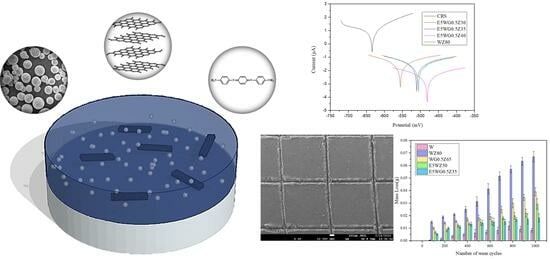
Green and Heavy-Duty Anticorrosion Coatings: Waterborne Epoxy Thermoset Composites Modified through Variation of Zinc Dust Loading and Incorporation of Amine-Capped Aniline Trimer and Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
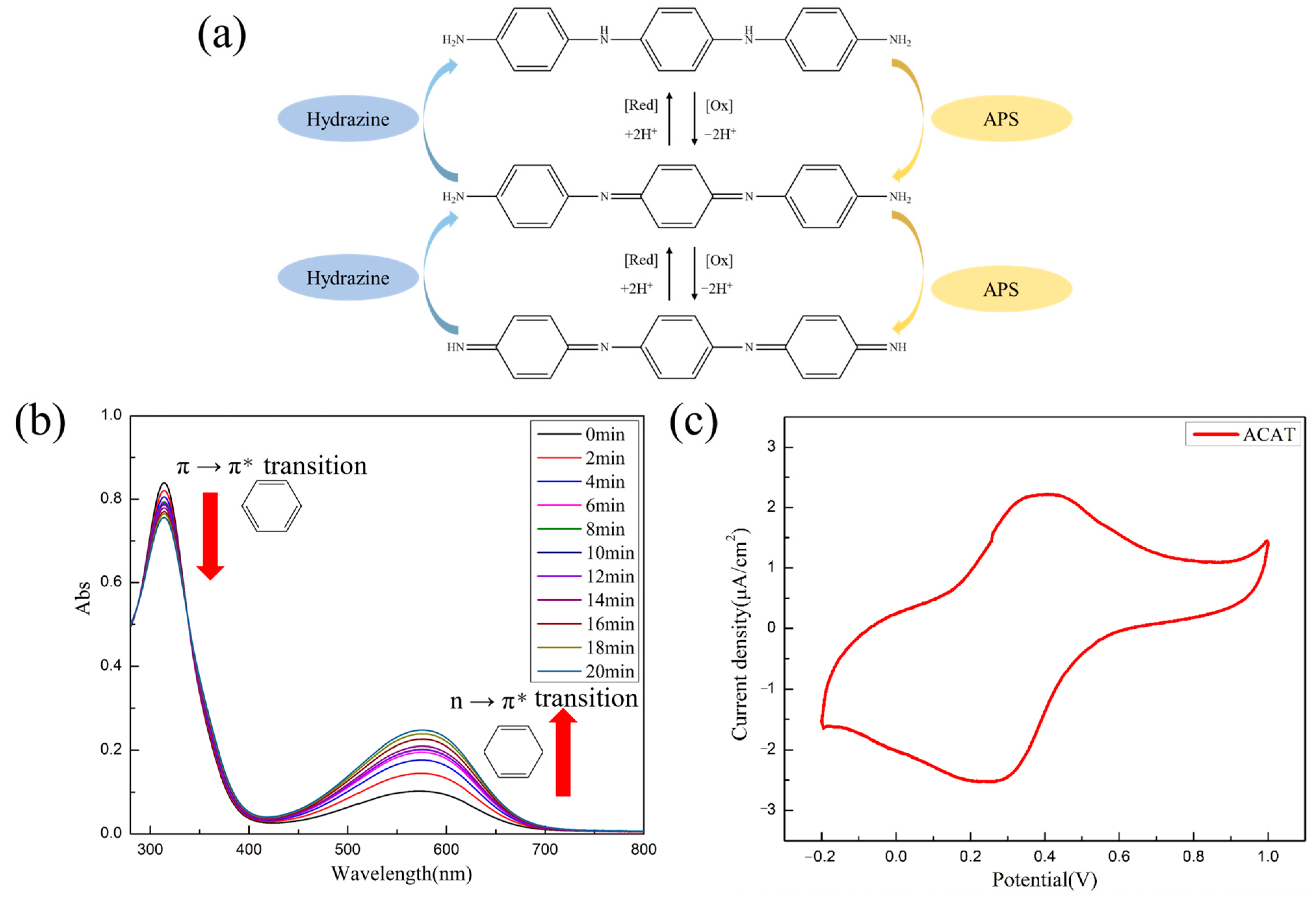
2.2. Preparation of Amino-Capped Aniline Trimer (ACAT) [45]
2.3. Preparation of Graphene Oxide (GO)
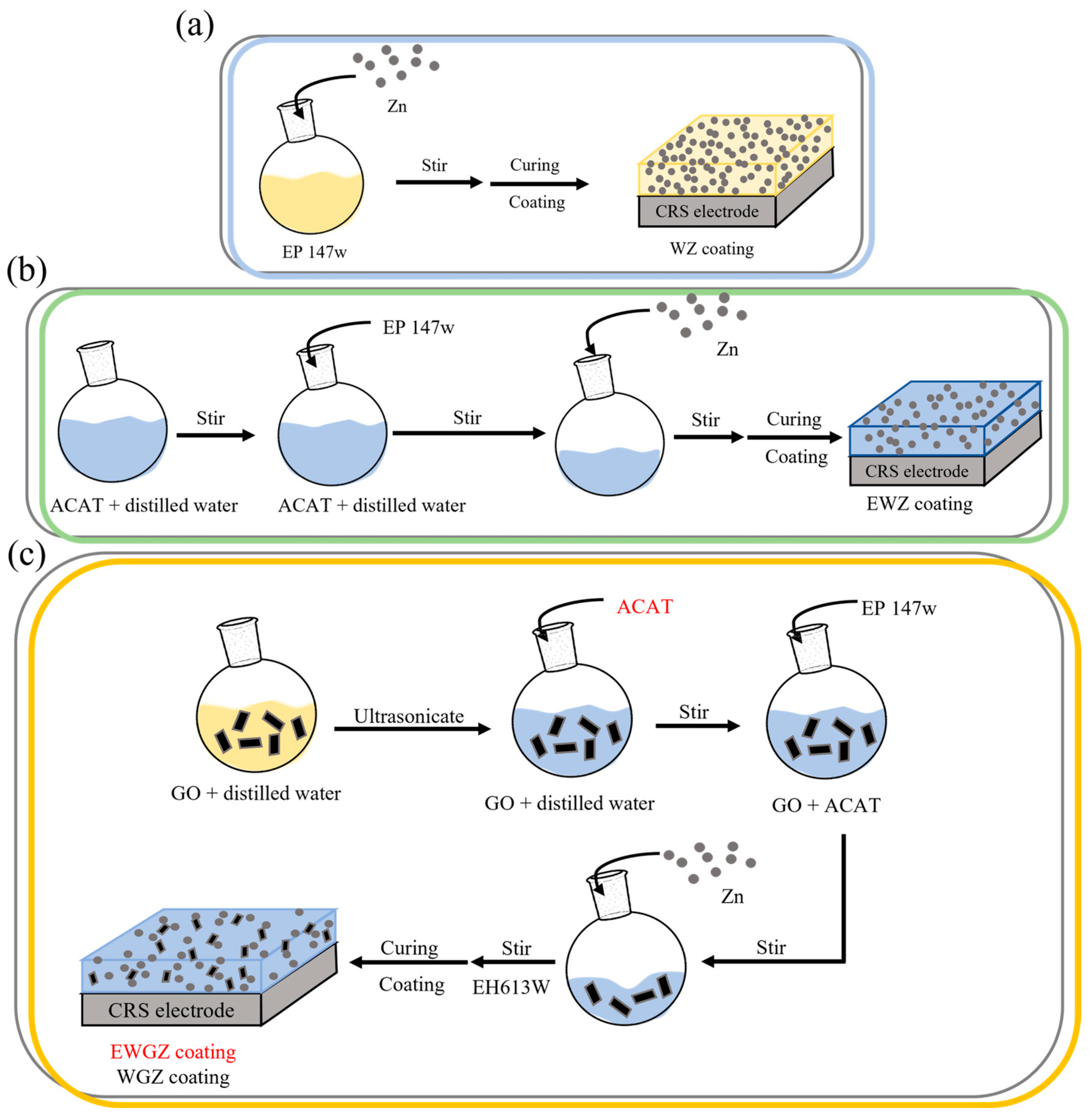
2.4. Preparation of Waterborne Epoxy Thermoset Coatings (WETCs)
2.5. Preparation of Waterborne Epoxy Thermoset Composite Coatings Containing 1, 3, and 5 wt-% of ACAT with ZD (EWZ), Respectively
2.6. Preparation of Waterborne Epoxy Thermoset Composite Coatings Containing 0.25, 0.5, and 0.75% w/w GO with ZD (WGZ), Respectively
2.7. Preparation of Waterborne Epoxy Thermoset Composite (WETC) Coatings Containing 5% w/w ACAT and 0.5% w/w GO (EWGZ)
2.8. Preparation of WETC Coatings for Electrochemical Corrosion Measurements
2.9. Preparation of WETC Coatings for Salt Spray Testing
2.10. Preparation of WETC Coatings for Adhesion Testing and Wear Resistance Testing
3. Results and Discussion
3.1. Characterization of Commercial Waterborne Epoxy Thermoset Coating (WETC)
3.2. Characterization of Commercial ZD
3.3. Characterization of ACAT
3.4. Characterization of GO
3.5. Characterization of Coatings
3.6. Potentiodynamic Measurements of Composite Coatings
3.7. Salt Spray Test of CS Coated with Epoxy, WGZ, or EWGZ
3.8. Adhesion of the Coatings
3.9. Abrasion of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Zhang, Z.; Han, M. Han. Metal Corrosion for Nanofabrication. Small 2012, 8, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, H.; Zeze, A.L.P.; Liu, X.; Tao, M. Coating performance, durability and anti-corrosion mechanism of organic modified geopolymer composite for marine concrete protection. Cem. Concr. Compos. 2022, 129, 104495. [Google Scholar] [CrossRef]
