Microfluidic-Assisted Formulation of ε-Polycaprolactone Nanoparticles and Evaluation of Their Properties and In Vitro Cell Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
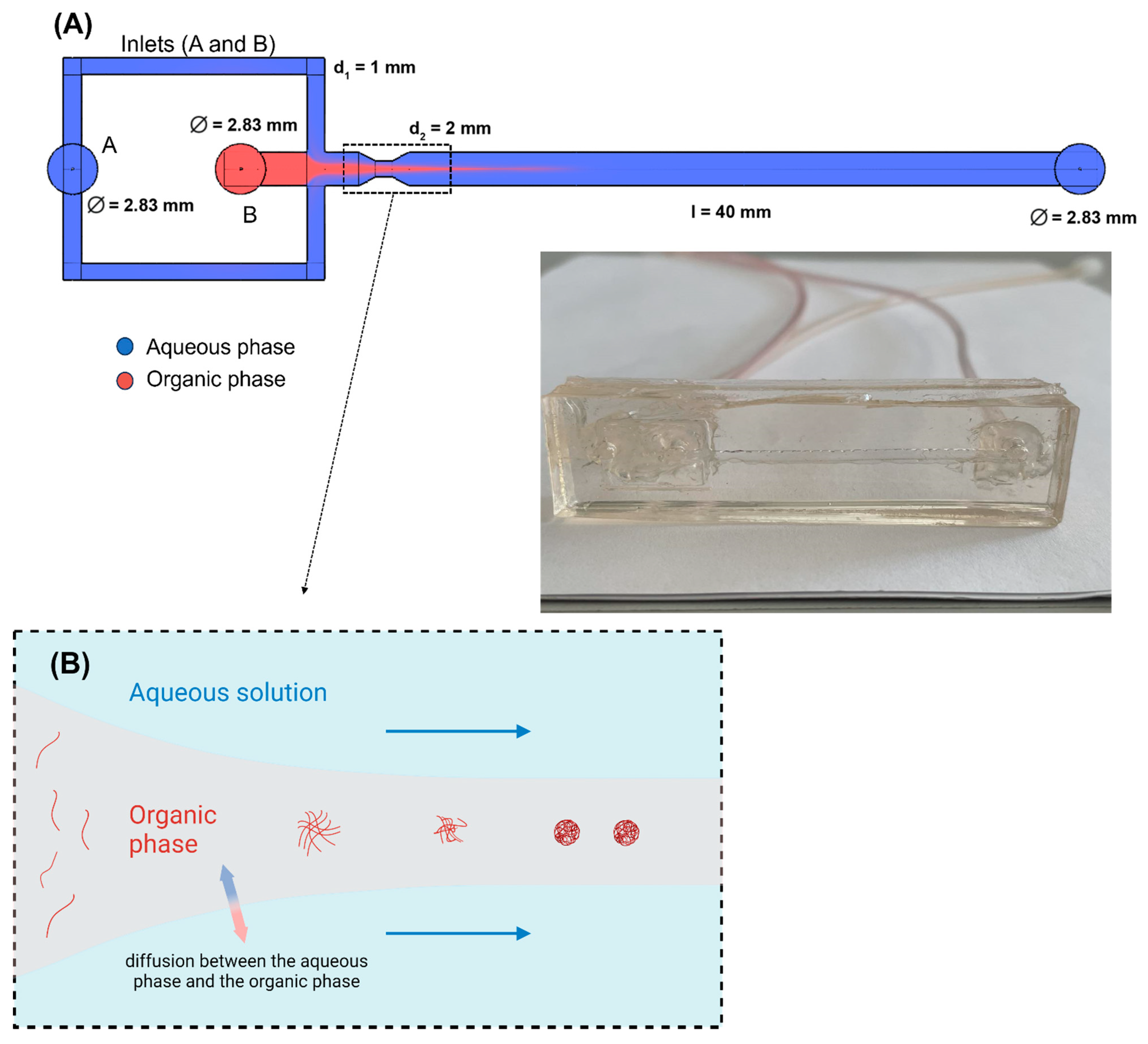
2.2. Microfluidic Device
2.3. Methods
2.3.1. Preparation of Polymeric Nanoparticles
Preparation of Solutions
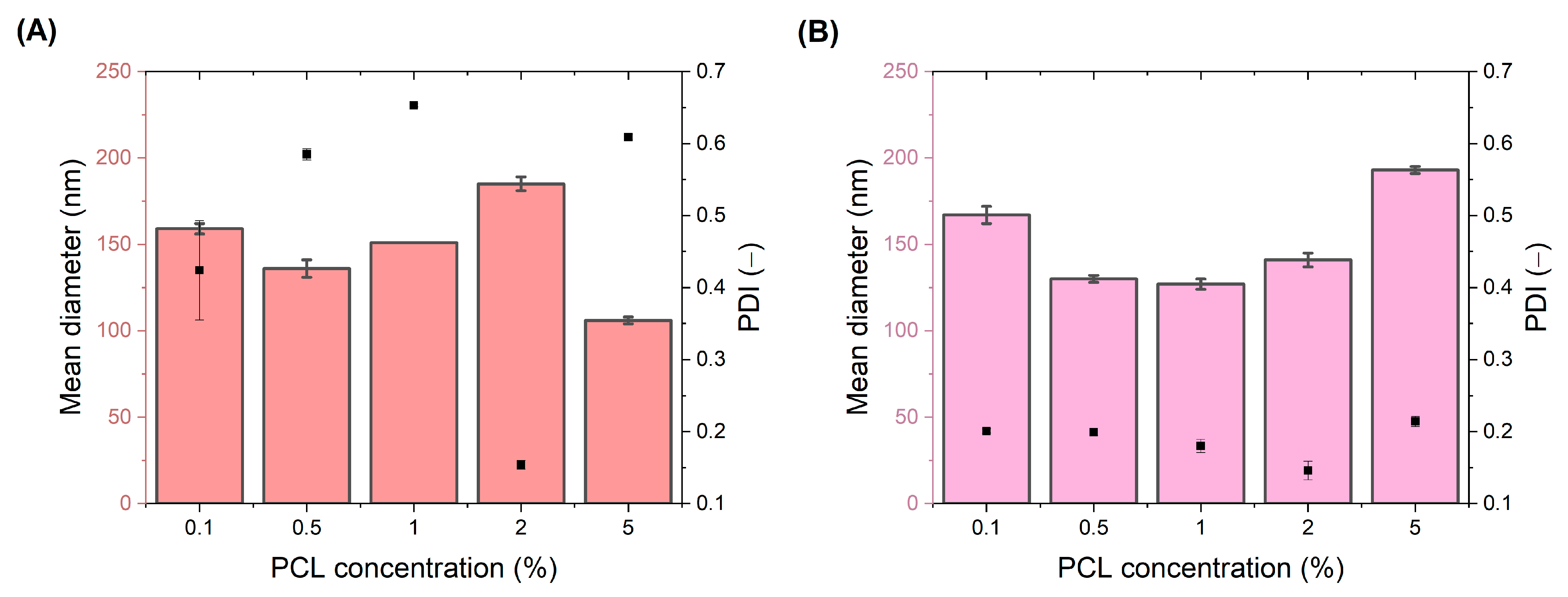
Formulation of Nanoparticles
2.3.2. Particle Size and Zeta Potential Analysis
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. FTIR Analysis
2.3.5. In Vitro Release Studies
2.3.6. Cellular Uptake and Cytotoxicity Assay
Cytotoxicity Assay
Confocal Microscopy
3. Results and Discussion
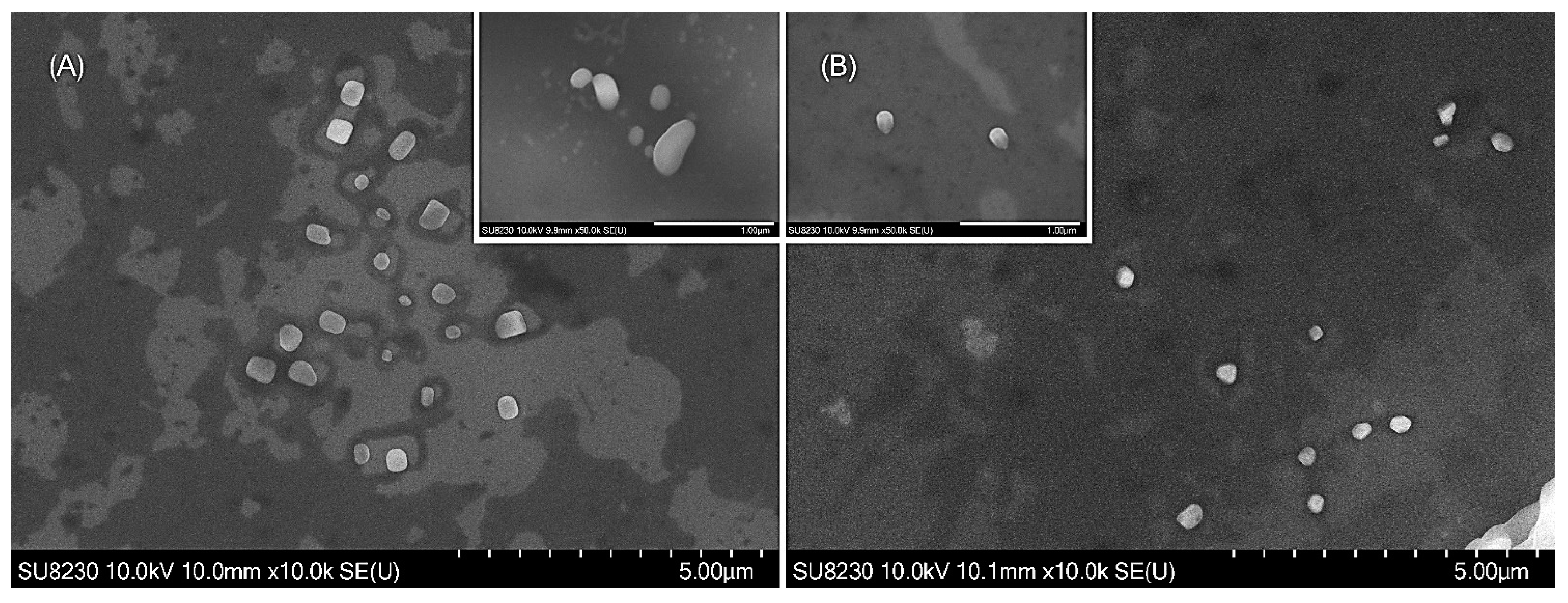
3.1. SEM Analysis
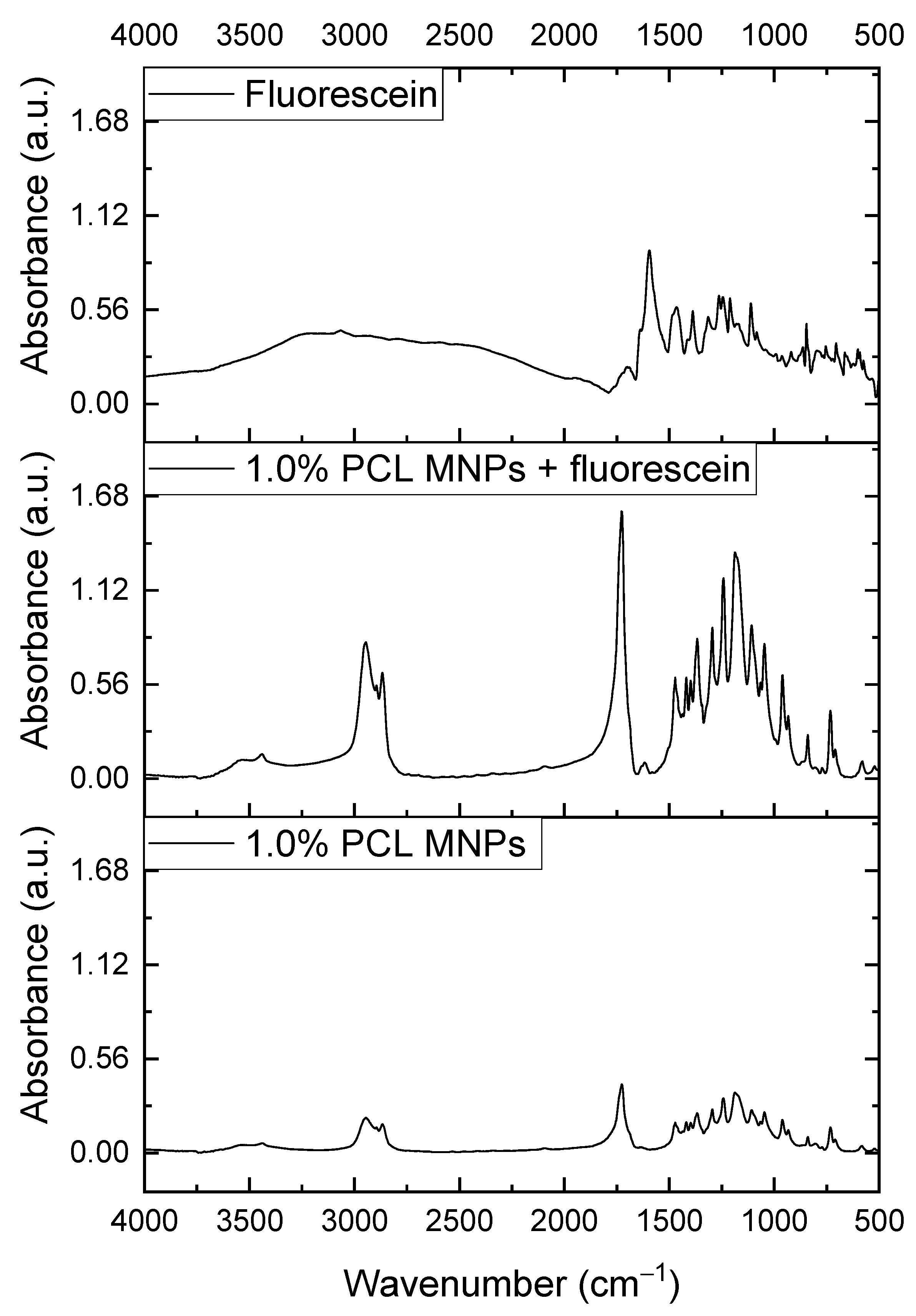
3.2. FTIR Analysis
3.3. In Vitro Release Studies
3.4. In Vitro Cytotoxicity and Cellular Uptake Studies
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, P.; Tran, T.T.-D.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef]
- Gui, R.; Wang, Y.; Sun, J. Encapsulating magnetic and fluorescent mesoporous silica into thermosensitive chitosan microspheres for cell imaging and controlled drug release in vitro. Colloids Surf. B Biointerfaces 2014, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Powell, R.; Chan, P. Frontiers in Research Review: Cutting-Edge Molecular Approaches to Therapeutics Polymeric Core-Shell Nanoparticles For Therapeutics. Clin. Exp. Pharmacol. Physiol. 2006, 33, 557–562. [Google Scholar] [CrossRef]
- Pathak, Y.; Thassu, D. Drug Delivery Nanoparticles Formulation and Characterization; CRC Press: Boca Raton, FL, USA, 2016; Volume 191. [Google Scholar]
- Nehilla, B.J.; Bergkvist, M.; Popat, K.C.; Desai, T.A. Purified and surfactant-free coenzyme Q10-loaded biodegradable nanoparticles. Int. J. Pharm. 2008, 348, 107–114. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Seyler, I.; Appel, M.; Devissaguet, J.-P.; Legrand, P.; Barratt, G. Macrophage activation by a lipophilic derivative of muramyldipeptide within nanocapsules: Investigation of the mechanism of drug delivery. J. Nanoparticle Res. 1999, 1, 91–97. [Google Scholar] [CrossRef]
