Advances in the Production of Sustainable Bacterial Nanocellulose from Banana Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of the Physical and Chemical Properties of Banana Midrib Juice, Green Tea Infusion, and SCOBY
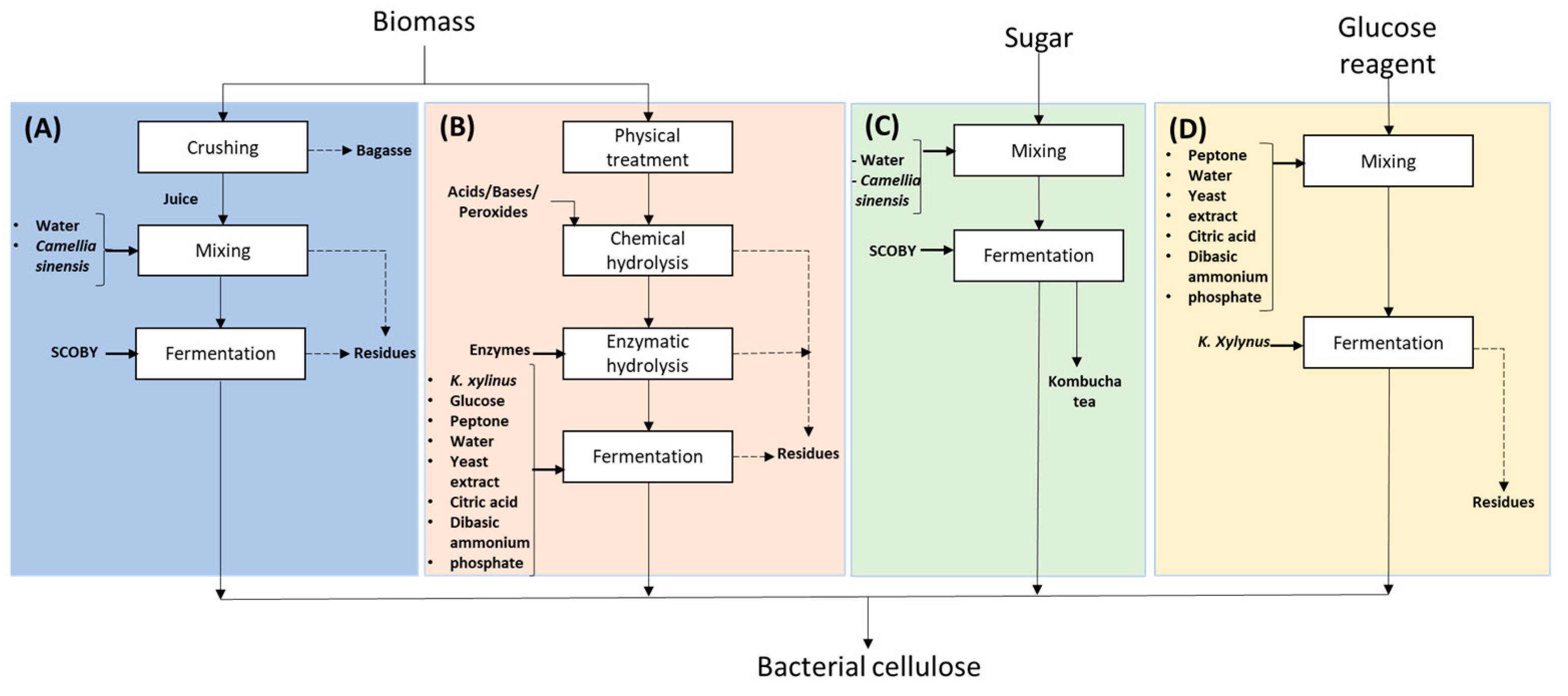
2.3. Experimental Design for Bacterial Nanocellulose Production
Culture Medium Optimization
2.4. Production of Bacterial Nanocellulose Using Optimal Values
2.4.1. Scanning Electron Microscopy
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. Thermogravimetric Analysis
2.4.4. X-ray Diffraction
3. Results and Discussion
3.1. Physical and Chemical Characteristics of the Components Utilized in the Preparation of the Culture Medium
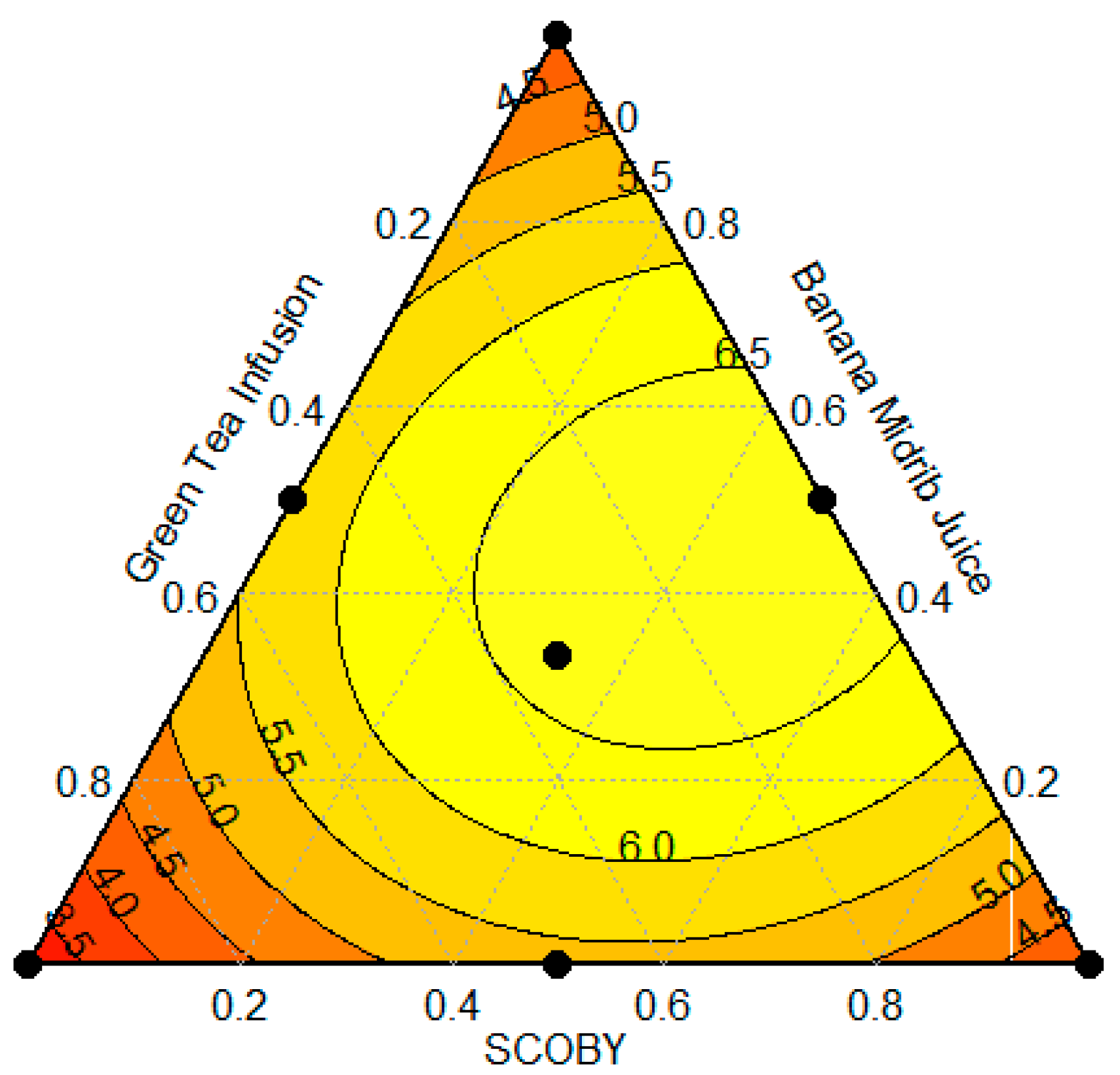
3.2. Optimized Culture Medium Using Simplex-Centroid Design
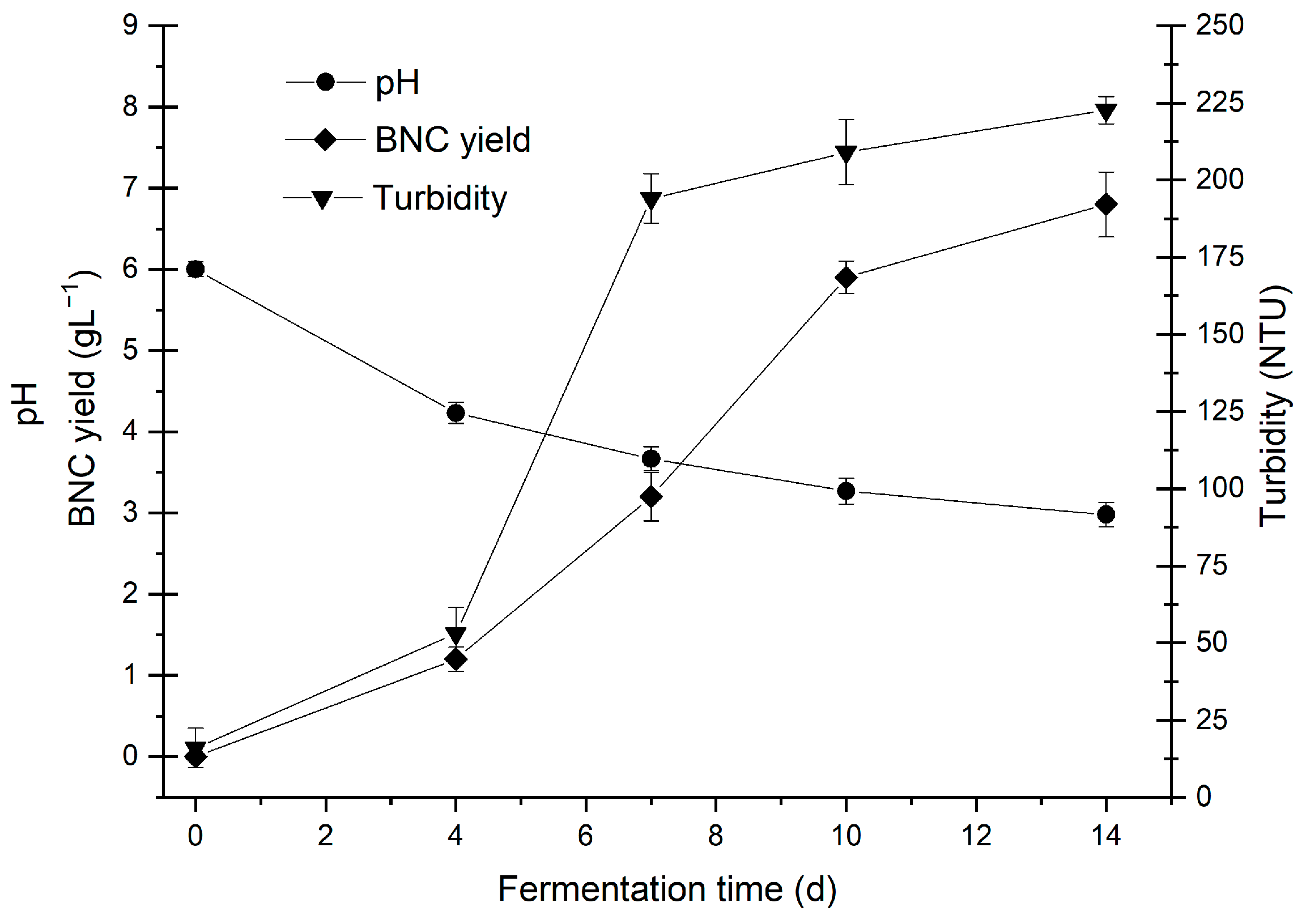
3.3. Kinetics of BNC Production Using Optimal Values
3.4. Physical and Chemical Properties of Bacterial Nanocellulose
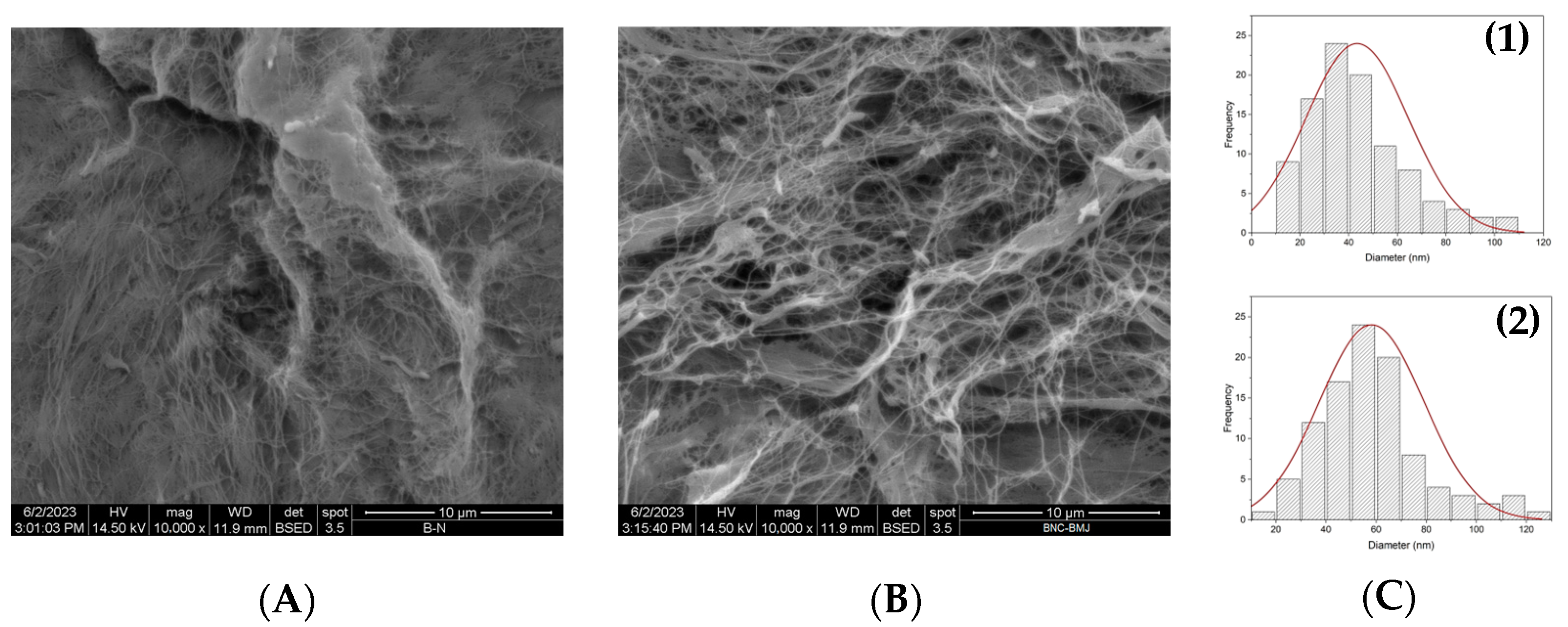
3.4.1. SEM Analysis
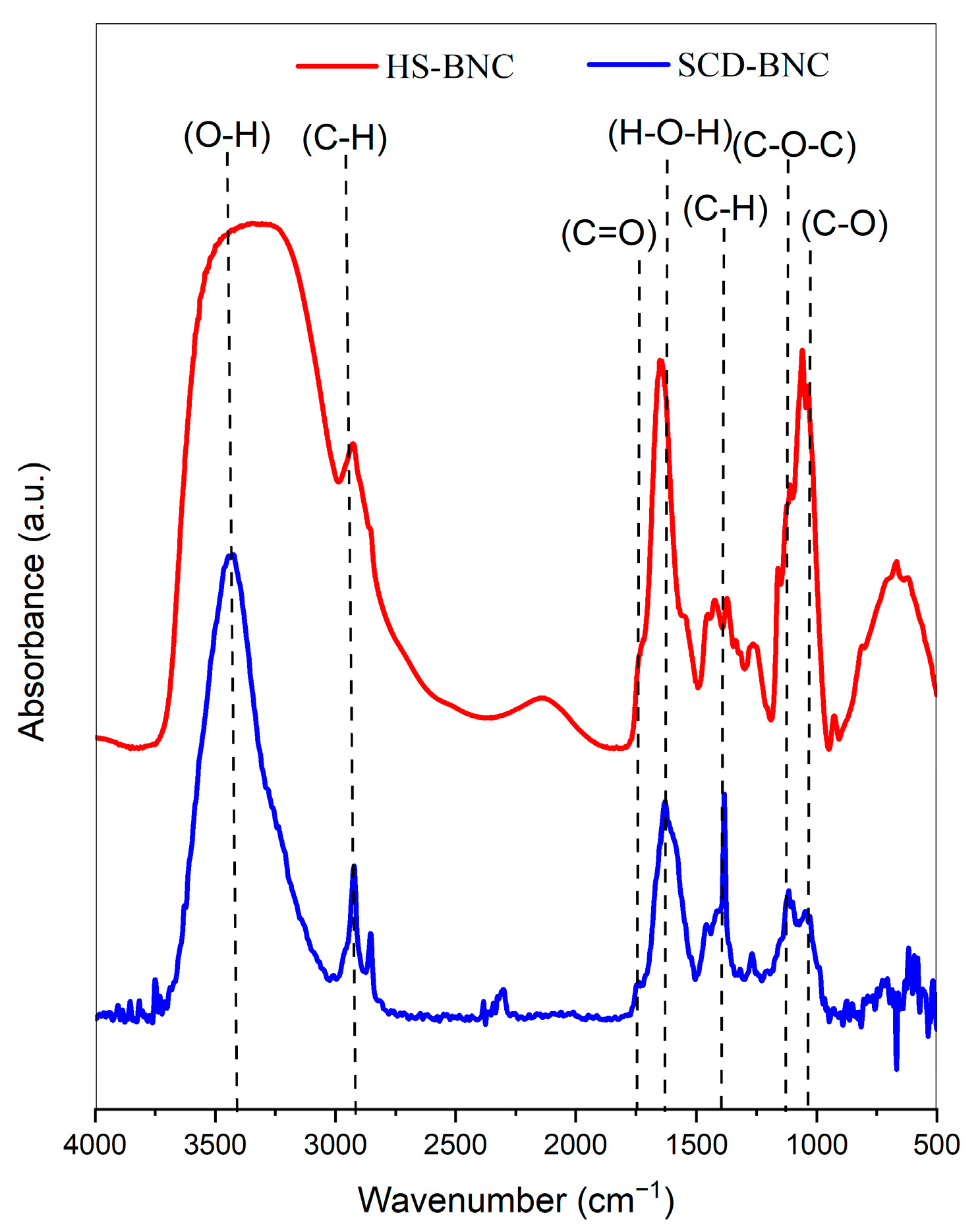
3.4.2. FTIR Analysis
3.4.3. TGA Analysis
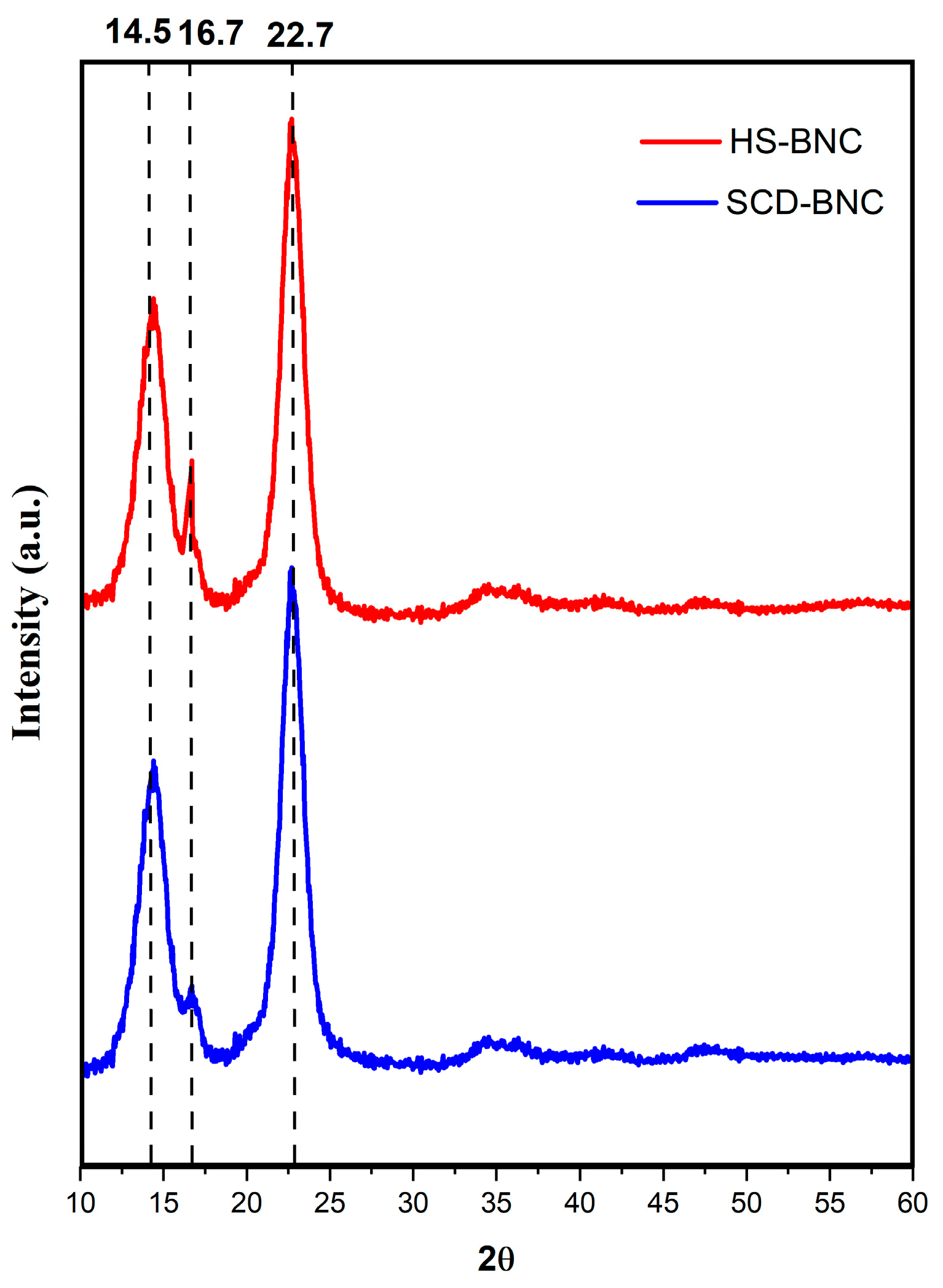
3.4.4. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Zhang, T.; Shen, Y.; Han, L.; Peng, Z.; Xie, Z.; Zhong, C.; Jia, S. Bacterial cellulose production from ethylenediamine pretreated Caragana korshinskii Kom. In Industrial Crops and Products; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 164. [Google Scholar] [CrossRef]
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr. Polym. 2023, 302, 120383. [Google Scholar] [CrossRef] [PubMed]
- Gromovykh, T.I.; Sadykova, V.S.; Lutcenko, S.V.; Dmitrenok, A.S.; Feldman, N.B.; Danilchuk, T.N.; Kashirin, V.V. Bacterial cellulose synthesized by Gluconacetobacter hansenii for medical applications. Appl. Biochem. Microbiol. 2017, 53, 60–67. [Google Scholar] [CrossRef]
- Saleh, A.K.; El-Gendi, H.; El-Fakharany, E.M.; Owda, M.E.; Awad, M.A.; Kamoun, E.A. Exploitation of cantaloupe peels for bacterial cellulose production and functionalization with green synthesized Copper oxide nanoparticles for diverse biological applications. Sci. Rep. 2022, 12, 19241. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial cellulose films: Influence of bacterial strain and drying route on film properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef]
- da Gama, F.M.P.; Dourado, F.; NanoCellulose, B. Bacterial NanoCellulose: What future? Bioimpacts 2018, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; El-Malkey, S.E.; Abu-Saied, M.A.; Mohammed, A.B.A. Exploration of a novel and efficient source for production of bacterial nanocellulose, bioprocess optimization and characterization. Sci. Rep. 2022, 12, 18533. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Liu, H.; Huang, X.; Xu, B.; Zhong, C.; Jia, S. Bioconversion of lignocellulosic biomass into bacterial nanocellulose: Challenges and perspectives. Green Chem. Eng. 2023, 4, 160–172. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. Available online: https://www.frontiersin.org/articles/10.3389/fsufs.2019.00007 (accessed on 14 February 2024). [CrossRef]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.E.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, N.; Panthapulakkal, S.; Sain, M.; Dalai, A.K. Production of nanocellulose fibers from pinecone biomass: Evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crops Prod. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Hafemann, E.; Battisti, R.; Marangoni, C.; Machado, R.A.F. Valorization of royal palm tree agroindustrial waste by isolating cellulose nanocrystals. Carbohydr. Polym. 2019, 218, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Pola, L.; Collado, S.; Oulego, P.; Díaz, M. Kraft black liquor as a renewable source of value-added chemicals. Chem. Eng. J. 2022, 448, 137728. [Google Scholar] [CrossRef]
- Feng, X.; Ge, Z.; Wang, Y.; Xia, X.; Zhao, B.; Dong, M. Production and characterization of bacterial cellulose from kombucha-fermented soy whey. Food Prod. Process. Nutr. 2024, 6, 20. [Google Scholar] [CrossRef]
- Tan, J.S.; Phapugrangkul, P.; Lee, C.K.; Lai, Z.-W.; Bakar, M.H.A.; Murugan, P. Banana frond juice as novel fermentation substrate for bioethanol production by Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2019, 21, 101293. [Google Scholar] [CrossRef]
- Fiallos-Cárdenas, M.; Ramirez, A.D.; Pérez-Martínez, S.; Bonilla, H.R.; Ordoñez-Viñan, M.; Ruiz-Barzola, O.; Reinoso, M.A. Bacterial Nanocellulose Derived from Banana Leaf Extract: Yield and Variation Factors. Resources 2021, 10, 121. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from agricultural Residues by two Komagataeibacter sp. Strains. Bioengineered 2022, 13, 10010–10025. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.D.P.; Costa, A.F.S.; Galdino, C.J.S.; Vinhas, G.M.; Santos, E.M.S.; Sarubbo, L.A. Bacterial cellulose production using fruit residues as substract to industrial application. Chem. Eng. Trans. 2019, 74, 1165–1170. [Google Scholar] [CrossRef]
- Lee, J.; An, H.-E.; Lee, K.H.; Kim, S.; Park, C.; Kim, C.-B.; Yoo, H.Y. Identification of Gluconacetobacter xylinus LYP25 and application to bacterial cellulose production in biomass hydrolysate with acetic acid. Int. J. Biol. Macromol. 2024, 261, 129597. [Google Scholar] [CrossRef] [PubMed]
- Anguluri, K.; La China, S.; Brugnoli, M.; Cassanelli, S.; Gullo, M. Better under stress: Improving bacterial cellulose production by Komagataeibacter xylinus K2G30 (UMCC 2756) using adaptive laboratory evolution. Front. Microbiol. 2022, 13, 994097. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, R.S.; Anandham, R.; Kumutha, K.; Gayathry, G.; Mageshwaran, V.; Uthandi, S. Characterization and optimization of bacterial cellulose produced by Acetobacter spp. J. Environ. Biol. 2020, 41, 207–215. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, J.-J. Improvement of bacterial cellulose fermentation by metabolic perturbation with mixed carbon sources. Process Biochem. 2022, 122, 95–102. [Google Scholar] [CrossRef]
- Chandana, A.; Mallick, S.P.; Dikshit, P.K.; Singh, B.N.; Sahi, A.K. Recent Developments in Bacterial Nanocellulose Production and its Biomedical Applications. J. Polym. Environ. 2022, 30, 4040–4067. [Google Scholar] [CrossRef]
- Öztürk, B.E.T.; Eroğlu, B.; Delik, E.; Çiçek, M.; Çiçek, E. Comprehensive Evaluation of Three Important Herbs for Kombucha Fermentation. Food Technol. Biotechnol. 2023, 61, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.N.Y.; Surek, M.; Sartori, D.; Serrato, R.V.; Spinosa, W.A. Role of Acetic Acid Bacteria in Food and Beverages. Food Technol. Biotechnol. 2023, 61, 85–103. [Google Scholar] [CrossRef]