- Chen, F.; Liu, P. Conducting Polyaniline Nanoparticles and Their Dispersion for Waterborne Corrosion Protection Coatings. ACS Appl. Mater. Interfaces 2011, 3, 2694–2702. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Chen, J.; Liu, S.; Zhang, G.; Zhao, H.; Wang, L.; Xue, Q. Anticorrosive performance of waterborne epoxy coatings containing water-dispersible hexagonal boron nitride (h-BN) nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Kek-Merl, D.; Zorko, M.; Verpoest, I. Mechanical and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Appl. Surf. Sci. 2014, 292, 432–437. [Google Scholar] [CrossRef]
- Simovich, T.; Wu, A.H.; Lamb, R.N. Hierarchically rough, mechanically durable and superhydrophobic epoxy coatings through rapid evaporation spray method. Thin Solid Films 2015, 589, 472–478. [Google Scholar] [CrossRef]
- Weng, C.-J.; Chang, C.-H.; Peng, C.-W.; Chen, S.-W.; Yeh, J.-M.; Hsu, C.-L.; Wei, Y. Advanced Anticorrosive Coatings Prepared from the Mimicked Xanthosoma Sagittifolium-leaf-like Electroactive Epoxy with Synergistic Effects of Superhydrophobicity and Redox Catalytic Capability. Chem. Mater. 2011, 23, 2075–2083. [Google Scholar] [CrossRef]
- Gao, X.-Z.; Liu, H.-J.; Cheng, F.; Chen, Y. Thermoresponsive polyaniline nanoparticles: Preparation, characterization, and their potential application in waterborne anticorrosion coatings. Chem. Eng. J. 2016, 283, 682–691. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, W.; Wang, L.; Li, S.; Zhu, T.; Liu, G. Liquid-phase exfoliated fluorographene as a two dimensional coating filler for enhanced corrosion protection performance. Corros. Sci. 2016, 103, 312–318. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Husain, E.; Narayanan, T.N.; Taha-Tijerina, J.J.; Vinod, S.; Vajtai, R.; Ajayan, P.M. Marine Corrosion Protective Coatings of Hexagonal Boron Nitride Thin Films on Stainless Steel. ACS Appl. Mater. Interfaces 2013, 5, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shon, M. Effects of multi-walled carbon nano tubes on corrosion protection of zinc rich epoxy resin coating. J. Ind. Eng. Chem. 2015, 21, 1258–1264. [Google Scholar] [CrossRef]