- Legrand, P.; Lesieur, S.; Bochot, A.; Gref, R.; Raatjes, W.; Barratt, G.; Vauthier, C. Influence of polymer behaviour in organic solution on the production of polylactide nanoparticles by nanoprecipitation. Int. J. Pharm. 2007, 344, 33–43. [Google Scholar] [CrossRef]
- Tavares, M.R.; de Menezes, L.R.; Filho, J.C.D.; Cabral, L.M.; Tavares, M.I.B. Surface-coated polycaprolactone nanoparticles with pharmaceutical application: Structural and molecular mobility evaluation by TD-NMR. Polym. Test. 2017, 60, 39–48. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Reddy, J. Polycaprolactone nano fi bers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]
- Bilensoy, E.; Sarisozen, C.; Esendağlı, G.; Doğan, A.L.; Aktaş, Y.; Şen, M.; Mungan, N.A. Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. Int. J. Pharm. 2009, 371, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Payyappilly, S.S.; Panja, S.; Mandal, P.; Dhara, S.; Chattopadhyay, S. Organic Solvent-Free Low Temperature Method of Preparation for Self Assembled Amphiphilic Poly(ϵ-Caprolactone)–Poly(Ethylene Glycol) Block Copolymer Based Nanocarriers for Protein Delivery. Colloids Surf. B Biointerfaces 2015, 135, 510–517. [Google Scholar] [CrossRef]
- Othman, R.; Vladisavljević, G.T.; Nagy, Z.K. Preparation of biodegradable polymeric nanoparticles for pharmaceutical applications using glass capillary microfluidics. Chem. Eng. Sci. 2015, 137, 119–130. [Google Scholar] [CrossRef]
- Badilescu, S.; Packirisamy, M. Microfluidics-nano-integration for synthesis and sensing. Polymers 2012, 4, 1278–1310. [Google Scholar] [CrossRef]
