Novel Photothermal Graphene-Based Hydrogels in Biomedical Applications
Abstract
1. Introduction
2. Hydrogel Classification and Physico-Chemical Property
3. Fabrication of Graphene-Based Hydrogels
3.1. Physical Crosslinking Approach
3.2. Chemical Crosslinking Approach
3.3. In-Situ Polymerization
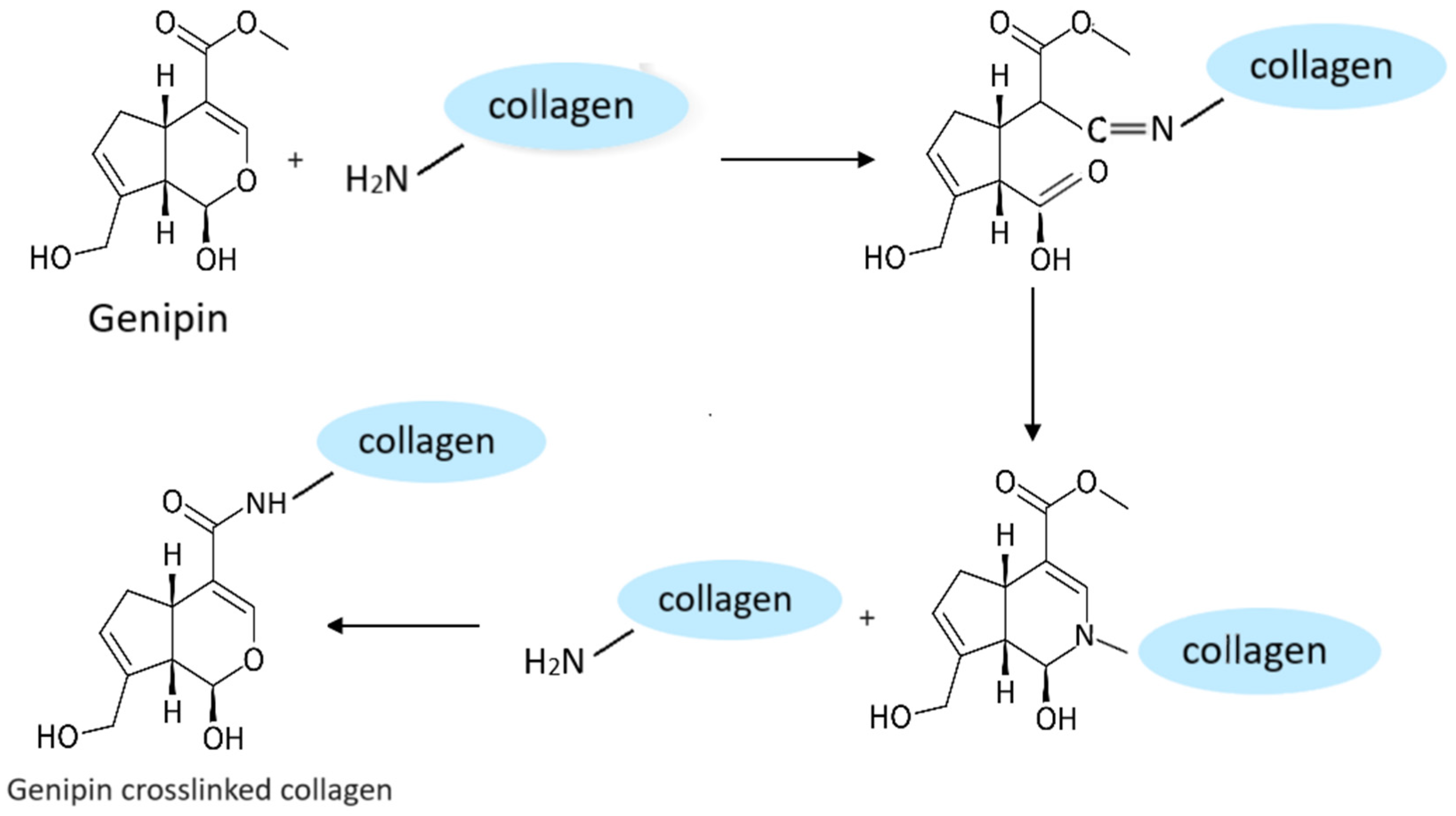
4. Role of Crosslinking Agents in Formation of Graphene-Based Hydrogels
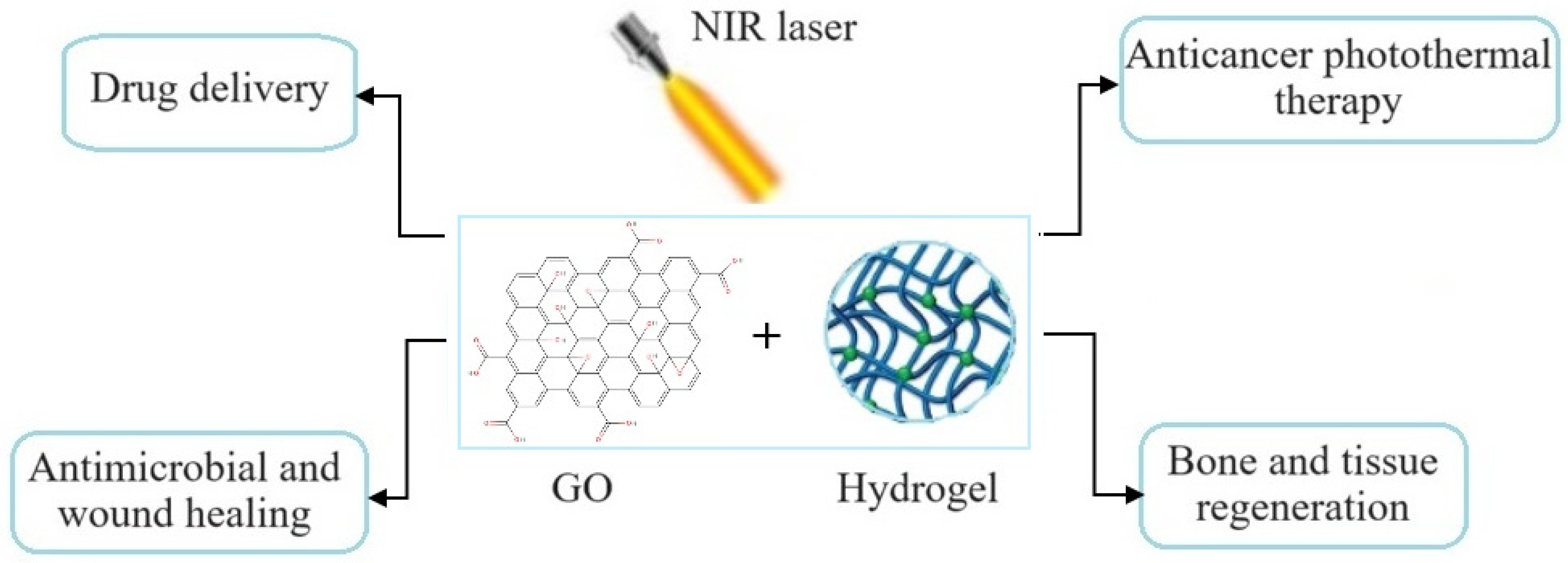
5. Photothermal Property of Graphene-Based Hydrogels and Their Biomedical Applications
5.1. Cancer Photothermal Therapy
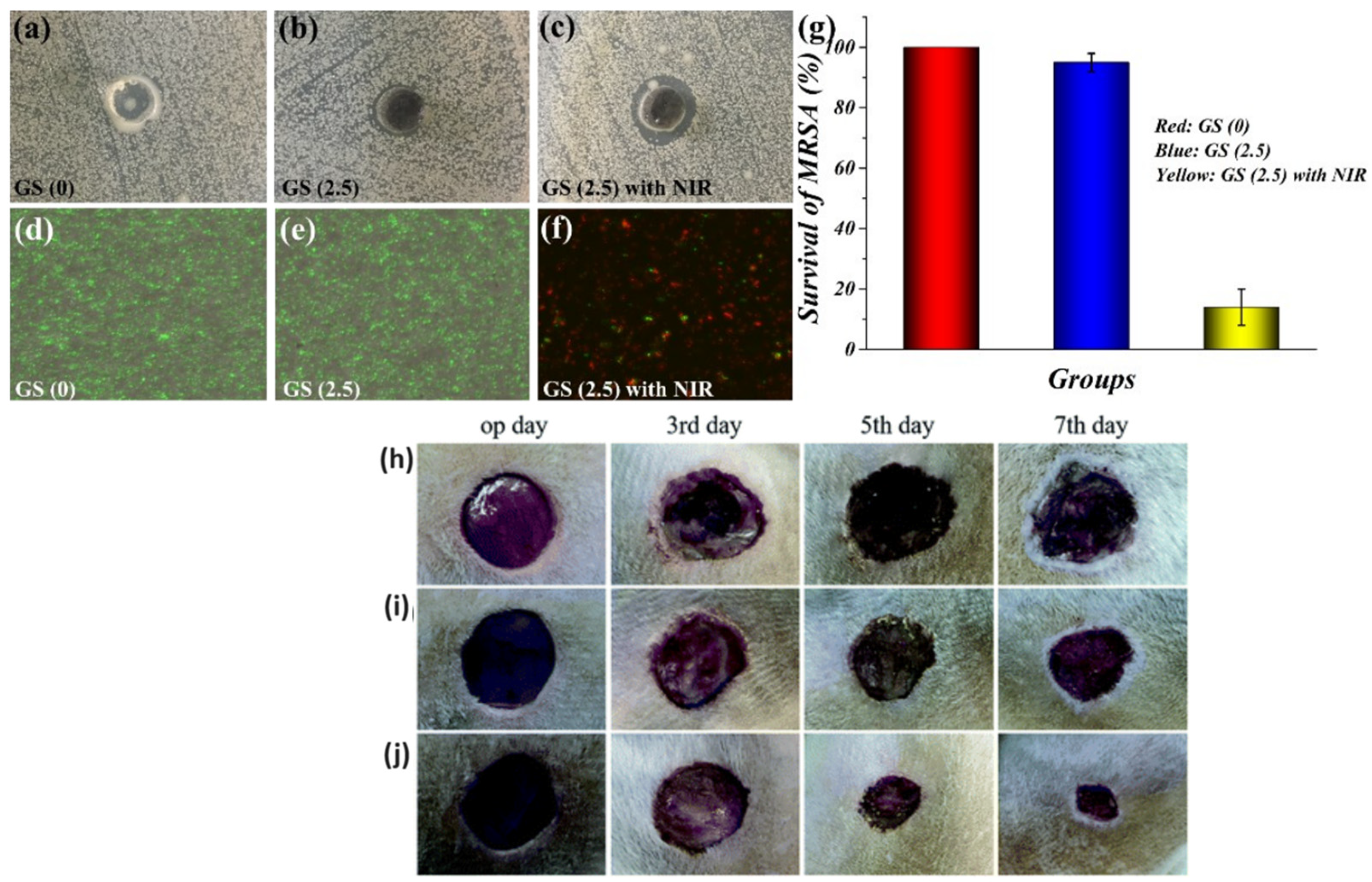
5.2. Bacterial Killing and Wound Healing
5.3. Bone Tissue Regeneration
5.4. Other Biomedical Applications
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, S.E.; Chamberlayne, C.F.; Zare, R.N. Electrically controlled drug release using pH-sensitive polymer films. Nanoscale 2018, 10, 10087–10093. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.N.; Guo, B.L. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Chen, P.; Chang, P.; Chen, S. A High-Sensitivity and Low-Power Theranostic Nanosystem for Cell SERS Imaging and Selectively Photothermal Therapy Using Anti-EGFR-Conjugated Reduced Graphene Oxide/Mesoporous Silica/AuNPs Nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.T.; Vo, T.A.T.; Hoang, T.X.; Cho, S. Graphene Integrated Hydrogels Based Biomaterials in Photothermal Biomedicine. Nanomaterials 2021, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, Z.; Ju, E.; Wang, Z.; Dong, K.; Ren, J.; Qu, X. Multifunctional upconverting nanoparticles for near-infrared triggered and synergistic antibacterial resistance therapy. Chem. Commun. 2014, 50, 10488–10490. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor. Sci. Rep. 2021, 11, 23094. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.X.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, S.; Meng, J.; Chen, W.; Li, J. Thermo-Responsive Hydrogels Coupled with Photothermal Agents for Biomedical Applications. Macromol. Biosci. 2023, 23, e2300214. [Google Scholar] [CrossRef]
- Liu, Y.; He, W.; Zhang, Z.; Lee, B.P. Recent Developments in Tough Hydrogels for Biomedical Applications. Gels 2018, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Thareja, P. Hydrogels differentiated by length scales: A review of biopolymer-based hydrogel preparation methods, characterization techniques, and targeted applications. Eur. Polym. J. 2022, 163, 110935. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C 2021, 118, 111447. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, L.; Chen, X.; Yin, W.; Ni, L.; Wang, M. Photothermal nanohybrid hydrogels for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 1066617. [Google Scholar] [CrossRef]
