Three-Dimensional Cross-Linking Network Coating for the Flame Retardant of Bio-Based Polyamide 56 Fabric by Weak Bonds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyelectrolyte Solution
2.3. Preparation of Melamine Solution
2.4. Preparation of Citric Acid Buffer
2.5. Preparation of Flame-Retardant Fabrics
2.6. Characterization
3. Results and Discussion
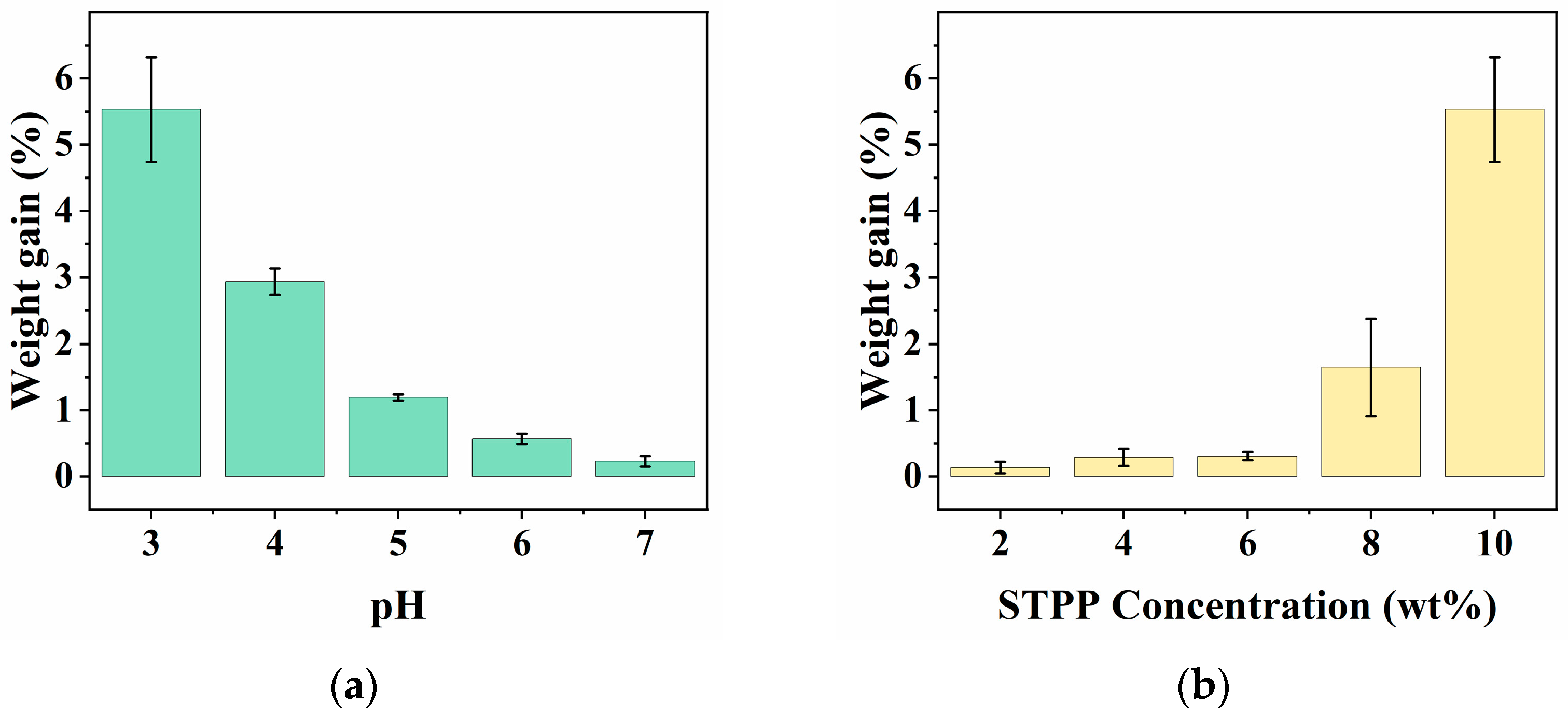
3.1. Effect of Preparation Conditions on Fabric Weight Gain
3.2. Characterization of Coated Fabric