- Kumar, A.; Sawant, K. Encapsulation of exemestane in polycaprolactone nanoparticles: Optimization, characterization, and release kinetics. Cancer Nanotechnol. 2013, 4, 57–71. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2018, 9, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, D.L.; Barresi, A.A.; Garbero, M. Nucleation, growth, and agglomeration in barium sulfate turbulent precipitation. AIChE J. 2002, 48, 2039–2050. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’Homme, R.K. Flash NanoPrecipitation of Organic Actives and Block Copolymers using a Confined Impinging Jets Mixer. Aust. J. Chem. 2003, 56, 1021–1024. [Google Scholar] [CrossRef]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Park, S.; Park, H.; Tae, H.; Jackman, J.A.; Cho, N. Lipid Nanoparticle Technologies for Nucleic Acid Delivery: A Nanoarchitectonics Perspective. Adv. Funct. Mater. 2022, 32, 2203669. [Google Scholar] [CrossRef]
- Ariga, K. Materials Nanoarchitectonics for Advanced Physics Research. Adv. Phys. Res. 2023, 2, 2200113. [Google Scholar] [CrossRef]
- Ariga, K. Materials nanoarchitectonics in a two-dimensional world within a nanoscale distance from the liquid phase. Nanoscale 2022, 14, 10610–10629. [Google Scholar] [CrossRef]
- Heshmatnezhad, F.; Nazar, A.R.S.; Aghaei, H.; Varshosaz, J. Production of doxorubicin-loaded PCL nanoparticles through a flow-focusing microfluidic device: Encapsulation efficacy and drug release. Soft Matter 2021, 17, 10675–10682. [Google Scholar] [CrossRef]
- Bramosanti, M.; Chronopoulou, L.; Grillo, F.; Valletta, A.; Palocci, C. Microfluidic-assisted nanoprecipitation of antiviral-loaded polymeric nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 369–376. [Google Scholar] [CrossRef]
- Jamwal, S.; Ram, B.; Ranote, S.; Dharela, R.; Chauhan, G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int. J. Biol. Macromol. 2018, 123, 968–978. [Google Scholar] [CrossRef]
- Yan, X.; Bernard, J.; Ganachaud, F. Nanoprecipitation as a simple and straightforward process to create complex polymeric colloidal morphologies. Adv. Colloid Interface Sci. 2021, 294, 102474. [Google Scholar] [CrossRef] [PubMed]
