Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pectin Extraction
2.2.1. Cold-Water Extraction (CW)
2.2.2. Hot-Water Extraction (HW)
2.2.3. Ultrasonic-Assisted Extraction (US)
2.2.4. Enzyme-Assisted Extraction (EZX)
2.3. Color Analysis
2.4. Monosaccharide Composition of Pectins
2.5. Molecular Weight of Pectins
2.6. FTIR Structural Analysis and DE Quantification
2.7. Viscosity of Pectin Solutions
2.8. Emulsifying Properties of Pectin Solutions
2.9. Betacyanin Content of Pectins
2.10. Antioxidant Activity of Pectins
2.11. Prebiotic Property of Pectins
2.12. Statistical Analysis
3. Results and Discussion
3.1. Yield and Composition of Pectins
3.2. Color and Betacyanin Content of Pectins
3.3. Molecular Weight of Pectins
3.4. Structural Analysis and DE Quantification of Pectins
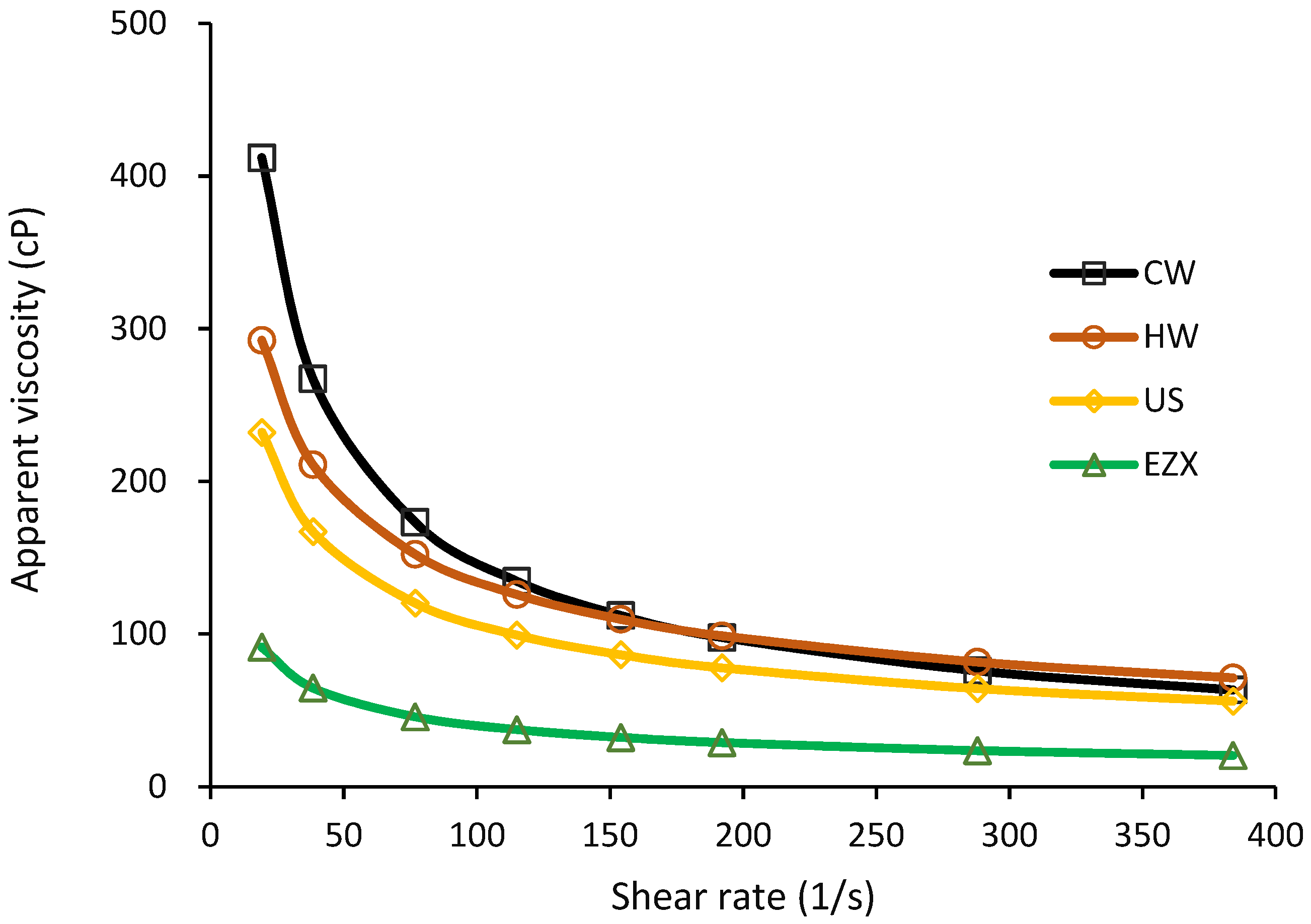
3.5. Viscosity of Pectin Solutions
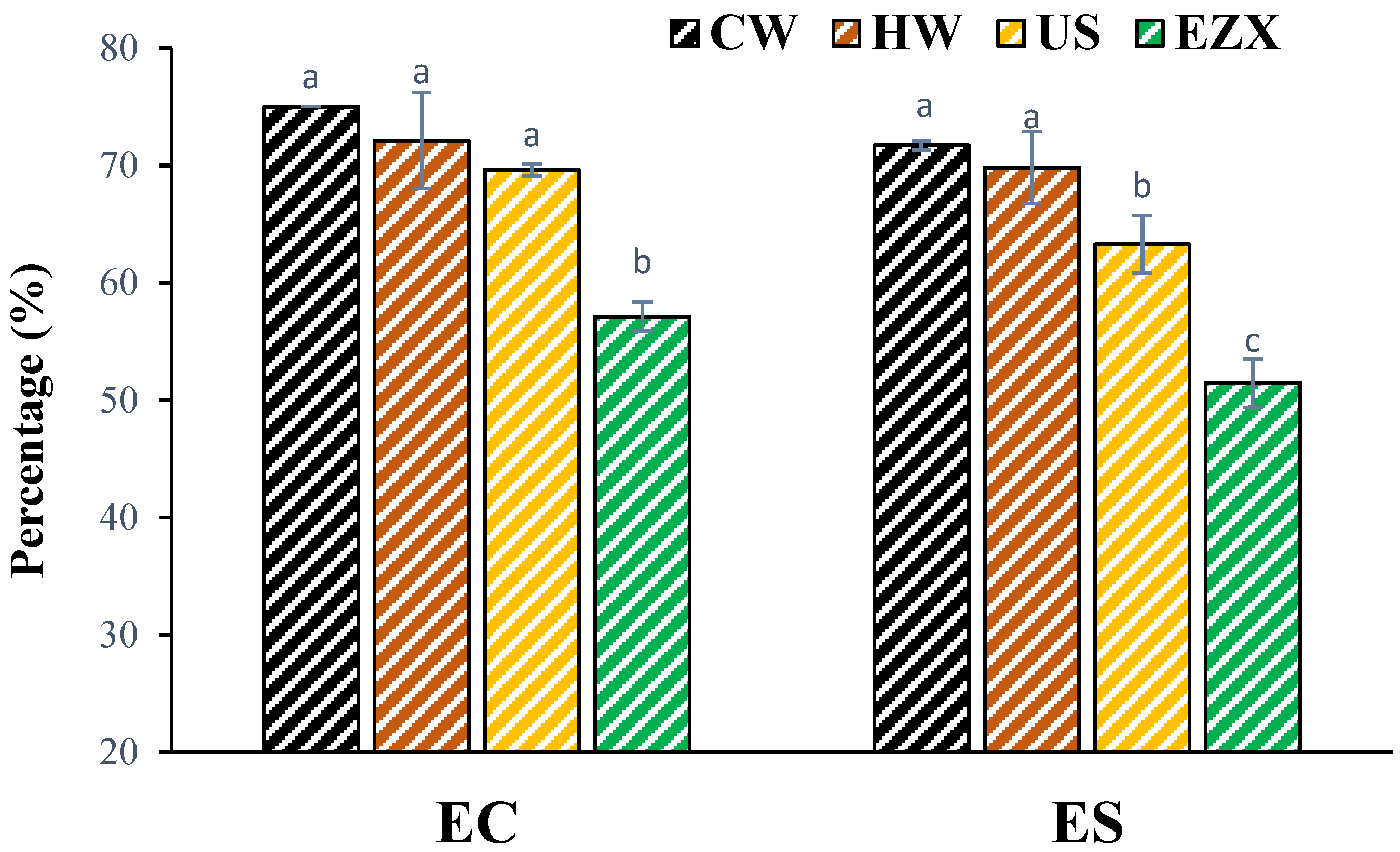
3.6. Emulsifying Properties of Pectins
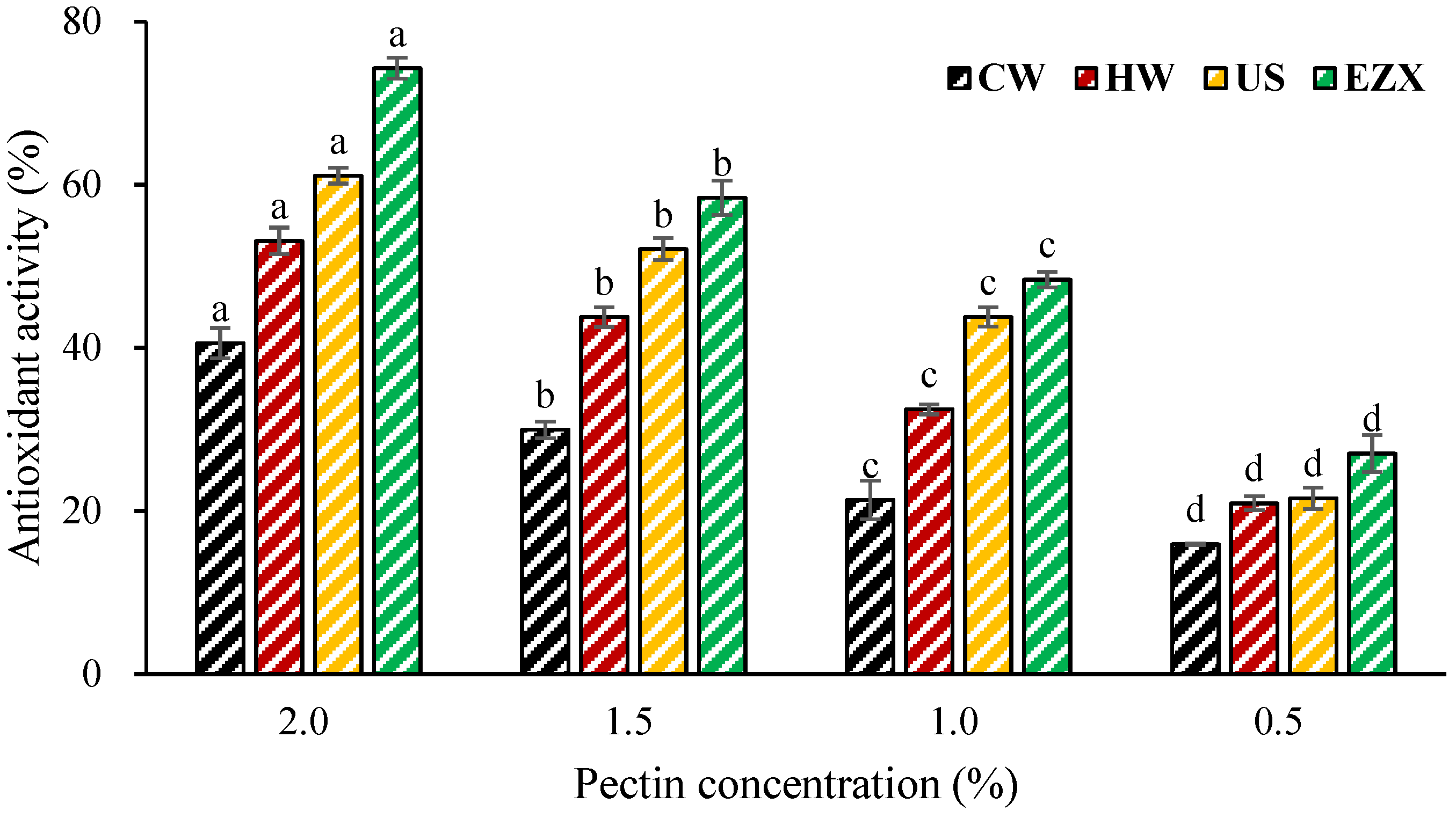
3.7. Antioxidant Activity of Pectins
3.8. Prebiotic Function of Pectin Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CW | Cold-water extraction |
| HW | Hot-water extraction |
| US | Ultrasound-assisted extraction |
| EZX | Enzyme-assisted extraction (Xylanase) |
| Man | Mannose |
| Rha | Rhamnose |
| GalA | Galacturonic acid |
| Glu | Glucose |
| Gal | Galactose |
| Ara | Arabinose |
| HG | Homogalacturonan |
| RG-I | Rhamnogalacturonan-I |
| DE | Degree of esterification |
| Mw | Molecular weight |
| EC | Emulsifying capacity |
| ES | Emulsifying stability |
References
- Jeong, U.S.; Kim, S.; Chae, Y.-W. Analysis on the cultivation trends and main producing areas of subtropical crops in Korea. J. Korea Acad.-Ind. Coop. Soc. 2020, 21, 524–535. [Google Scholar]
- Chua, B.L.; Tang, S.F.; Ali, A.; Chow, Y.H. Optimisation of pectin production from dragon fruit peels waste: Drying, extraction and characterisation studies. SN Appl. Sci. 2020, 2, 621. [Google Scholar] [CrossRef]