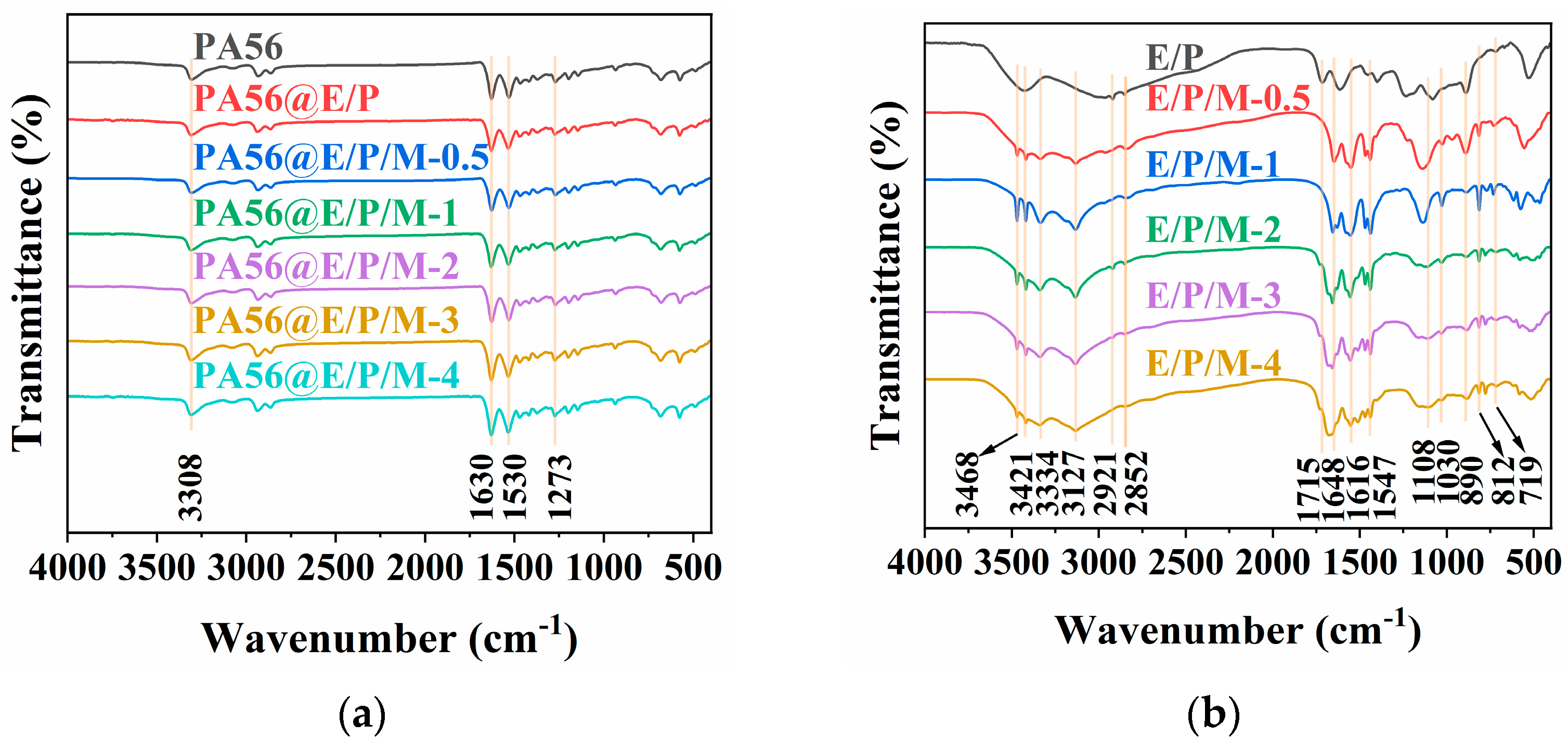
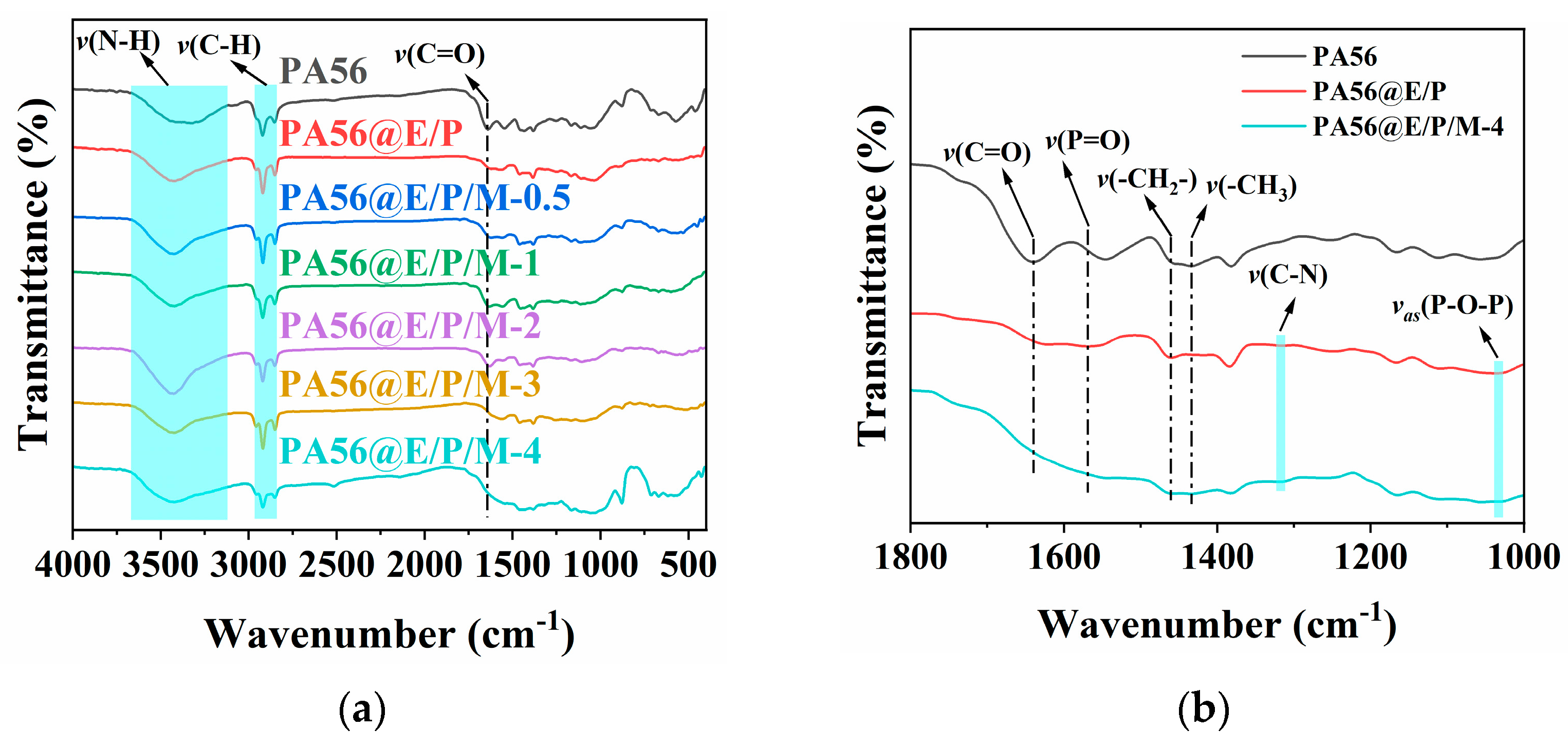
3.2.1. FTIR
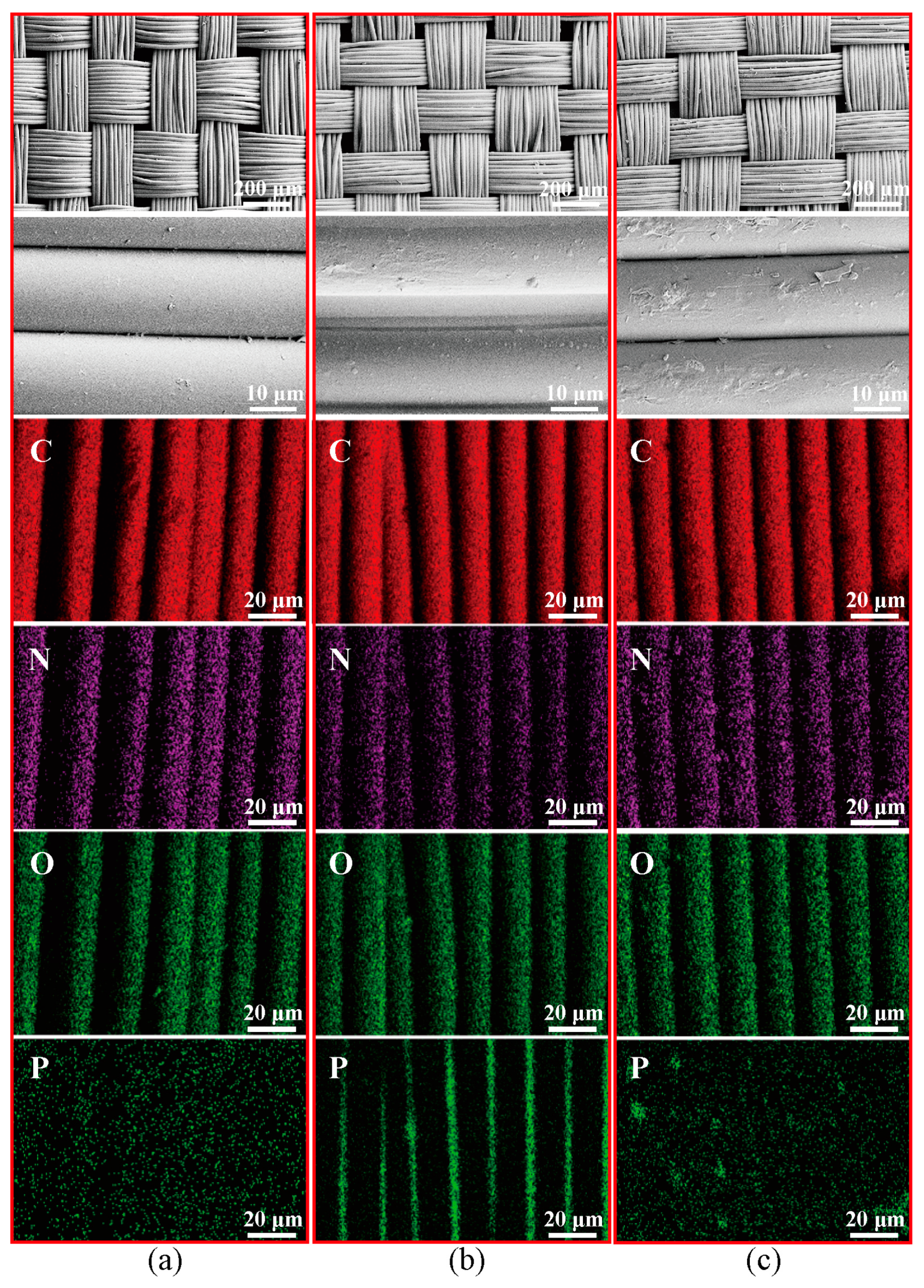
3.2.2. SEM and EDS
