.4–41.1 | - | - | - | - | - | [45,46] |
| Aeromonas salmonicida 741 | 2.8–3.8 | 13.3–30.0 * | - | - | - | - | - | [45] |
| Klebsiella pneumoniae ZMd31 | 0.6–0.7 | 11.0–18.3 * | - | - | - | - | - | [45] |
| Burkholderia sp. | 1.4–2.1 | 8–69 | - | - | - | - | - | [45] |
| Bacillus cereus SPV | 0.51 | 61.81 | - | - | - | - | - | [47] |
| Myristic acid | ||||||||
| Alcaligenes sp. AK 201 | ~2.9 | 55% | 1416 | 3.1 | 106 | - | - | [44] |
| C. necator DSM 545 | 1.43 | - | - | - | - | - | - | [48] |
| D. tsuruhatensis Bet002 | - | 76.7 | 131 | 1.1 | 173.2 | 289.8 | - | [49] |
| Burkholderia sp. | 1.1–1.9 | 1–49 | - | - | - | - | - | [50] |
| Palmitic acid | ||||||||
| Alcaligenes sp. AK 201 | ~3.2 | 55% | 1442 | 2.5 | 108 | - | - | [44] |
| C. necator DSM 545 | 1.97 | - | - | - | - | - | - | [48] |
| D. tsuruhatensis Bet002 | - | 53.8 | 166 | 1.5 | 175.7 | 302.9 | - | [49] |
| Burkholderia sp. | 0.6–1.5 | tr-9 | - | - | - | - | - | [50] |
| Rhodococcus pyridinivorans KY703220 | 1.435 (OD) | 40 | - | - | - | - | - | [51] |
| Stearic acid | ||||||||
| Alcaligenes sp. AK 201 | ~2.3 | 30% | 986 | 1.9 | 116 | - | - | [44] |
| C. necator DSM 545 | 2.11 | - | - | - | - | - | - | [48] |
| D. tsuruhatensis Bet002 | - | 45.0 | 188 | 1.8 | 177.4 | 391.8 | - | [49] |
| Burkholderia sp. | 0.5–1.0 | tr-1 | - | - | - | - | - | [50] |
| Fatty Acid (FA) | X, g/L | PHAs, g/L | Xres, g/L | PHAs, % | YX, g/g | YPHA, g/g | Biomass Productivity | PHA Productivity, PPHA, g/L·h | Degree of Use of FAs, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| PX, g/L·h | PXres, g/L·h | |||||||||
| Lauric C12:0 | 6.7 a | 5.0 a | 1.7 a | 75 a | 0.69 a | 0.52 a | 0.093 a | 0.024 a | 0.069 a | 64.7 a |
| Myristic C14:0 | 7.5 a | 5.6 a | 1.9 a | 74 a | 0.83 b | 0.62 a | 0.104 a | 0.026 a | 0.078 a | 60.0 a |
| Palmitic C16:0 | 3.9 b | 1.8 b | 2.1 a | 47 b | 0.67 a | 0.31 b | 0.054 b | 0.029 a | 0.025 b | 38.7 b |
| Stearic C18:0 | 2.5 c | 0.7 c | 1.8 a | 28 c | 0.66 a | 0.18 c | 0.035 c | 0.025 a | 0.010 c | 25.3 c |
| Mixture Number | FA Composition in the Mixture | FA Concentration, g/L | Total of FA, g/L | FA Ratio |
|---|---|---|---|---|
| 1 | Lauric C12:0 | 3.75 | 15.0 | C12:0/C14:0/C16:0/C18:0 = 1.0/1.0/1.0/1.0 |
| Myristic C14:0 | 3.75 | |||
| Palmitic C16:0 | 3.75 | |||
| Stearic C18:0 | 3.75 | |||
| 2 | Myristic C14:0 | 5.0 | 15.0 | C14:0/C16:0/C18:0 = 1.0/1.0/1.0 |
| Palmitic C16:0 | 5.0 | |||
| Stearic C18:0 | 5.0 | |||
| 3 | Myristic C14:0 | 1.5 | 15.0 | C14:0/C16:0/C18:0 = 1.0/7.8/1.2 |
| Palmitic C16:0 | 11.7 | |||
| Stearic C18:0 | 1.8 | |||
| 4 | Lauric C12:0 | 4.0 | 20.0 | C12:0/C14:0/C16:0/C18:0/C18:1ω9 = 1.0/1.0/1.0/1.0/1.0 |
| Myristic C14:0 | 4.0 | |||
| Palmitic C16:0 | 4.0 | |||
| Stearic C18:0 | 4.0 | |||
| Oleic C18:1ω9 | 4.0 | |||
| 5 | Myristic C14:0 | 1.5 | 20.0 | C14:0/C16:0/C18:0/C18:1ω9 = 1.0/6.0/1.0/5.3 |
| Palmitic C16:0 | 9.0 | |||
| Stearic C18:0 | 1.5 | |||