- Zhang, M.-y.; Cai, J. Preparation of branched RG-I-rich pectin from red dragon fruit peel and the characterization of its probiotic properties. Carbohydr. Polym. 2023, 299, 120144. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit wastes as a valuable source of value-added compounds: A collaborative perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- de la Luz Cadiz-Gurrea, M.; del Carmen Villegas-Aguilar, M.; Leyva-Jiménez, F.J.; Pimentel-Moral, S.; Fernandez-Ochoa, A.; Alañón, M.E.; Segura-Carretero, A. Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Res. Int. 2020, 138, 109786. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- del Amo-Mateos, E.; Cáceres, B.; Coca, M.; García-Cubero, M.T.; Lucas, S. Recovering rhamnogalacturonan-I pectin from sugar beet pulp using a sequential ultrasound and microwave-assisted extraction: Study on extraction optimization and membrane purification. Bioresour. Technol. 2024, 394, 130263. [Google Scholar] [CrossRef]
- Jamilah, B.; Shu, C.; Kharidah, M.; Dzulkily, M.; Noranizan, A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J. 2011, 18, 282. [Google Scholar]
- Taharuddin, N.; Jumaidin, R.; Mansor, M.; Hazrati, K.; Tarique, J.; Asyraf, M.; Razman, M. Unlocking the Potential of Lignocellulosic Biomass Dragon Fruit (Hylocereus polyrhizus) in Bioplastics, Biocomposites and Various Commercial Applications. Polymers 2023, 15, 2654. [Google Scholar] [CrossRef]
- Hotmaida, Y. Non-destructive determination of the main chemical components of red dragon fruit peel flour by using Near-Infrared Reflectance Spectroscopy (NIRS). In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012007. [Google Scholar]
- Yong, Y.Y.; Dykes, G.; Lee, S.M.; Choo, W.S. Effect of refrigerated storage on betacyanin composition, antibacterial activity of red pitahaya (Hylocereus polyrhizus) and cytotoxicity evaluation of betacyanin rich extract on normal human cell lines. LWT 2018, 91, 491–497. [Google Scholar] [CrossRef]
- Huang, Y.; Brennan, M.A.; Kasapis, S.; Richardson, S.J.; Brennan, C.S. Maturation process, nutritional profile, bioactivities and utilisation in food products of red pitaya fruits: A Review. Foods 2021, 10, 2862. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Diwan, D.; Shukla, A.C.; Gaffey, J.; Pathak, N.; Dashora, K.; Pandey, A.; Sharma, M.; Guleria, S.; Varjani, S. Valorization of dragon fruit waste to value-added bioproducts and formulations: A review. Crit. Rev. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Chen, S.; Zhang, Y.; Zhang, J.; Zhu, X.; Li, W.; Liu, J.; Jiang, Y.; Li, D. Pectin-rich dragon fruit peel extracts: An environmentally friendly emulsifier of natural origin. Food Chem. 2023, 429, 136955. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.N.T.; Nguyen, H.T.; Le, N.L.; Khoi, T.T.; Richel, A. Biodegradable films from dragon fruit (Hylocereus polyrhizus) peel pectin and potato starches crosslinked with glutaraldehyde. Food Packag. Shelf Life 2023, 37, 101084. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, characterization, and applications of pectins from plant by-products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Yu, M.; Xia, Y.; Zhou, M.; Guo, Y.; Zheng, J.; Zhang, Y. Effects of different extraction methods on structural and physicochemical properties of pectins from finger citron pomace. Carbohydr. Polym. 2021, 258, 117662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, Y.; Dorado, C.; Chau, H.K.; Hotchkiss, A.T.; Cameron, R.G. Modification of pectin with high-pressure processing treatment of fresh orange peel before pectin extraction: Part I. The effects on pectin extraction and structural properties. Food Hydrocoll. 2024, 149, 109516. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Zhang, J.; Jiang, Y.; Li, D. Pectin extracted from dragon fruit Peel: An exploration as a natural emulsifier. Int. J. Biol. Macromol. 2022, 221, 976–985. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar]
- Nguyen, B.M.N.; Pirak, T. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric. 2019, 5, 1633076. [Google Scholar] [CrossRef]
- Lim, J.; Yoo, J.; Ko, S.; Lee, S. Extraction and characterization of pectin from Yuza (Citrus junos) pomace: A comparison of conventional-chemical and combined physical–enzymatic extractions. Food Hydrocoll. 2012, 29, 160–165. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2020, 98, 105246. [Google Scholar] [CrossRef]