- Javaid, S.; Ahmad, N.M.; Mahmood, A.; Nasir, H.; Iqbal, M.; Ahmad, N.; Irshad, S. Cefotaxime loaded polycaprolactone based polymeric nanoparticles with antifouling properties for in-vitro drug release applications. Polymers 2021, 13, 2180. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Ali Bhatti, A.S.; Ahmad, N.M.; Ahmed, N.; Shahnaz, G.; Lebaz, N.; Elaissari, A. Amphotericin B loaded polymeric nanoparticles for treatment of Leishmania infections. Nanomaterials 2020, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Torrieri, G.; Santos, H.A. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin. Drug Deliv. 2018, 15, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, S.; Hasani-Sadrabadi, M.M.; Majedi, F.S.; Dashtimoghadam, E.; Tondar, M.; Jacob, K.I. Understanding biophysical behaviours of microfluidic-synthesized nanoparticles at nano-biointerface. Colloids Surf. B Biointerfaces 2016, 145, 802–811. [Google Scholar] [CrossRef]
- Michelon, M.; Oliveira, D.R.B.; de Figueiredo Furtado, G.; De La Torre, L.G.; Cunha, R.L. High-throughput continuous production of liposomes using hydrodynamic flow-focusing microfluidic devices. Colloids Surf. B Biointerfaces 2017, 156, 349–357. [Google Scholar] [CrossRef]
- Lari, A.S.; Khatibi, A.; Zahedi, P.; Ghourchian, H. Microfluidic-assisted production of poly(ε-caprolactone) and cellulose acetate nanoparticles: Effects of polymers, surfactants, and flow rate ratios. Polym. Bull. 2021, 78, 5449–5466. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18, e2106580. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials synthesis through microfluidic methods: An updated overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Baby, T.; Jin, S.; Liu, Y.; Yang, G.; Zhao, C.-X. Insight into drug encapsulation in polymeric nanoparticles using microfluidic nanoprecipitation. Chem. Eng. Sci. 2021, 235, 116468. [Google Scholar] [CrossRef]