- Marchebois, H.; Joiret, S.; Savall, C.; Bernard, J.; Touzain, S. Characterization of zinc-rich powder coatings by EIS and Raman spectroscopy. Surf. Coat. Technol. 2002, 157, 151–161. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Marchebois, H.; Keddam, M.; Savall, C.; Bernard, J.; Touzain, S. Zinc-rich powder coatings characterisation in artificial sea water: EIS analysis of the galvanic action. Electrochim. Acta 2004, 49, 1719–1729. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Arman, S.Y.; Ramezanzadeh, B.; Farghadani, S.; Mehdipour, M.; Rajabi, A. Application of the electrochemical noise to investigate the corrosion resistance of an epoxy zinc-rich coating loaded with lamellar aluminum and micaceous iron oxide particles. Corros. Sci. 2013, 77, 118–127. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nanoparticulate zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Zuo, Y. The influence of cathodic polarization on performance of two epoxy coatings on steel. Int. J. Electrochem. Sci. 2014, 9, 6266–6280. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Sun, W.; Yang, Z.; Wang, S.; Liu, G. Effect of chemical conversion induced by self-corrosion of zinc powders on enhancing corrosion protection performance of zinc-rich coatings. Corros. Sci. 2022, 194, 109942. [Google Scholar] [CrossRef]
- Cho, S.; Chiu, T.-M.; Castaneda, H. Electrical and electrochemical behavior of a zinc-rich epoxy coating system with carbon nanotubes as a diode-like material. Electrochim. Acta 2019, 316, 189–201. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Wijesinghe, S.; Blackwood, D.J. Revisiting Corrosion Protection Mechanisms of a Steel Surface by Damaged Zinc-Rich Paints. Corrosion 2019, 75, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Shreepathi, S.; Bajaj, P.; Mallik, B.P. Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: Role of Zn content on corrosion protection mechanism. Electrochim. Acta 2010, 55, 5129–5134. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Steinsmo, U.; Bjordal, M. Zinc-rich primers—Test performance and electrochemical properties. Prog. Org. Coat. 2005, 54, 224–229. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, L.; Zhu, Q.; Wang, Z. The unique flake ZnAl alloy and OIT anti-corrosion and anti-mildew waterborne epoxy coatings. Inorg. Chem. Commun. 2023, 156, 111120. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Xie, J. Synergistic Effect between Zinc Particles and Graphene on the Anti-Corrosion Performance of Epoxy Coatings. Int. J. Electrochem. Sci. 2022, 17, 221238. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Wang, Z.; Wang, X.; Yan, J. Flake-like ZnAl alloy powder modified waterborne epoxy coatings with enhanced corrosion resistance. Prog. Org. Coat. 2023, 175, 107367. [Google Scholar] [CrossRef]
- Amirbeygi, H.; Khosravi, H.; Tohidlou, E. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar] [CrossRef]
- Aghili, M.; Yazdi, M.K.; Ranjbar, Z.; Jafari, S.H. Anticorrosion performance of electro-deposited epoxy/ amine functionalized graphene oxide nanocomposite coatings. Corros. Sci. 2021, 179, 109143. [Google Scholar] [CrossRef]
- Mostovoy, A.; Bekeshev, A.; Brudnik, S.; Yakovlev, A.; Shcherbakov, A.; Zhanturina, N.; Zhumabekova, A.; Yakovleva, E.; Tseluikin, V.; Lopukhova, M. Studying the Structure and Properties of Epoxy Composites Modified by Original and Functionalized with Hexamethylenediamine by Electrochemically Synthesized Graphene Oxide. Nanomaterials 2024, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hu, Y.; Guo, W. Enhancement of active anti-corrosion properties of waterborne epoxy resin by mussel bionic modified halloysite nanotube. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132018. [Google Scholar] [CrossRef]
- Ho, J.; Mudraboyina, B.; Spence-Elder, C.; Resendes, R.; Cunningham, M.F.; Jessop, P.G. Water-borne coatings that share the mechanism of action of oil-based coatings. Green Chem. 2018, 20, 1899–1905. [Google Scholar] [CrossRef]
- Javadi, A.; Cobaj, A.; Soucek, M.D. Chapter 12—Commercial waterborne coatings. In Handbook of Waterborne Coatings; Zarras, P., Soucek, M.D., Tiwari, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–344. [Google Scholar]