- Ripoll, C.S.S.; Hincapié-Llanos, G.A. Evaluation of sources and methods of pectin extraction from fruit and Vegetable wastes: A Systematic Literature Review (SLR). Food Biosci. 2023, 51, 102278. [Google Scholar]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current advancements in pectin: Extraction, properties and multifunctional applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Liaotrakoon, W.; Van Buggenhout, S.; Christiaens, S.; Houben, K.; De Clercq, N.; Dewettinck, K.; Hendrickx, M.E. An explorative study on the cell wall polysaccharides in the pulp and peel of dragon fruits (Hylocereus spp.). Eur. Food Res. Technol. 2013, 237, 341–351. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Dalagnol, L.M.; Silveira, V.C.; da Silva, H.B.; Manfroi, V.; Rodrigues, R.C. Improvement of pectinase, xylanase and cellulase activities by ultrasound: Effects on enzymes and substrates, kinetics and thermodynamic parameters. Process Biochem. 2017, 61, 80–87. [Google Scholar] [CrossRef]
- Nguyen, K.X.; Mai, H.C.; Tran, T.K.N.; Nguyen, T.V. Evaluation of parameters affecting the process of extraction pectin from red flesh dragon fruit peel. Mater. Today Proc. 2022, 51, 1448–1454. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Park, J.J.; Park, G.D.; Lee, W.Y. Enzymatic hydrolysis modifies emulsifying properties of okra pectin. Foods 2022, 11, 1497. [Google Scholar] [CrossRef] [PubMed]
- Said, N.S.; Olawuyi, I.F.; Cho, H.-S.; Lee, W.-Y. Novel edible films fabricated with HG-type pectin extracted from different types of hybrid citrus peels: Effects of pectin composition on film properties. Int. J. Biol. Macromol. 2023, 253, 127238. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Ruiz-Torralba, A.; Méndez-Albiñana, P.; Guerra-Hernández, E.; García-Villanova, B.; Moreno, R.; Villamiel, M.; Montilla, A. Berry fruits as source of pectin: Conventional and non-conventional extraction techniques. Int. J. Biol. Macromol. 2021, 186, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Sutor, K.; Wybraniec, S. Identification and determination of betacyanins in fruit extracts of Melocactus species. J. Agric. Food Chem. 2020, 68, 11459–11467. [Google Scholar] [CrossRef] [PubMed]