- Bendre, A.; Bhat, M.P.; Lee, K.-H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent developments in microfluidic technology for synthesis and toxicity-efficiency studies of biomedical nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Wojasiński, M.; Wasiak, I.; Ciach, T. Investigation of controlled solvent exchange precipitation of fluorescent organic nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 86–92. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Saeidian, H.; Mirjafary, Z.; Mokhtari, J. Preparation and characterization of memantine loaded polycaprolactone nanocapsules for Alzheimer’s disease. J. Porous Mater. 2020, 28, 205–212. [Google Scholar] [CrossRef]
- Badran, M.M.; Alanazi, A.E.; Ibrahim, M.A.; Alshora, D.H.; Taha, E.; HAlomrani, A. Optimization of Bromocriptine-Mesylate-Loaded Polycaprolactone Nanoparticles Coated with Chitosan for Nose-to-Brain Delivery: In Vitro and In Vivo Studies. Polymers 2023, 15, 3890. [Google Scholar] [CrossRef]
- Tapia-Guerrero, Y.S.; Del Prado-Audelo, M.L.; Borbolla-Jiménez, F.V.; Gomez, D.M.G.; García-Aguirre, I.; Colín-Castro, C.A.; Morales-González, J.A.; Leyva-Gómez, G.; Magaña, J.J. Effect of UV and gamma irradiation sterilization processes in the properties of different polymeric nanoparticles for biomedical applications. Materials 2020, 13, 1090. [Google Scholar] [CrossRef]
- Boken, J.; Soni, S.K.; Kumar, D. Microfluidic Synthesis of Nanoparticles and their Biosensing Applications. Crit. Rev. Anal. Chem. 2016, 46, 538–561. [Google Scholar] [CrossRef]
- Heshmatnezhad, F.; Nazar, A.R.S. On-chip controlled synthesis of polycaprolactone nanoparticles using continuous-flow microfluidic devices. J. Flow Chem. 2020, 10, 533–543. [Google Scholar] [CrossRef]
- Nair, B.P.; Vaikkath, D.; Mohan, D.S.; Nair, P.D. Fabrication of a microvesicles-incorporated fibrous membrane for controlled delivery applications in tissue engineering. Biofabrication 2014, 6, 045008. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, S.; Godara, S.K.; Kaur, V. Synthesis of fluorescein dye using microwave radiations and its application on textile substrates. Fibres Text. East. Eur. 2021, 29, 100–105. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic therapy of ovarian carcinoma cells with curcumin-loaded biodegradable polymeric nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Tariq, I.; Sohail, M.F.; Ali, M.Y.; Preis, E.; Ambreen, G.; Pinnapireddy, S.R.; Jedelská, J.; Schäfer, J.; et al. Lipoparticles for synergistic chemo-photodynamic therapy to ovarian carcinoma cells: In vitro and in vivo assessments. Int. J. Nanomed. 2021, 16, 951–976. [Google Scholar] [CrossRef]
- Dinarvand, R.; Moghadam, S.H.; Mohammadyari-Fard, L.; Atyabi, F. Preparation of biodegradable microspheres and matrix devices containing naltrexone. AAPS PharmSciTech 2003, 4, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, Y.; Zhang, C.Y.; Fang, T. Co-delivery of paclitaxel and doxorubicin by ph-responsive prodrug micelles for cancer therapy. Int. J. Nanomed. 2020, 15, 3319–3331. [Google Scholar] [CrossRef] [PubMed]
- Dayanandan, A.P.; Cho, W.J.; Kang, H.; Bello, A.B.; Kim, B.J.; Arai, Y.; Lee, S.-H. Emerging nano-scale delivery systems for the treatment of osteoporosis. Biomater. Res. 2023, 27, 68. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Bailey, J.L.; Janeczek, A.A.; Stumpf, P.S.; Johnston, A.H.; Oreffo, R.O.C.; Woo, Y.L.; Cheong, Y.C.; Evans, N.D.; Newman, T.A. Quantification of intracellular payload release from polymersome nanoparticles. Sci. Rep. 2016, 6, 29460. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qian, H.; Sun, S.; Sun, D.; Yin, H.; Cai, X.; Liu, Z.; Wu, J.; Jiang, T.; Liu, X. Hollow mesoporous silica nanoparticles for intracellular delivery of fluorescent dye. Chem. Central J. 2011, 5, 1. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
- Lee, K.D.; Nir, S.; Papahadjopoulos, D. Quantitative Analysis of Liposome-Cell Interactions In Vitro: Rate Constants of Binding and Endocytosis with Suspension and Adherent J774 Cells and Human Monocytes. Biochemistry 1993, 32, 889–899. [Google Scholar] [CrossRef]