- Kim, B.K. Aqueous polyurethane dispersions. Colloid Polym. Sci. 1996, 274, 599–611. [Google Scholar] [CrossRef]
- Quites, D.; Somers, A.; Forsyth, M.; Paulis, M. Development of waterborne anticorrosive coatings by the incorporation of coumarate based corrosion inhibitors and phosphate functionalization. Prog. Org. Coat. 2023, 183, 107781. [Google Scholar] [CrossRef]
- Cui, J.; Bao, Y.; Sun, Y.; Wang, H.; Li, J. Critical factors on corrosion protective waterborne coatings containing functionalized graphene oxide: A review. Compos. Part A Appl. Sci. Manuf. 2023, 174, 107729. [Google Scholar] [CrossRef]
- Gao, X.; Yan, R.; Lv, Y.; Ma, H.; Ma, H. In situ pretreatment and self-healing smart anti-corrosion coating prepared through eco-friendly water-base epoxy resin combined with non-toxic chelating agents decorated biomass porous carbon. J. Clean. Prod. 2020, 266, 121920. [Google Scholar] [CrossRef]
- Sheng, C.; Cheng, L.; Chen, X.; Zhang, Y.; Guo, W. Synergistic effect of 2D/0D mixed graphitic carbon nitride/Fe2O3 on the excellent corrosion behavior of epoxy-based waterborne coatings. Colloid Polym. Sci. 2021, 299, 883–897. [Google Scholar] [CrossRef]
- Lan, Y.-X.; Cho, Y.-C.; Liu, W.-R.; Wong, W.-T.; Sun, C.-F.; Yeh, J.-M. Small-load rGO as partial replacement for the large amount of zinc dust in epoxy zinc-rich composites applied in heavy-duty anticorrosion coatings. Prog. Org. Coat. 2023, 175, 107332. [Google Scholar] [CrossRef]
- Lan, Y.-X.; Hu, K.-W.; Yan, M.; Liu, W.-R.; Wong, W.-T.; Sun, C.-F.; Yeh, J.-M. Synergistic Effect of Electrocatalytic Characteristics of a Redox Segment and Gas Barrier of rGO to Significantly Replace the Loading of Zinc Dust in Solvent-Based Epoxy Composites Applied for Heavy-Duty Anticorrosion Coatings. ACS Appl. Eng. Mater. 2023, 1, 955–969. [Google Scholar] [CrossRef]
- ASTM B-117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM: West Conshehoken, PA, USA, 2024.
- ASTM-D3359; Standard Test Methods for Rating Adhesion by Tape Test. ASTM: West Conshehoken, PA, USA, 2023.
- ASTM D4060; Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM: West Conshehoken, PA, USA, 2019.