- Halib, N.; Amin, M.C.I.M. Physicochemical Properties and Characterization of Nata de Coco from Local Food Industries as a Source of Cellulose. Sifat Fizikokimia dan Pencirian Nata de Coco daripada Industri Makanan Tempatan Sebagai Sumber Selulosa. 2012. Available online: https://www.ukm.my/jsm/pdf_files/SM-PDF-41-2-2012/08%20Nadia.pdf (accessed on 30 July 2023).
- Zhang, J.; Yang, Y.; Deng, J.; Wang, Y.; Hu, Q.; Li, C.; Liu, S. Dynamic profile of the microbiota during coconut water pre-fermentation for nata de coco production. LWT-Food Sci. Technol. 2017, 81, 87–93. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic Biomass Valorization for Bioethanol Production: A Circular Bioeconomy Approach. BioEnergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, S.; Gao, F.; Wang, Y.; Taherzadeh, M.J.; Wang, Y.; Qin, X.; Wang, X.; Luo, H.; Yao, B.; et al. Exploring the cellulolytic and hemicellulolytic activities of manganese peroxidase for lignocellulose deconstruction. Biotechnol. Biofuels Bioprod. 2023, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef]
- Chaiyachet, O.A.; Thongmoon, S.; Udomchai, T. Utilization of Yam Bean Juice as Nutrient Source for Bacterial Cellulose Production by Komagataeibacter nataicola TISTR 975. Curr. Nutr. Food Sci. 2023, 19, 564–571. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kaewkod, T.; Sangboonruang, S.; Khacha-Ananda, S.; Charoenrak, S.; Bovonsombut, S.; Tragoolpua, Y. Combinations of traditional kombucha tea with medicinal plant extracts for enhancement of beneficial substances and activation of apoptosis signaling pathways in colorectal cancer cells. Food Sci. Technol. Braz. 2022, 42, e107521. [Google Scholar] [CrossRef]
- Neves, E.Z.; Kumineck Junior, S.R.; Katrucha, G.P.; Silveira, V.F.; Silveira ML, L.; Pezzin AP, T.; dos Santos Schneider, A.L.; Garcia MC, F.; Apati, G.P. Development of Bacterial Cellulose Membranes Incorporated with Plant Extracts. Macromol. Symp. 2022, 406, 2200031. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Bekatorou, A.; Plioni, I.; Sparou, K.; Maroutsiou, R.; Tsafrakidou, P.; Petsi, T.; Kordouli, E. Bacterial Cellulose Production Using the Corinthian Currant Finishing Side-Stream and Cheese Whey: Process Optimization and Textural Characterization. Foods 2019, 8, 193. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016, 133, 14. [Google Scholar] [CrossRef]
- Öz, Y.E.; Kalender, M. Optimization Of Bacterial Cellulose Production From Sugar Beet Molasses By Gluconacetobacter Xylinus Nrrl B-759 In Static Culture. Cellul. Chem. Technol. 2021, 55, 1051–1060. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Fontão, A.I.; Coelho, A.; Leal, M.; da Silva, F.A.S.; Wan, Y.; Dourado, F.; Gama, M. Response surface statistical optimization of bacterial nanocellulose fermentation in static culture using a low-cost medium. New Biotechnol. 2019, 49, 19–27. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Yang, H.; Liu, C.; Zhang, Z.; Chen, G. Biodegradable polyhydroxyalkanoates production from wheat straw by recombinant Halomonas elongata A1. Int. J. Biol. Macromol. 2021, 187, 675–682. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef]