- Li, S.; Molina, I.; Martinez, M.B.; Vert, M. Hydrolytic and enzymatic degradations of physically crosslinked hydrogels prepared from PLA/PEO/PLA triblock copolymers. J. Mater. Sci. Maed. Med. 2002, 13, 81–86. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discover Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T.F. Production of nanoparticle drug delivery systems with microfluidics tools. Expert. Opin. Drug Deliv. 2015, 12, 547–562. [Google Scholar] [CrossRef]
- Génot, V.; Desportes, S.; Croushore, C.; Lefèvre, J.-P.; Pansu, R.B.; Delaire, J.A.; von Rohr, P.R. Synthesis of organic nanoparticles in a 3D flow focusing microreactor. Chem. Eng. J. 2010, 161, 234–239. [Google Scholar] [CrossRef]
- Laouini, A.; Charcosset, C.; Fessi, H.; Holdich, R.; Vladisavljević, G. Preparation of liposomes: A novel application of microengineered membranes—Investigation of the process parameters and application to the encapsulation of vitamin E. RSC Adv. 2013, 3, 4985–4994. [Google Scholar] [CrossRef]
- Laouini, A.; Charcosset, C.; Fessi, H.; Holdich, R.; Vladisavljević, G. Preparation of liposomes: A novel application of microengineered membranes—From laboratory scale to large scale. Colloids Surf. B Biointerfaces 2013, 112, 272–278. [Google Scholar] [CrossRef][Green Version]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; DeVoe, D.L.; Gaitan, M. Microfluidic Mixing and the Formation of Nanoscale Lipid Vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef]
- Thode, K.; Müller, R.H.; Kresse, M. Two-time window and multiangle photon correlation spectroscopy size and zeta potential analysis—Highly sensitive rapid assay for dispersion stability. J. Pharm. Sci. 2000, 89, 1317–1324. [Google Scholar] [CrossRef]