- Sandate-Flores, L.; Rodríguez-Hernández, D.V.; Rostro-Alanis, M.; Melchor-Martínez, E.M.; Brambila-Paz, C.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Rodríguez-Rodríguez, J.; Iqbal, H.M. Evaluation of three methods for betanin quantification in fruits from cacti. MethodsX 2022, 9, 101746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, H.; Wei, M.; Zhu, C. Effects of enzymatic treatment on the physicochemical properties and antioxidant activity of hawthorn pectin. Mater. Today Commun. 2022, 30, 103225. [Google Scholar] [CrossRef]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Mada, T.; Duraisamy, R.; Guesh, F. Optimization and characterization of pectin extracted from banana and papaya mixed peels using response surface methodology. Food Sci. Nutr. 2022, 10, 1222–1238. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Hromadkova, Z.; Ebringerova, A.; Valachovič, P. Ultrasound-assisted extraction of water-soluble polysaccharides from the roots of valerian (Valeriana officinalis L.). Ultrason. Sonochem. 2002, 9, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.-I.; Teodorescu, F.; Rovinaru, C.; Banu, A. Characterization of Pectin Oligosaccharides Obtained from Citrus Peel Pectin. Fermentation 2023, 9, 312. [Google Scholar] [CrossRef]
- Muhammad, K.; Zahari, N.I.M.; Gannasin, S.P.; Adzahan, N.M.; Bakar, J. High methoxyl pectin from dragon fruit (Hylocereus polyrhizus) peel. Food Hydrocoll. 2014, 42, 289–297. [Google Scholar] [CrossRef]
- Rahmati, S.; Abdullah, A.; Kang, O.L. Effects of different microwave intensity on the extraction yield and physicochemical properties of pectin from dragon fruit (Hylocereus polyrhizus) peels. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100186. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Khubber, S.; Kazemi, M.; Amiri Samani, S.; Lorenzo, J.M.; Simal-Gandara, J.; Barba, F.J. Structural-functional variability in pectin and effect of innovative extraction methods: An integrated analysis for tailored applications. Food Rev. Int. 2023, 39, 2352–2377. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Yang, W.; Sang, Y.; Guo, M.; Liu, D.; Zhou, Y.; Wang, H.; Cheng, S.; Chen, G. The effects of drying temperature on the kinetics, color, structure, and pectin composition of Zizyphus jujuba Mill. cv. Junzao. J. Food Process. Preserv. 2021, 45, e15942. [Google Scholar] [CrossRef]