- Galvan, D.; Effting, L.; Cremasco, H.; Conte-Junior, C.A. Recent Applications of Mixture Designs in Beverages, Foods, and Pharmaceutical Health: A Systematic Review and Meta-Analysis. Foods 2021, 10, 1941. [Google Scholar] [CrossRef]
- Lawson, J.; Willden, C. Mixture Experiments in R Using mixexp. J. Stat. Softw. 2016, 72, 1–20. [Google Scholar] [CrossRef]
- Cini, J.R.D.M.; Borsato, D.; Guedes, C.L.B.; da Silva, H.C.; Coppo, R.L. Comparison of methods for determination of oxidative stability of B100 biodiesel mixed with synthetic antioxidants: Application of simplex-centroid design with process variable. Quím. Nova 2013, 36, 79–84. [Google Scholar] [CrossRef][Green Version]
- Correia, I.; Borsato, D.; Savada, F.; Pauli, E.; Mantovani, A.; Cremasco, H.; Chendynski, L. Inhibition of the biodiesel oxidation by alcoholic extracts of green and black tea leaves and plum pulp: Application of the simplex-centroid design. Renew. Energy 2020, 160, 288–296. [Google Scholar] [CrossRef]
- Pauli, E.D.; Malta, G.B.; Sanchez, P.M.; Moreira, I.C.; Scarminio, I.S. Mixture design analysis of solvent extractor effects on epicatechin, epigallocatechin gallate, epigallocatechin and antioxidant activities of the Camellia sinensis L. leaves. Anal. Chem. Res. 2014, 2, 23–29. [Google Scholar] [CrossRef]
- Squeo, G.; De Angelis, D.; Leardi, R.; Summo, C.; Caponio, F. Background, Applications and Issues of the Experimental Designs for Mixture in the Food Sector. Foods 2021, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J.A. A Primer on Experiments with Mixtures; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 351. [Google Scholar] [CrossRef]
- Filho, R.C.N.; Galvan, D.; Effting, L.; Terhaag, M.M.; Yamashita, F.; Benassi, M.D.T.; Spinosa, W.A. Effects of adding spices with antioxidants compounds in red ale style craft beer: A simplex-centroid mixture design approach. Food Chem. 2021, 365, 130478. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Saha, C.K.; Ward, A.J.; Møller, H.B.; Alam, M.M. Anaerobic co-digestions of agro-industrial waste blends using mixture design. Biomass Bioenergy 2019, 122, 156–164. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Huo, X.; Ali, S.; Sun, J.; Qin, J.; Liang, K.; Liu, Y. Early hydration and compressive strength of steam cured high-strength concrete based on simplex centroid design method. Case Stud. Constr. Mater. 2022, 17, e01583. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Sha, F.; He, S.; Lu, G.; Yu, Z.; Chen, H. Preparation and performance of the ultra-high performance mortar based on simplex-centroid design method. J. Mater. Res. Technol. 2021, 15, 3060–3077. [Google Scholar] [CrossRef]
- Jiao, D.; Shi, C.; Yuan, Q.; An, X.; Liu, Y. Mixture design of concrete using simplex centroid design method. Cem. Concr. Compos. 2018, 89, 76–88. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. ACS Publications. Available online: https://pubs.acs.org/doi/pdf/10.1021/ac60147a030 (accessed on 14 November 2023).
- Liu, X.; Cao, L.; Wang, S.; Huang, L.; Zhang, Y.; Tian, M.; Li, X.; Zhang, J. Isolation and characterization of bacterial cellulose produced from soybean whey and soybean hydrolyzate. Sci. Rep. 2023, 13, 16024. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, Q.Q.; Ji, K.H.; Zeng, B.; Li, G.Q. Homogeneous and porous modified bacterial cellulose achieved by in situ modification with low amounts of carboxymethyl cellulose. Cellulose 2014, 21, 2637–2646. [Google Scholar] [CrossRef]