- Nishimura, S.-N.; Sato, D.; Koga, T. Mechanically Tunable Hydrogels with Self-Healing and Shape Memory Capabilities from Thermo-Responsive Amino Acid-Derived Vinyl Polymers. Gels 2023, 9, 829. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Luo, C.; Qian, B.; Liu, S.; Zeng, Y.; Hou, J.; Deng, B.; Sun, Y.; Yang, J.; et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics 2019, 9, 5839–5853. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Hasan, A. Graphene Oxide Loaded Hydrogel for Enhanced Wound Healing in Diabetic Patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3943–3946. [Google Scholar]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sheng, S.; Dong, X.; Zhang, Y.; Li, X.; Zhu, D.; Lv, F. A photo-triggered hydrogel for bidirectional regulation with imaging visualization. Soft Matter 2020, 16, 7598–7605. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Shan, X.; Hao, L.; Feng, Q.; Zhang, Z. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomater. 2017, 54, 307–320. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Costa, F.J.P.; Nave, M.; Moreira, A.F.; Mendonça, A.G.; Correia, I.J.; de Melo-Diogo, D. Injectable hydrogels for the delivery of nanomaterials for cancer combinatorial photothermal therapy. Biomater. Sci. 2023, 11, 6082–6108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Shi, J.; Jiang, Z.; Zhang, C.Y. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact. Mater. 2022, 14, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Hu, Y.; Zhao, Y.; Tang, K.; Zhang, Z.; Liu, Z.; Wang, Y.; Guo, H.; Miao, Y.; Du, H.; et al. Nanomaterials for photothermal cancer therapy. RSC Adv. 2023, 13, 14443–14460. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, A.; Ali, I.; Jo, S.H.; Vu, T.T.; Gal, Y.S.; Kim, Y.H.; Park, S.H.; Lim, K.T. Facile Fabrication of NIR-Responsive Alginate/CMC Hydrogels Derived through IEDDA Click Chemistry for Photothermal-Photodynamic Anti-Tumor Therapy. Gels 2023, 9, 961. [Google Scholar] [CrossRef]
- Xu, K.; Sun, X.; Chong, C.; Ren, L.; Tan, L.; Sun, H.; Wang, X.; Li, L.; Xia, J.; Zhang, R.; et al. Green Starch-Based Hydrogels with Excellent Injectability, Self-Healing, Adhesion, Photothermal Effect, and Antibacterial Activity for Promoting Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 2027–2040. [Google Scholar] [CrossRef]