| Oleic C18:1ω9 | 8.0 |
| FA Mixture Number | X, g/L | PHAs, g/L | PHAs, % | Xres, g/L | YX, g/g | YPHA, g/g | Biomass Productivity | PHA Productivity, PPHA, g/L·h | Degree of the Use of FAs, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| PX, g/L·h | PXres, g/L·h | |||||||||
| Saturated FAs | ||||||||||
| 1 | 8.9 a | 7.0 a | 79 a | 1.9 a | 0.63 ab | 0.49 ac | 0.124 a | 0.026 a | 0.097 a | 94.9 ab |
| 2 | 8.2 ab | 6.0 b | 73 a | 2.2 a | 0.59 a | 0.43 b | 0.114 ab | 0.031 a | 0.083 b | 92.7 a |
| 3 | 8.0 b | 5.7 b | 71 a | 2.4 a | 0.62 ab | 0.44 abc | 0.111 b | 0.033 a | 0.079 b | 86.1 bcd |
| Saturated FAs + Oleic acid | ||||||||||
| 4 | 8.6 ab | 6.5 ab | 76 a | 2.1 a | 0.69 b | 0.52 ac | 0.119 ab | 0.029 a | 0.090 ab | 83.1 c |
| 5 | 8.2 ab | 6.1 ab | 75 a | 2.1 a | 0.61 ab | 0.45 abc | 0.114 ab | 0.029 a | 0.085 ab | 90.4 ad |
| P(3HB) Sample Number | Substrate | Mw, kDa | Đ | Cx, % | Tmelt, °C | Hmelt, J/g | Tdegr, °C | Tg, °C | Tcryst, °C |
|---|---|---|---|---|---|---|---|---|---|
| Saturated FAs | |||||||||
| 1 | Lauric (12:0) | 305.5 | 3.74 | 49.7 | 169.3 | 72.6 | 127.1 (19.5%) 284.7 | - | 65.2 47.5 |
| 2 | Myristic (14:0) | 364.9 | 3.71 | 52.8 | 169.2 | 77.1 | 113.0 (19.4%) 284.9 | 1.8 | 67.4 45.5 |
| 3 | Palmitic (16:0) | 423.7 | 3.46 | 50.7 | 170.1 | 74.1 | 113.0 (21.4%) 284.7 | 2.0 | 60.7 48.3 |
| 4 | Stearic (18:0) | 447.1 | 2.88 | 47.5 | 166.0 | 69.3 | 282.9 | - | 53.2 |
| FA Mixture * | |||||||||
| 5 | Mixture 1 | 289.3 | 2.72 | 39.2 | 157.4 168.6 | 7.9 49.3 | 92.4 (14.9%) 264.5 | - | 74.1 |
| 6 | Mixture 2 | 333.4 | 2.74 | 53.2 | 171.5 | 77.7 | 127.5 (9.2%) 295.7 | 3.0 | 62.1 |
| 7 | Mixture 3 | 464.5 | 3.48 | 56.0 | 171.0 | 81.8 | 115.3 (17%) 267.6 | - | 66.0 |
| 8 | Mixture 4 | 325.0 | 3.22 | 57.1 | 169.8 | 83.4 | 111.3 (22.8%) 281.1 | - | 65.3 |
| 9 | Mixture 5 | 315.5 | 3.32 | 49.4 | 150.3 163.1 | 10.4 61.7 | 77.1 (3.6%) 138.5 (8.1%) 252.4 | - | 64.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Biosynthesis of Polyhydroxyalkanoates in Cupriavidus necator B-10646 on Saturated Fatty Acids. Polymers 2024, 16, 1294. https://doi.org/10.3390/polym16091294
Zhila NO, Sapozhnikova KY, Kiselev EG, Shishatskaya EI, Volova TG. Biosynthesis of Polyhydroxyalkanoates in Cupriavidus necator B-10646 on Saturated Fatty Acids. Polymers. 2024; 16(9):1294. https://doi.org/10.3390/polym16091294
Chicago/Turabian StyleZhila, Natalia O., Kristina Yu. Sapozhnikova, Evgeniy G. Kiselev, Ekaterina I. Shishatskaya, and Tatiana G. Volova. 2024. "Biosynthesis of Polyhydroxyalkanoates in Cupriavidus necator B-10646 on Saturated Fatty Acids" Polymers 16, no. 9: 1294. https://doi.org/10.3390/polym16091294
APA StyleZhila, N. O., Sapozhnikova, K. Y., Kiselev, E. G., Shishatskaya, E. I., & Volova, T. G. (2024). Biosynthesis of Polyhydroxyalkanoates in Cupriavidus necator B-10646 on Saturated Fatty Acids. Polymers, 16(9), 1294. https://doi.org/10.3390/polym16091294