- Mago, M.; Yadav, A.; Gupta, R.; Garg, V.K. Management of banana crop waste biomass using vermicomposting technology. Bioresour. Technol. 2021, 326, 124742. [Google Scholar] [CrossRef]
- Fiallos-Cárdenas, M.; Pérez-Martínez, S.; Ramirez, A.D. Prospectives for the development of a circular bioeconomy around the banana value chain. Sustain. Prod. Consum. 2022, 30, 541–555. [Google Scholar] [CrossRef]
- Bai, F.; Chen, G.; Niu, H.; Zhu, H.; Huang, Y.; Zhao, M.; Hou, R.; Peng, C.; Li, H.; Wan, X.; et al. The types of brewing water affect tea infusion flavor by changing the tea mineral dissolution. Food Chem. X 2023, 18, 100681. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, K.-G.; Kim, M.K. Volatile and non-volatile compounds in green tea affected in harvesting time and their correlation to consumer preference. J. Food Sci. Technol. 2016, 53, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Ojukwu, M.; Lee, L.-X.; Easa, A.M. Quality characteristics of green Tea’s infusion as influenced by brands and types of brewing water. Heliyon 2023, 9, 2. [Google Scholar] [CrossRef]
- Costa, M.A.D.C.; Moreira, L.D.P.D.; Duarte, V.D.S.; Cardoso, R.R.; José, V.P.B.D.S.; da Silva, B.P.; Grancieri, M.; Corich, V.; Giacomini, A.; Bressan, J.; et al. Kombuchas from Green and Black Tea Modulate the Gut Microbiota and Improve the Intestinal Health of Wistar Rats Fed a High-Fat High-Fructose Diet. Nutrients 2022, 14, 5234. [Google Scholar] [CrossRef]
- Ulusoy, A.; Tamer, C.E. Determination of suitability of black carrot (Daucus carota L. spp. sativus var. atrorubens Alef.) juice concentrate, cherry laurel (Prunus laurocerasus), blackthorn (Prunus spinosa) and red raspberry (Rubus ideaus) for kombucha beverage production. J. Food Meas. Charact. 2019, 13, 1524–1536. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Lin, Q.; Jin, T.; Zang, X.; Yun, T.; Ding, Z.; Rekaby, S.A.; Zhao, Z.; Eissa, M.A. Physio-biochemical evaluation of Si-rich biochar amendment to improve the salt stress tolerance of Grand Nain and Williams banana genotypes. Ind. Crops Prod. 2023, 204, 117333. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Marie-Magdeleine, C.; Boval, M.; Philibert, L.; Borde, A.; Archimède, H. Effect of banana foliage (Musa x paradisiaca) on nutrition, parasite infection and growth of lambs. Livest. Sci. 2010, 131, 234–239. [Google Scholar] [CrossRef]
- Lucaciu, L.A.; Seicean, R.; Seicean, A. Small molecule drugs in the treatment of inflammatory bowel diseases: Which one, when and why?—A systematic review. Eur. J. Gastroenterol. Hepatol. 2020, 32, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Nurcholis, W.; Marliani, N.; Asyhar, R.; Minarni, M. Optimized Solvents for the Maceration of Phenolic Antioxidants from Curcuma xanthorrhiza Rhizome using a Simplex Centroid Design. J. Pharm. Bioallied Sci. 2023, 15, 35. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Dartora, B.; Hickert, L.R.; Fabricio, M.F.; Ayub, M.A.Z.; Furlan, J.M.; Wagner, R.; Perez, K.J.; Sant’Anna, V. Understanding the effect of fermentation time on physicochemical characteristics, sensory attributes, and volatile compounds in green tea kombucha. Food Res. Int. 2023, 174, 113569. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Aditiawati, P.; Taufik, I.; Alexis, J.J.G.; Dungani, R. Bacterial Nanocellulose from Symbiotic Culture of Bacteria and Yeast Kombucha Prepared with Lemongrass Tea and Sucrose: Optimization and Characterization. BioResources 2023, 18, 3178–3197. [Google Scholar] [CrossRef]