- Báez, D.F. Graphene-Based Nanomaterials for Photothermal Therapy in Cancer Treatment. Pharmaceutics 2023, 15, 2286. [Google Scholar] [CrossRef]
- Liu, J.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar] [CrossRef]
- Xie, C.; Luo, J.; Luo, Y.; Zhou, J.; Guo, X.; Lu, X. Electroactive Hydrogels with Photothermal/Photodynamic Effects for Effective Wound Healing Assisted by Polydopamine-Modified Graphene Oxide. ACS Appl. Mater. Interfaces 2023, 15, 42329–42340. [Google Scholar] [CrossRef]
- Lu, Q.; Jang, H.S.; Han, W.J.; Lee, J.H.; Choi, H.J. Stimuli-Responsive Graphene Oxide-Polymer Nanocomposites. Macromol. Res. 2019, 27, 1061–1070. [Google Scholar] [CrossRef]
- Olate-Moya, F.; Palza, H. Effect of graphene oxide on the pH-responsive drug release from supramolecular hydrogels. J. Appl. Polym. Sci. 2022, 139, 51420. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Chi, R.; Shi, J.; Yang, Y.; Zhang, X. Reduced graphene oxide loaded with MoS2 and Ag3PO4 nanoparticles/PVA interpenetrating hydrogels for improved mechanical and antibacterial properties. Mater. Des. 2019, 183, 108166. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Hubner, P.; Marcilio, N.R.; Tessaro, I.C. Gelatin/poly(vinyl alcohol) based hydrogel film—A potential biomaterial for wound dressing: Experimental design and optimization followed by rotatable central composite design. J. Biomater. Appl. 2021, 36, 682–700. [Google Scholar] [CrossRef]
- Hussain, S.; Maktedar, S.S. Structural, functional and mechanical performance of advanced Graphene-based composite hydrogels. Results Chem. 2023, 6, 101029. [Google Scholar] [CrossRef]
- Trembecka-Wójciga, K.; Sobczak, J.J.; Sobczak, N. A comprehensive review of graphene-based aerogels for biomedical applications. The impact of synthesis parameters onto material microstructure and porosity. Arch. Civ. Mech. Eng. 2023, 23, 133. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. A Review on Synthesis Methods of Phyllosilicate- and Graphene-Filled Composite Hydrogels. J. Compos. Sci. 2022, 6, 15. [Google Scholar] [CrossRef]
- Xie, R.H.; Ren, P.G.; Hui, J.; Ren, F.; Ren, L.Z.; Sun, Z.F. Preparation and properties of graphene oxide-regenerated cellulose/polyvinyl alcohol hydrogel with pH-sensitive behavior. Carbohyd. Polym. 2016, 138, 222–228. [Google Scholar]