3.2.3. XPS
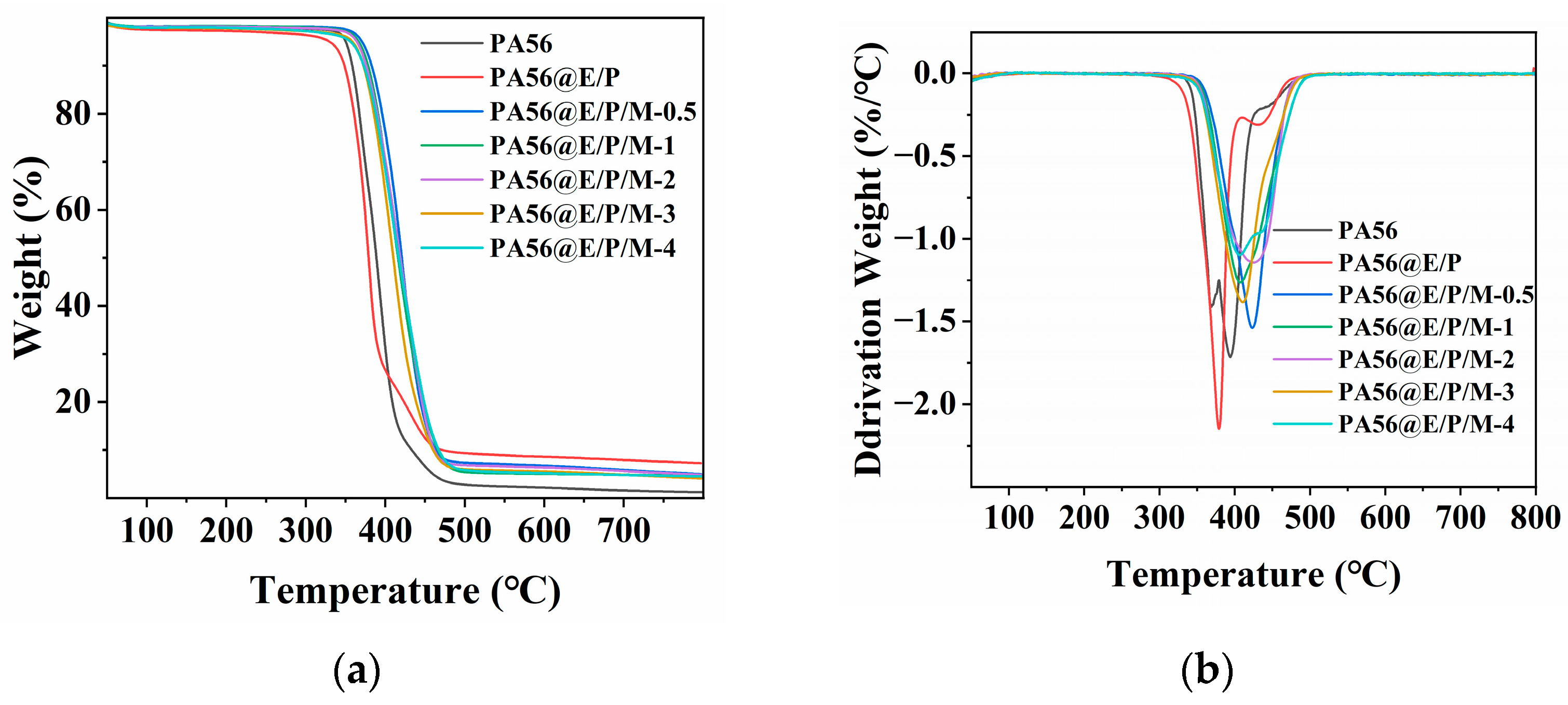
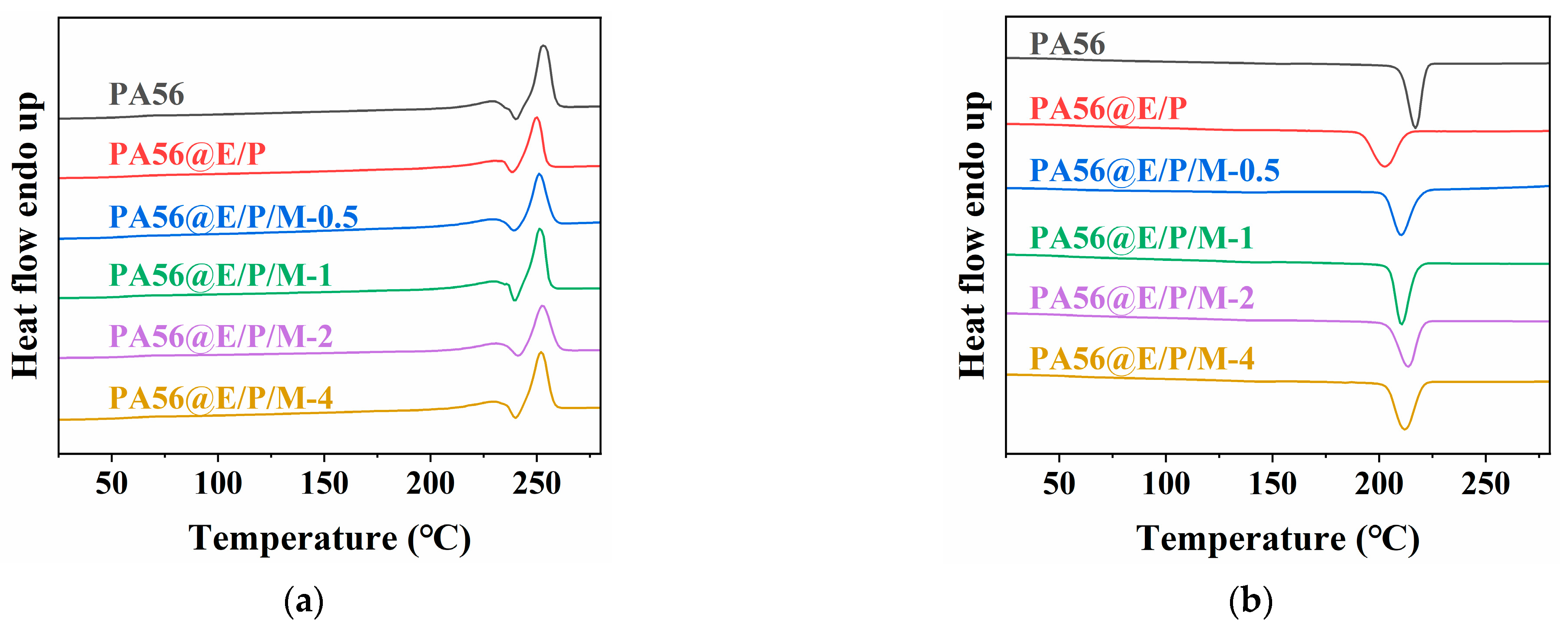
3.3. Thermal Properties
3.4. Flame Retardants’ Properties