- Hatiboruah, D.; Devi, D.Y.; Namsa, N.D.; Nath, P. Turbidimetric analysis of growth kinetics of bacteria in the laboratory environment using smartphone. J. Biophotonics 2020, 13, e201960159. [Google Scholar] [CrossRef] [PubMed]
- Leonarski, E.; Cesca, K.; Borges, O.M.A.; de Oliveira, D.; Poletto, P. Typical kombucha fermentation: Kinetic evaluation of beverage and morphological characterization of bacterial cellulose. J. Food Process. Preserv. 2021, 45, e16100. [Google Scholar] [CrossRef]
- Hamed, D.A.; Maghrawy, H.H.; Kareem, H.A. Biosynthesis of bacterial cellulose nanofibrils in black tea media by a symbiotic culture of bacteria and yeast isolated from commercial kombucha beverage. World J. Microbiol. Biotechnol. 2022, 39, 48. [Google Scholar] [CrossRef] [PubMed]
- Skiba, E.A.; Shavyrkina, N.A.; Skiba, M.A.; Mironova, G.F.; Budaeva, V.V. Biosynthesis of Bacterial Nanocellulose from Low-Cost Cellulosic Feedstocks: Effect of Microbial Producer. Int. J. Mol. Sci. 2023, 24, 14401. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Azi, F.; Ge, Z.-W.; Liu, Y.-F.; Yin, X.-T.; Dong, M.-S. Bio-conversion of kitchen waste into bacterial cellulose using a new multiple carbon utilizing Komagataeibacter rhaeticus: Fermentation profiles and genome-wide analysis. Int. J. Biol. Macromol. 2021, 191, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Khattak, W.A.; Khan, T.; Ul-Islam, M.; Wahid, F.; Park, J.K. Production, Characterization and Physico-mechanical Properties of Bacterial Cellulose from Industrial Wastes. J. Polym. Environ. 2015, 23, 45–53. [Google Scholar] [CrossRef]
- Khattak, W.A.; Khan, T.; Ul-Islam, M.; Wahid, F.; Park, J.K. Production, characterization and biological features of bacterial cellulose from scum obtained during preparation of sugarcane jaggery (gur). J. Food Sci. Technol. 2015, 52, 8343–8349. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Pacheco, G.; Nogueira, C.R.; Meneguin, A.B.; Trovatti, E.; Silva, M.C.C.; Machado, R.T.A.; Ribeiro, S.J.L.; da Silva Filho, E.C.; Barud, H.D.S. Development and characterization of bacterial cellulose produced by cashew tree residues as alternative carbon source. Ind. Crops Prod. 2017, 107, 13–19. [Google Scholar] [CrossRef]
- Taokaew, S.; Seetabhawang, S.; Siripong, P.; Phisalaphong, M. Biosynthesis and Characterization of Nanocellulose-Gelatin Films. Materials 2013, 6, 782. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Masruchin, N.; Rusmana, D.; Chalid, M. Preparation and characterization of Arenga pinnata thermoplastic starch/bacterial cellulose nanofiber biocomposites via in-situ twin screw extrusion. Int. J. Biol. Macromol. 2024, 261, 129792. [Google Scholar] [CrossRef] [PubMed]
- El-Shall, F.N.; Al-Shemy, M.T.; Dawwam, G.E. Multifunction smart nanocomposite film for food packaging based on carboxymethyl cellulose/Kombucha SCOBY/pomegranate anthocyanin pigment. Int. J. Biol. Macromol. 2023, 242, 125101. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Ul-Islam, M.; Kamal, T.; Ahmad, W.; Abbas, Y.; Manan, S.; Ullah, M.W.; Yang, G. Ex situ development and characterization of green antibacterial bacterial cellulose-based composites for potential biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 307–321. [Google Scholar] [CrossRef]