- Hanif, W.; Hardiansyah, A.; Randy, A.; Asri, L.A.T.W. Physically crosslinked PVA/graphene-based materials/aloe vera hydrogel with antibacterial activity. RSC Adv. 2021, 11, 29029–29041. [Google Scholar] [CrossRef] [PubMed]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.J. Preparation and characterization of cellulose/N,N′-methylene bisacrylamide/graphene oxide hybrid hydrogels and aerogels. Carbohyd. Polym. 2018, 196, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Study of the effect of mixing approach on cross-linking efficiency of hyaluronic acid-based hydrogel cross-linked with 1,4-butanediol diglycidyl ether. Eur. J. Pharm. Sci. 2016, 91, 131–137. [Google Scholar] [CrossRef]
- Basak, P.; Pahari, P.; Das, P.; Das, N.; Samanta, S.K.; Roy, S. Synthesis and Characterisation of Gelatin-Pva/Hydroxyapetite(Hap) Composite for Medical Applications. IOP Conf. Ser. Mat. Sci. 2018, 410, 012021. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Vo, N.T.N.; Huang, L.; Lemos, H.; Mellor, A.L.; Novakovic, K. Genipin-crosslinked chitosan hydrogels: Preliminary evaluation of the in vitro biocompatibility and biodegradation. J. Appl. Polym. Sci. 2021, 138, 50848. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Vlasceanu, G.M.; Crica, L.E.; Pandele, A.M.; Ionita, M. Graphene Oxide Reinforcing Genipin Crosslinked Chitosan-Gelatin Blend Films. Coatings 2020, 10, 189. [Google Scholar] [CrossRef]
- Marapureddy, S.G.; Thareja, P. Synergistic effect of chemical crosslinking and addition of graphene-oxide in Chitosan-Hydrogels, films, and drug delivery. Mater. Today Commun. 2022, 31, 103430. [Google Scholar] [CrossRef]
- Li, B.G.; Wu, C.; Wang, C.Y.; Luo, Z.Y.; Cao, J.P. Fabrication of tough, self-recoverable, and electrically conductive hydrogels by reduction of poly(acrylic acid) grafted graphene oxide in polyacrylamide hydrogel matrix. J. Appl. Polym. Sci. 2020, 137, 48781. [Google Scholar] [CrossRef]
- Zhang, N.; Li, R.; Zhang, L.; Chen, H.; Wang, W.; Liu, Y.; Wu, T.; Wang, X.; Wang, W.; Li, Y.; et al. Actuator materials based on graphene oxide/polyacrylamide composite hydrogels prepared by in situ polymerization. Soft Matter 2011, 7, 7231–7239. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, H.; Shen, J. Synthesis and molecular dynamics simulation of CuS@GO–CS hydrogel for enhanced photothermal antibacterial effect. New J. Chem. 2021, 45, 6895–6903. [Google Scholar] [CrossRef]
- Liao, G.C.; Hu, J.F.; Chen, Z.; Zhang, R.Q.; Wang, G.C.; Kuang, T.R. Preparation, Properties, and Applications of Graphene-Based Hydrogels. Front. Chem. 2018, 6, 450. [Google Scholar] [CrossRef]