- Feng, S.-S.; Huang, G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol®) from nanospheres of biodegradable polymers. J. Control Release 2001, 71, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, M.; Kost, B.; Gonciarz, W.; Krupa, A.; Socka, M.; Rogala, M. Nanocarriers based on block copolymers of L-proline and lactide: The effect of core crosslinking versus its pH-sensitivity on their cellular uptake. Eur. Polym. J. 2021, 156, 110572. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Kusumasari, F.C.; Samada, L.H.; Budianto, E. Preparation, Characterization and In Vitro Release Study of Microcapsule Simvastatin Using Biodegradable Polymeric Blend of Poly(L-Lactic Acid) and Poly(ε-Caprolactone) with Double Emulsifier. Mater. Sci. Forum 2020, 977, 178–183. [Google Scholar] [CrossRef]
- Nozal, V.; Rojas-Prats, E.; Maestro, I.; Gil, C.; Perez, D.I.; Martinez, A. Improved controlled release and brain penetration of the small molecule S14 using PLGA nanoparticles. Int. J. Mol. Sci. 2021, 22, 3206. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybak, E.; Kowalczyk, P.; Czarnocka-Śniadała, S.; Wojasiński, M.; Trzciński, J.; Ciach, T. Microfluidic-Assisted Formulation of ε-Polycaprolactone Nanoparticles and Evaluation of Their Properties and In Vitro Cell Uptake. Polymers 2023, 15, 4375. https://doi.org/10.3390/polym15224375
Rybak E, Kowalczyk P, Czarnocka-Śniadała S, Wojasiński M, Trzciński J, Ciach T. Microfluidic-Assisted Formulation of ε-Polycaprolactone Nanoparticles and Evaluation of Their Properties and In Vitro Cell Uptake. Polymers. 2023; 15(22):4375. https://doi.org/10.3390/polym15224375
Chicago/Turabian StyleRybak, Ewa, Piotr Kowalczyk, Sylwia Czarnocka-Śniadała, Michał Wojasiński, Jakub Trzciński, and Tomasz Ciach. 2023. "Microfluidic-Assisted Formulation of ε-Polycaprolactone Nanoparticles and Evaluation of Their Properties and In Vitro Cell Uptake" Polymers 15, no. 22: 4375. https://doi.org/10.3390/polym15224375
APA StyleRybak, E., Kowalczyk, P., Czarnocka-Śniadała, S., Wojasiński, M., Trzciński, J., & Ciach, T. (2023). Microfluidic-Assisted Formulation of ε-Polycaprolactone Nanoparticles and Evaluation of Their Properties and In Vitro Cell Uptake. Polymers, 15(22), 4375. https://doi.org/10.3390/polym15224375