- Thorat, M.N.; Dastager, S.G. High yield production of cellulose by a: Komagataeibacter rhaeticus PG2 strain isolated from pomegranate as a new host. RSC Adv. 2018, 8, 29797–29805. [Google Scholar] [CrossRef] [PubMed]
- Jiamsawat, C.; Wasanapiarnpong, T.; Srikulkit, K. Improving carbon yield of bacterial cellulose derived carbon by phosphorus/nitrogen doping. Mater. Today Commun. 2022, 33, 104382. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Saha, N.; Ngwabebhoh, F.A.; Zandraa, O.; Saha, T.; Saha, P. Kombucha-derived bacterial cellulose from diverse wastes: A prudent leather alternative. Cellulose 2021, 28, 9335–9353. [Google Scholar] [CrossRef]
- Pereira, E.H.D.S.; Attallah, O.A.; Tas, C.E.; Chee, B.S.; Freitas, F.; Garcia, E.L.; Mc Auliffe, M.A.; Mojicevic, M.; Batista, M.N.; Reis, M.A.; et al. Boosting bacterial nanocellulose production from chemically recycled post-consumer polyethylene terephthalate. Sustain. Mater. Technol. 2024, 39, e00784. [Google Scholar] [CrossRef]
- Amin, M.Z.; Islam, T.; Mostofa, F.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.). Heliyon 2019, 5, e02994. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef]
- Öz, Y.E.; Kalender, M. A novel static cultivation of bacterial cellulose production from sugar beet molasses: Series static culture (SSC) system. Int. J. Biol. Macromol. 2023, 225, 1306–1314. [Google Scholar] [CrossRef]
- Machado, R.T.; Meneguin, A.B.; Sábio, R.M.; Franco, D.F.; Antonio, S.G.; Gutierrez, J.; Tercjak, A.; Berretta, A.A.; Ribeiro, S.J.; Lazarini, S.C.; et al. Komagataeibacter rhaeticus grown in sugarcane molasses-supplemented culture medium as a strategy for enhancing bacterial cellulose production. Ind. Crops Prod. 2018, 122, 637–646. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, Y.-H.; Hsu, K.-D.; Lai, Y.-J.; Chen, Y.-K.; Cheng, K.-C. Isolation and identification of cellulose-producing strain Komagataeibacter intermedius from fermented fruit juice. Carbohydr. Polym. 2016, 151, 827–833. [Google Scholar] [CrossRef]
- Naeem, M.A.; Siddiqui, Q.; Khan, M.R.; Mushtaq, M.; Wasim, M.; Farooq, A.; Naveed, T.; Wei, Q. Bacterial cellulose-natural fiber composites produced by fibers extracted from banana peel waste. J. Ind. Text. 2022, 51, 990S–1006S. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Chen, Y.; Wang, J. Enhanced bacterial cellulose production in Gluconacetobacter xylinus by overexpression of two genes (bscC and bcsD) and a modified static culture. Int. J. Biol. Macromol. 2024, 260, 129552. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhao, F.; Peng, Q.; Zhou, Z.; Han, Y. Production and characterization of bacterial cellulose produced by Gluconacetobacter xylinus isolated from Chinese persimmon vinegar. Carbohydr. Polym. 2018, 194, 200–207. [Google Scholar] [CrossRef]
- Gomes, F.P.; Silva, N.H.C.S.; Trovatti, E.; Serafim, L.S.; Duarte, M.F.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenergy 2013, 55, 205–211. [Google Scholar] [CrossRef]