- Woo, K.; Ngou, F.; Ngo, L.; Soong, W.; Tang, P. Stability of betalain pigment from red dragon fruit (Hylocereus polyrhizus). Am. J. Food Technol. 2011, 6, 140–148. [Google Scholar] [CrossRef][Green Version]
- Cai, Y.; Corke, H. Amaranthus betacyanin pigments applied in model food systems. J. Food Sci. 1999, 64, 869–873. [Google Scholar] [CrossRef]
- Van, M.P.; Duc, D.T.; Thanh, H.D.T.; Chi, H.T. Comparison of ultrasound assisted extraction and enzyme assisted extraction of betacyanin from red dragon fruit peel. In Proceedings of the E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; p. 04004. [Google Scholar]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Process. Process Intensif. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Rogošić, M.; Mencer, H.; Gomzi, Z. Polydispersity index and molecular weight distributions of polymers. Eur. Polym. J. 1996, 32, 1337–1344. [Google Scholar] [CrossRef]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Chatjigakis, A.; Pappas, C.; Proxenia, N.; Kalantzi, O.; Rodis, P.; Polissiou, M. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohydr. Polym. 1998, 37, 395–408. [Google Scholar] [CrossRef]
- Liang, W.-l.; Liao, J.-s.; Qi, J.-R.; Jiang, W.-x.; Yang, X.-q. Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll. 2012, 29, 356–362. [Google Scholar] [CrossRef]
- Cho, E.-H.; Jung, H.-T.; Lee, B.-H.; Kim, H.-S.; Rhee, J.-K.; Yoo, S.-H. Green process development for apple-peel pectin production by organic acid extraction. Carbohydr. Polym. 2019, 204, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Verkempinck, S.H.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Targeted modifications of citrus pectin to improve interfacial properties and the impact on emulsion stability. Food Hydrocoll. 2022, 132, 107841. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Liu, J.-P.; Huang, X.; Du, L.-P.; Shi, F.-L.; Dong, R.; Huang, X.-T.; Zheng, K.; Liu, Y.; Cheong, K.