- Huang, H.; Chen, P.; Zhang, X.; Lu, Y.; Zhan, W. Edge-to-Edge Assembled Graphene Oxide Aerogels with Outstanding Mechanical Performance and Superhigh Chemical Activity. Small 2013, 9, 1397–1404. [Google Scholar] [CrossRef]
- Aidun, A.; Firoozabady, A.S.; Moharrami, M.; Ahmadi, A.; Haghighipour, N.; Bonakdar, S.; Faghihi, S. Graphene oxide incorporated polycaprolactone/chitosan/collagen electrospun scaffold: Enhanced osteogenic properties for bone tissue engineering. Artif. Organs. 2019, 43, E264–E281. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Liang, H.; Chen, J.; Yu, S. Ultralight Multifunctional Carbon-Based Aerogels by Combining Graphene Oxide and Bacterial Cellulose. Small 2017, 13, 1700453. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Zou, B.; Palacios, A.; Navarro, M.; Qiao, G.; Ding, Y. Thickening and gelling agents for formulation of thermal energy storage materials—A critical review. Renew. Sustain. Energy Rev. 2022, 155, 111906. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Q.; Zhang, G.; Ye, L. In situ crosslinking of poly(vinyl alcohol)/graphene oxide Nano-composite hydrogel: Intercalation structure and adsorption mechanism for advanced Pb(II) removal. J. Polym. Res. 2018, 25, 168. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119824. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Su, Y.-L.; Hu, S.-H.; Chen, S.-Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-crosslinked dynamic hydrogels for biomedical applications. Mater. Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef]
- Sharma, S.S.A.; Bashir, S.; Kasi, R.; Subramaniam, R.T. The significance of graphene based composite hydrogels as smart materials: A review on the fabrication, properties, and its applications. FlatChem 2022, 33, 100352. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, G.-M.; Xian, G.-Y.; Su, X.-Q.; Yu, L.-L.; Yao, F. Mechanistic biosynthesis of SN-38 coated reduced graphene oxide sheets for photothermal treatment and care of patients with gastric cancer. J. Photochem. Photobiol. B Biol. 2020, 204, 111736. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ye, M.; Wu, Y.; Wu, L.; Lan, K.; Wu, Z. Hyperthermia combined with immune checkpoint inhibitor therapy: Synergistic sensitization and clinical outcomes. Cancer Med. 2024, 12, 3201–3221. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postoperative Recurrence of Breast Cancer, ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar]