- French, A.D.; Cintrón, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Jung, H.-I.; Lee, O.-M.; Jeong, J.-H.; Jeon, Y.-D.; Park, K.-H.; Kim, H.-S.; An, W.-G.; Son, H.-J. Production and Characterization of Cellulose by Acetobacter sp. V6 Using a Cost-Effective Molasses–Corn Steep Liquor Medium. Appl. Biochem. Biotechnol. 2010, 162, 486–497. [Google Scholar] [CrossRef]
- Leonarski, E.; Martini, G.V.; Cesca, K.; SILVA, M.F.D.A.; Goldbeck, R.; Poletto, P. Enzymatic upcycling of bacterial cellulose from kombucha to obtain cellobiose. Cellul. Chem. Technol. 2023, 57, 125–132. [Google Scholar] [CrossRef]

| Parameters | BMJ | GTI | SCOBY |
|---|---|---|---|
| pH | 5.64 ± 0.02 | 5.37 ± 0.02 | 3.20 ± 0.02 |
| Density (g/mL) | 1.02 ± 5.0 × 10−3 | 1.01 ± 5.0 × 10−3 | 1.05 ± 5.0 × 10−3 |
| °Brix | 4.000 ± 0.32 | 1.33 ± 0.32 | 12.83 ± 0.32 |
| Reducing sugars (g/L) | 15.97 ± 0.49 | 0.00 ± 0.00 | 5.67 ± 0.49 |
| Conductivity (µS/cm) | 16.57 ± 5.70 a | 39.40 ± 5.70 a | 75.13 ± 5.70 |
| Antioxidant activity (DPPH) (%) | 71.00 ± 0.11 | 70.27 ± 0.11 | 16.18 ± 0.11 |
| Trolox (µM) | 45.07 ± 0.58 b | 45.47 ± 0.58 b | 8.17 ± 0.58 |
| L* | 27.55 ± 0.13 | 92.23 ± 0.13 | 90.97 ± 0.13 |
| a* | 0.49 ± 0.15 | −5.24 ± 0.15 | −0.42 ± 0.15 |
| b* | −0.35 ± 0.32 | 34.48 ± 0.32 | 15.33 ± 0.32 |
| chroma | 0.62 ± 0.16 | 27.43 ± 0.16 | 15.13 ± 0.16 |
| Hue | 324.03 ± 0.32 | 210.33 ± 0.32 | 90.50 ± 0.32 |
| Treatment | BMJ | GTI | SCOBY | Yield (g L−1) |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 4.17 ± 0.10 |
| 2 | 0 | 1 | 0 | 3.25 ± 0.08 |
| 3 | 0 | 0 | 1 | 4.13 ± 0.12 |
| 4 | 0.5 | 0.5 | 0 | 4.75 ± 0.15 |
| 5 | 0.5 | 0 | 0.5 | 6.17 ± 0.11 |
| 6 | 0 | 0.5 | 0.5 | 5.12 ± 0.09 |
| 7 | 0.33 | 0.33 | 0.33 | 7.13 ± 0.05 |
| 8 | 0.33 | 0.33 | 0.33 | 7.10 ± 0.10 |
| 9 | 0.33 | 0.33 | 0.33 | 6.94 ± 0.13 |
| Factors | Coefficient | Std. Error | t-Value | p-Value | |
|---|---|---|---|---|---|
| x1 | 4.170 | 0.1018 | 40.973 | <0.001 | Very Significant |
| x2 | 3.250 | 0.1018 | 31.933 | <0.001 | Very Significant |
| x3 | 4.130 | 0.1018 | 40.580 | <0.001 | Very Significant |
| x1:x2 | 4.160 | 0.4986 | 8.344 | <0.05 | Low Significant |
| x1:x3 | 8.080 | 0.4986 | 16.206 | <0.01 | Significant |
| x2:x3 | 5.720 | 0.4986 | 11.472 | <0.01 | Significant |
| x1:x2:x3 | 32.722 | 2.6968 | 12.133 | <0.01 | Significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dáger-López, D.; Chenché, Ó.; Ricaurte-Párraga, R.; Núñez-Rodríguez, P.; Bajaña, J.M.; Fiallos-Cárdenas, M. Advances in the Production of Sustainable Bacterial Nanocellulose from Banana Leaves. Polymers 2024, 16, 1157. https://doi.org/10.3390/polym16081157
Dáger-López D, Chenché Ó, Ricaurte-Párraga R, Núñez-Rodríguez P, Bajaña JM, Fiallos-Cárdenas M. Advances in the Production of Sustainable Bacterial Nanocellulose from Banana Leaves. Polymers. 2024; 16(8):1157. https://doi.org/10.3390/polym16081157
Chicago/Turabian StyleDáger-López, David, Óscar Chenché, Rayner Ricaurte-Párraga, Pablo Núñez-Rodríguez, Joaquin Morán Bajaña, and Manuel Fiallos-Cárdenas. 2024. "Advances in the Production of Sustainable Bacterial Nanocellulose from Banana Leaves" Polymers 16, no. 8: 1157. https://doi.org/10.3390/polym16081157
APA StyleDáger-López, D., Chenché, Ó., Ricaurte-Párraga, R., Núñez-Rodríguez, P., Bajaña, J. M., & Fiallos-Cárdenas, M. (2024). Advances in the Production of Sustainable Bacterial Nanocellulose from Banana Leaves. Polymers, 16(8), 1157. https://doi.org/10.3390/polym16081157