-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Li, M.; Ma, F.; Li, R.; Ren, G.; Yan, D.; Zhang, H.; Zhu, X.; Wu, R.; Wu, J. Degradation of Tremella fuciformis polysaccharide by a combined ultrasound and hydrogen peroxide treatment: Process parameters, structural characteristics, and antioxidant activities. Int. J. Biol. Macromol. 2020, 160, 979–990. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Ultrasound-assisted extraction of pectin from Citrus limetta peels: Optimization, characterization, and its comparison with commercial pectin. Food Biosci. 2023, 51, 102231. [Google Scholar] [CrossRef]
- Esquivel, P.; Stintzing, F.C.; Carle, R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Z. Für Naturforschung C 2007, 62, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of low molecular weight pectins via electron beam irradiation and their potential prebiotic functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Yeung, Y.K.; Kang, Y.-R.; So, B.R.; Jung, S.K.; Chang, Y.H. Structural, antioxidant, prebiotic and anti-inflammatory properties of pectic oligosaccharides hydrolyzed from okra pectin by Fenton reaction. Food Hydrocoll. 2021, 118, 106779. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Cesca, K.; Poletto, P.; de Oliveira, D. New perspectives for banana peel polysaccharides and their conversion to oligosaccharides. Food Res. Int. 2021, 149, 110706. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lazarte, A.; Kachrimanidou, V.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 2018, 199, 482–491. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. Effects of ultrasound on the degradation kinetics, physicochemical properties and prebiotic activity of Flammulina velutipes polysaccharide. Ultrason. Sonochem. 2022, 82, 105901. [Google Scholar] [CrossRef]


| CW | HW | US | EZX | |
|---|---|---|---|---|
| Yield (%) | 10.93 ± 1.21 c | 15.03 ± 1.44 b | 15.17 ± 0.75 b | 20.22 ± 1.75 a |
| Protein (%) | 3.97 ± 0.05 c | 4.17 ± 0.03 b | 4.56 ± 0.09 a | 3.37 ± 0.06 d |
| DE (%) | 50.88 ± 0.27 a | 46.82 ± 0.61 b | 51.79 ± 0.13 a | 47.74 ± 0.70 b |
| Mw (×103 kDa) | 1.21 ± 0.12 a | 1.01 ± 0.10 a | 0.97 ± 0.09 a | 0.84 ± 0.03 b |