- Liu, W.; Zhang, X.Y.; Zhou, L.; Shang, L.; Su, Z.Q. Reduced graphene oxide (rGO) hybridized hydrogel as a near-infrared (NIR)/pH dual-responsive platform for combined chemo-photothermal therapy. J. Colloid Interf. Sci. 2019, 536, 160–170. [Google Scholar] [CrossRef]
- Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gómez, S.C.; Serna, J.A.; Rueda-Gensini, L.; Ostos, C.; Muñoz-Camargo, C.; Cruz, J.C. Novel Photo- and Thermo-Responsive Nanocomposite Hydrogels Based on Functionalized rGO and Modified SIS/Chitosan Polymers for Localized Treatment of Malignant Cutaneous Melanoma. Front. Bioeng. Biotechnol. 2022, 10, 947616. [Google Scholar] [CrossRef] [PubMed]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111294. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Wang, S.; Chen, J.; Zhi, W.; Guan, Y.; Cai, B.; Zhu, Y.; Jia, Y.; Huang, S.; et al. Injectable in situ intelligent thermo-responsive hydrogel with glycyrrhetinic acid-conjugated nano graphene oxide for chemo-photothermal therapy of malignant hepatocellular tumor. J. Biomater. Appl. 2022, 37, 151–165. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; SamariKhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Huang, H.; Zhang, H.; Hou, L.; Zhang, Z. Functionalized graphene oxide-based thermosensitive hydrogel for near-infrared chemo-photothermal therapy on tumor. J. Biomater. Appl. 2016, 30, 1230–1241. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.P.; Zhao, X.; Hu, T.L.; Chen, B.J.; Yin, Z.H.; Ma, P.X.; Guo, B.L. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Han, K.; Bai, Q.; Zeng, Q.; Sun, N.; Zheng, C.; Wu, W.; Zhang, Y.; Lu, T. A multifunctional mussel-inspired hydrogel with antioxidant, electrical conductivity and photothermal activity loaded with mupirocin for burn healing. Mater. Des. 2022, 217, 110598. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a Mesoporous Polydopamine@GO/Cellulose Nanofibril Composite Hydrogel with an Encapsulation Structure for Controllable Drug Release and Toxicity Shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Zheng, S.Y.; Chen, C.W.; Zhang, D.G. A graphene hybrid supramolecular hydrogel with high stretchability, self-healable and photothermally responsive properties for wound healing. Rsc. Adv. 2021, 11, 6367, Erratum in Rsc. Adv. 2022, 12, 2536. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.-C.; Burnouf, T.; Chiang, C.-W.; Jheng, P.-R.; Szunerits, S.; Yang, J.-C.; Chuang, E.-Y. Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J. Nanobiotechnol. 2023, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, L.; Tai, G.; Yan, F.; Cai, L.; Xin, C.; Al Islam, S. Graphene Oxide-loaded magnetic nanoparticles within 3D hydrogel form High-performance scaffolds for bone regeneration and tumour treatment. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106672. [Google Scholar] [CrossRef]