- Huang, T.-C.; Su, Y.-A.; Yeh, T.-C.; Huang, H.-Y.; Wu, C.-P.; Huang, K.-Y.; Chou, Y.-C.; Yeh, J.-M.; Wei, Y. Advanced anticorrosive coatings prepared from electroactive epoxy–SiO2 hybrid nanocomposite materials. Electrochim. Acta 2011, 56, 6142–6149. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.S.; Alemany, L.B.; Lu, W.; Tour, J.M. Correction to Improved Synthesis of Graphene Oxide. ACS Nano 2018, 12, 2078. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Zhao, C.; Qian, X. One-pot hydrothermal synthesis of ZnO/RGO/ZnO@Zn sensor for sunset yellow in soft drinks. Talanta 2018, 179, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Cui, L.; Lu, X.; Mao, H.; Zhang, W.; Wei, Y. Electroactive polyimide with oligoaniline in the main chain via oxidative coupling polymerization. Eur. Polym. J. 2007, 43, 2641–2647. [Google Scholar] [CrossRef]
- Chao, D.; Lu, X.; Chen, J.; Zhao, X.; Wang, L.; Zhang, W.; Wei, Y. New method of synthesis of electroactive polyamide with amine-capped aniline pentamer in the main chain. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 477–482. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Huang, T.-C.; Lin, J.-C.; Chang, J.-H.; Lee, Y.-T.; Yeh, J.-M. Advanced environmentally friendly coatings prepared from amine-capped aniline trimer-based waterborne electroactive polyurethane. Mater. Chem. Phys. 2013, 137, 772–780. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y. Study of Reduced Graphene Oxide Preparation by Hummers’ Method and Related Characterization. J. Nanomater. 2015, 2015, 168125. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.-J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Yoon, J.H.; Yang, W.J.; Ryu, S.H. Synthesis, characterization, and surface wettability properties of amine functionalized graphene oxide films with varying amine chain lengths. J. Colloid Interface Sci. 2013, 401, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, Y.; Wang, Y.; Wang, Y.; Zhou, J.; Song, L. Highly transparent superhydrophilic graphene oxide coating for antifogging. Mater. Lett. 2016, 182, 372–375. [Google Scholar] [CrossRef]
- Xiao, Y.-K.; Ji, W.-F.; Chang, K.-S.; Hsu, K.-T.; Yeh, J.-M.; Liu, W.-R. Sandwich-structured rGO/PVDF/PU multilayer coatings for anti-corrosion application. RSC Adv. 2017, 7, 33829–33836. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Lu, W.-K.; Elsenbaumer, R.L.; Wessling, B. Corrosion protection of mild steel by coatings containing polyaniline. Synth. Met. 1995, 71, 2163–2166. [Google Scholar] [CrossRef]
- Tallman, D.E.; Pae, Y.; Bierwagen, G.P. Conducting Polymers and Corrosion: Polyaniline on Steel. Corrosion 1999, 55, 779–786. [Google Scholar] [CrossRef]
- Ghamsarizade, R.; Ramezanzadeh, B.; Mohammadloo, H.E. Chapter 12—Corrosion measurements in coatings and paintings. In Electrochemical and Analytical Techniques for Sustainable Corrosion Monitoring; Aslam, J., Verma, C., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 217–264. [Google Scholar]
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mater. 1996, 10, 391–402. [Google Scholar] [CrossRef]
- Jalili, M.; Rostami, M.; Ramezanzadeh, B. An investigation of the electrochemical action of the epoxy zinc-rich coatings containing surface modified aluminum nanoparticle. Appl. Surf. Sci. 2015, 328, 95–108. [Google Scholar] [CrossRef]
- Qi, C.; Dam-Johansen, K.; Weinell, C.E.; Bi, H.; Wu, H. Enhanced anticorrosion performance of zinc rich epoxy coatings modified with stainless steel flakes. Prog. Org. Coat. 2022, 163, 106616. [Google Scholar] [CrossRef]