| Mw/Mn | 4.81 | 4.19 | 4.22 | 13.04 |
| L* | 65.00 ± 0.10 b | 64.10 ± 0.80 bc | 63.00 ± 0.45 c | 69.08 ± 0.88 a |
| a* | 20.06 ± 0.24 a | 17.01 ± 0.77 b | 19.90 ± 0.08 a | 9.13 ± 0.10 c |
| b* | 7.73 ± 0.04 b | 7.23 ± 0.32 b | 4.81 ± 0.22 c | 10.06 ± 0.16 a |
| Betacyanin (mg/L) | 50.65 ± 1.56 a | 31.01 ± 0.26 c | 43.49 ± 0.78 b | 20.74 ± 0.26 d |
| Mol% | CW | HW | US | EZX |
|---|---|---|---|---|
| Man | 1.21 ± 0.18 b | 1.27 ± 0.01 b | 0.59 ± 0.08 c | 1.63 ± 0.23 a |
| Rha | 8.76 ± 0.17 ab | 9.36 ± 0.15 a | 8.11 ± 0.07 b | 9.51 ± 0.47 a |
| GalA | 77.21 ± 0.42 c | 78.51 ± 0.35 b | 83.12 ± 0.31 a | 78.25 ± 2.26 bc |
| Glu | 5.51 ± 0.01 a | 3.62 ± 0.15 bc | 2.70 ± 0.06 c | 4.03 ± 0.60 b |
| Gal | 3.68 ± 0.10 b | 4.51 ± 0.07 a | 4.56 ± 0.09 a | 4.71 ± 0.42 a |
| Ara | 3.86 ± 0.06 a | 2.73 ± 0.02 b | 0.93 ± 0.02 d | 1.87 ± 0.54 c |
| HG | 68.45 ± 0.59 b | 69.15 ± 0.50 b | 75.02 ± 0.38 a | 68.73 ± 2.73 b |
| RG-I | 25.05 ± 0.37 a | 25.95 ± 0.35 a | 21.70 ± 0.25 b | 25.61 ± 1.90 a |
| HG/RG | 2.73 ± 0.06 b | 2.66 ± 0.05 b | 3.46 ± 0.06 a | 2.69 ± 0.31 b |
| Rha/GalA | 0.11 ± 0.00 a | 0.12 ± 0.00 a | 0.10 ± 0.00 b | 0.12 ± 0.01 a |
| Model | Parameter | Pectin Samples | |||
|---|---|---|---|---|---|
| CW | HW | US | EZX | ||
| η = Kγ(n−1) | K | 2613.51 | 1176.96 | 943.42 | 400.41 |
| n | 0.37 | 0.53 | 0.53 | 0.5 | |
| r2 | 1.00 | 1.00 | 0.99 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Olawuyi, I.F.; Said, N.S.; Lee, W.-Y. Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques. Polymers 2024, 16, 1097. https://doi.org/10.3390/polym16081097
Du H, Olawuyi IF, Said NS, Lee W-Y. Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques. Polymers. 2024; 16(8):1097. https://doi.org/10.3390/polym16081097
Chicago/Turabian StyleDu, Huimin, Ibukunoluwa Fola Olawuyi, Nurul Saadah Said, and Won-Young Lee. 2024. "Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques" Polymers 16, no. 8: 1097. https://doi.org/10.3390/polym16081097
APA StyleDu, H., Olawuyi, I. F., Said, N. S., & Lee, W.-Y. (2024). Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques. Polymers, 16(8), 1097. https://doi.org/10.3390/polym16081097