- Li, D.; Nie, W.; Chen, L.; McCoul, D.; Liu, D.; Zhang, X.; Ji, Y.; Yu, B.; He, C. Self-Assembled Hydroxyapatite-Graphene Scaffold for Photothermal Cancer Therapy and Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 2003–2017. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2021, 413, 127413. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef]
- Li, C.N.; Pagneux, Q.; Voronova, A.; Barras, A.; Abderrahmani, A.; Plaisance, V.; Pawlowski, V.; Hennuyer, N.; Staels, B.; Rosselle, L.; et al. Near-infrared light activatable hydrogels for metformin delivery. Nanoscale 2019, 11, 15810–15820. [Google Scholar]
- Mauri, E.; Salvati, A.; Cataldo, A.; Mozetic, P.; Basoli, F.; Abbruzzese, F.; Trombetta, M.; Bellucci, S.; Rainer, A. Graphene-laden hydrogels: A strategy for thermally triggered drug delivery. Mater. Sci. Eng. C 2021, 118, 111353. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, F.; Oz, Y.; Quéniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, A.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.P.; Su, J.H.; Meng, Q.S.; Yin, Q.; Chen, L.L.; Gu, W.W.; Zhang, Z.W.; Yu, H.J.; Zhang, P.C.; Wang, S.L.; et al. Cancer Cell Membrane-Coated Gold Nanocages with Hyperthermia-Triggered Drug Release and Homotypic Target Inhibit Growth and Metastasis of Breast Cancer. Adv. Funct. Mater. 2017, 27, 1604300, Erratum in Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Yang, L.; Wang, F. Photothermally controlled drug release system with high dose loading for synergistic chemo-photothermal therapy of multidrug resistance cancer. Colloids Surf. B Biointerfaces 2019, 175, 239–247. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages |
|---|---|---|
| Physically crosslinked hydrogels | - Easy to obtain (one-step fabrication) and low cost; - Stable and uniform dispersion solutions; - Porous network structure and ultra-low density; - Biocompatibility, good absorption properties, great electrical and thermal conductivity, and stability. | - Poor mechanical properties due to crystallization, separation, and the irregular crosslinking dispersion of GO. |