- Armelin, E.; Martí, M.; Liesa, F.; Iribarren, J.I.; Alemán, C. Partial replacement of metallic zinc dust in heavy duty protective coatings by conducting polymer. Prog. Org. Coat. 2010, 69, 26–30. [Google Scholar] [CrossRef]










| Sample Code | Composition | Electrochemistry | Thickness (µm) | Adhesion Test 1 | Mass Loss of Wear Test (g) 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Epoxy (wt%) | ZD (wt%) | ACAT (wt%) | GO (wt%) | Ecorr (mV) | Icorr (µA/cm2) | PEF (%) | ||||
| CRS | - | - | - | - | −660.1 | 27.0 | - | - | - | - |
| WETC | 100 | - | - | - | −645.6 | 0.189 | 99.30 | 150 ± 1.7 | 5B | 0.0130 |
| WZ80 | 20 | 80 | - | - | −506.4 | 0.0124 | 99.95 | 150 ± 2.2 | 4B | 0.0670 |
| WG0.5Z65 | 34.5 | 65 | - | 0.5 | −511.9 | 0.0116 | 99.95 | 151 ± 2.1 | 5B | 0.0390 |
| E5WZ50 | 45 | 50 | 5 | - | −496.8 | 0.00808 | 99.97 | 147 ± 1.1 | 5B | 0.0293 |
| E5WG0.5Z35 | 59.5 | 35 | 5 | 0.5 | −510.3 | 0.00120 | 99.96 | 149 ± 1.7 | 5B | 0.0183 |
| Researcher | Compound Used to Replace ZD | Compound Ratio (wt%) | ZD Replaced (wt%) | Ref. | |
|---|---|---|---|---|---|
| 1 | Carlos Alemán (2010) | polyaniline emeraldine salt | 0.3 | 19 | [65] |
| 2 | B. Ramezanzadeh (2013) | micaceous iron oxide (MIO) | 10 | 10 | [17] |
| Al | 10 | 10 | |||
| MIO + Al | 10 (5 + 5) | 10 | |||
| 3 | B. Ramezanzadeh (2015) | modified nano-aluminum powder | 2 | 2 | [63] |
| 4 | Hao Wu (2022) | stainless steel flakes (SSFs) | 2.5 | 5 | [64] |
| 5 | 10 | ||||
| 10 | 19 | ||||
| 5 | Jui-Ming Yeh (2023) | 300 °C rGO | 0.5 | 20 | [40] |
| 1400 °C rGO | 0.5 | 30 | |||
| 6 | Jui-Ming Yeh (2023) | rGO | 0.5 | 20 | [41] |
| aniline pentamer-based diamine (DAAP) | 1 | 30 | |||
| 5 | 50 | ||||
| rGO + DAAP | 1.5 (0.5 + 1) | 50 | |||
| 5 (0.5 + 5) | 70 | ||||
| 7 | This study | GO | 0.5 | 15 | - |
| ACAT | 5 | 30 | |||
| GO + ACAT | 5.5 (0.5 + 5) | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.-X.; Chen, Y.-H.; Chao, Y.-L.; Chang, Y.-H.; Huang, Y.-C.; Liu, W.-R.; Wong, W.-T.; Sun, A.C.-F.; Santiago, K.S.; Yeh, J.-M. Green and Heavy-Duty Anticorrosion Coatings: Waterborne Epoxy Thermoset Composites Modified through Variation of Zinc Dust Loading and Incorporation of Amine-Capped Aniline Trimer and Graphene Oxide. Polymers 2024, 16, 1252. https://doi.org/10.3390/polym16091252
Lan Y-X, Chen Y-H, Chao Y-L, Chang Y-H, Huang Y-C, Liu W-R, Wong W-T, Sun AC-F, Santiago KS, Yeh J-M. Green and Heavy-Duty Anticorrosion Coatings: Waterborne Epoxy Thermoset Composites Modified through Variation of Zinc Dust Loading and Incorporation of Amine-Capped Aniline Trimer and Graphene Oxide. Polymers. 2024; 16(9):1252. https://doi.org/10.3390/polym16091252
Chicago/Turabian StyleLan, Yun-Xiang, Yun-Hsuan Chen, Ying-Lung Chao, Yu-Hsuan Chang, Yu-Chi Huang, Wei-Ren Liu, Wei-Tsan Wong, Andrew Chi-Fa Sun, Karen S. Santiago, and Jui-Ming Yeh. 2024. "Green and Heavy-Duty Anticorrosion Coatings: Waterborne Epoxy Thermoset Composites Modified through Variation of Zinc Dust Loading and Incorporation of Amine-Capped Aniline Trimer and Graphene Oxide" Polymers 16, no. 9: 1252. https://doi.org/10.3390/polym16091252
APA StyleLan, Y.-X., Chen, Y.-H., Chao, Y.-L., Chang, Y.-H., Huang, Y.-C., Liu, W.-R., Wong, W.-T., Sun, A. C.-F., Santiago, K. S., & Yeh, J.-M. (2024). Green and Heavy-Duty Anticorrosion Coatings: Waterborne Epoxy Thermoset Composites Modified through Variation of Zinc Dust Loading and Incorporation of Amine-Capped Aniline Trimer and Graphene Oxide. Polymers, 16(9), 1252. https://doi.org/10.3390/polym16091252