| Chemically crosslinked hydrogels | - “Green” method, simple (one-step fabrication), and low cost; - Easy to obtain by the self-assembled ability of GO sheets; - High water retention capacity, biocompatibility, excellent electrical and thermal conductivities, and strong mechanical properties. | - Poor absorption properties, toxicity of the most commonly used reducing agents, and chemical moieties covalently bounded to the GO cannot be eliminated by washing steps. |
| In situ polymerization | - Easy to obtain and low cost; - Excellent pH sensitivity, swelling–deswelling ability, and strong interfacial interactions; - Favorable dispersibility of GO. | - Low stretching capacity and easily breakable at low deformation during elongation. |
| Hydrogel | Agent | Application | Ref. |
|---|---|---|---|
| CSMA/BPEI/BPEI-GO | DOX | PTT/cancer therapy | [75] |
| CMC-rGO/CS-PEG | DOX | PTT/cancer therapy | [76] |
| SISMA/CSMA/rGO | DOX | PTT/cancer therapy | [77] |
| CS/agarose/GO CS/agarose/rGO | DOX/IBU | PTT/cancer therapy | [78] |
| GA/NGO | BH | PTT/cancer therapy | [79] |
| GO/CS | docetaxel | PTT/cancer therapy | [81] |
| DA/rGO | HA | PTT/wound healing/antibacterial | [83] |
| GelDA/pGO | mupirocin | PTT/wound healing/antibacterial | [84] |
| MPDA/GO/CNF | TH | PTT/drug release | [85] |
| NAGA/GS | - | PTT/wound healing | [86] |
| PVA/rGO | MoS2/Ag3PO4 | PTT/wound healing/antibacterial | [37] |
| Gel/Alg/rGO | pEV | PTT/wound healing | [87] |
| MGO/PVA/SA/HA | - | PTT/bone regeneration/tissue repair | [88] |
| nHA-rGO | - | PTT/bone regeneration/cancer therapy | [89] |
| nHA/GO/CS | - | PTT/bone regeneration/tissue repair | [90] |
| CS/rGO | teriparatide | PTT/bone regeneration | [91] |
| GO/pNIPAM/GelMA | - | PTT/drug release | [92] |
| rGO-COOH | metformin | PTT/drug release/diabetes | [93] |
| PAA/FLG-HG | diclofenac | PTT/drug release | [94] |
| PEGDMA-rGO | insulin | PTT/drug release/diabetes | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, A.-M.; Ficai, D.; Ficai, A. Novel Photothermal Graphene-Based Hydrogels in Biomedical Applications. Polymers 2024, 16, 1098. https://doi.org/10.3390/polym16081098
Croitoru A-M, Ficai D, Ficai A. Novel Photothermal Graphene-Based Hydrogels in Biomedical Applications. Polymers. 2024; 16(8):1098. https://doi.org/10.3390/polym16081098
Chicago/Turabian StyleCroitoru, Alexa-Maria, Denisa Ficai, and Anton Ficai. 2024. "Novel Photothermal Graphene-Based Hydrogels in Biomedical Applications" Polymers 16, no. 8: 1098. https://doi.org/10.3390/polym16081098
APA StyleCroitoru, A.-M., Ficai, D., & Ficai, A. (2024). Novel Photothermal Graphene-Based Hydrogels in Biomedical Applications. Polymers, 16(8), 1098. https://doi.org/10.3390/polym16081098