3.4.1. Vertical Burning Test and Limiting Oxygen Index (LOI)
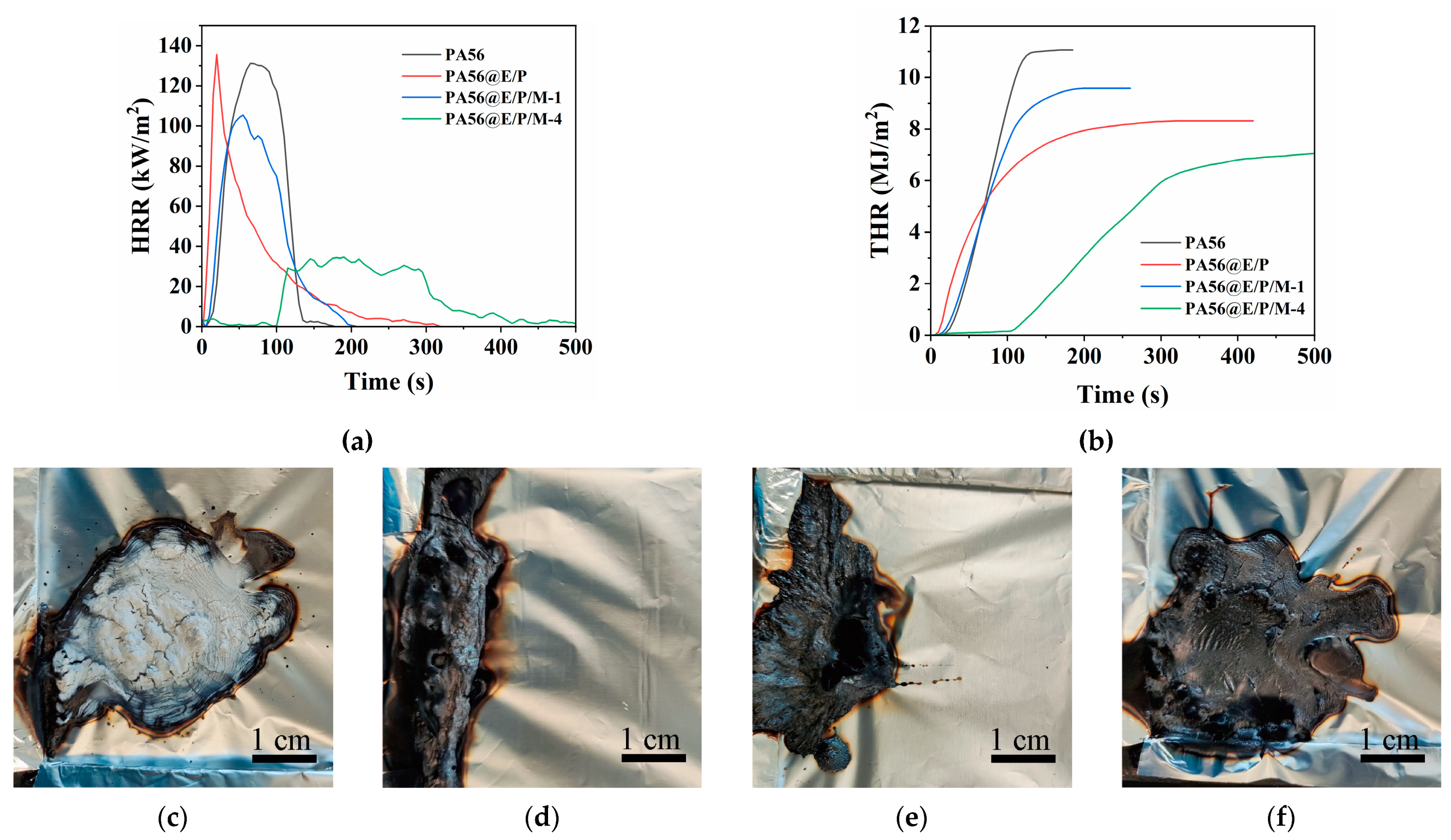
3.4.2. Cone Calorimetry Test
3.5. Flame Retardants’ Mechanical Properties
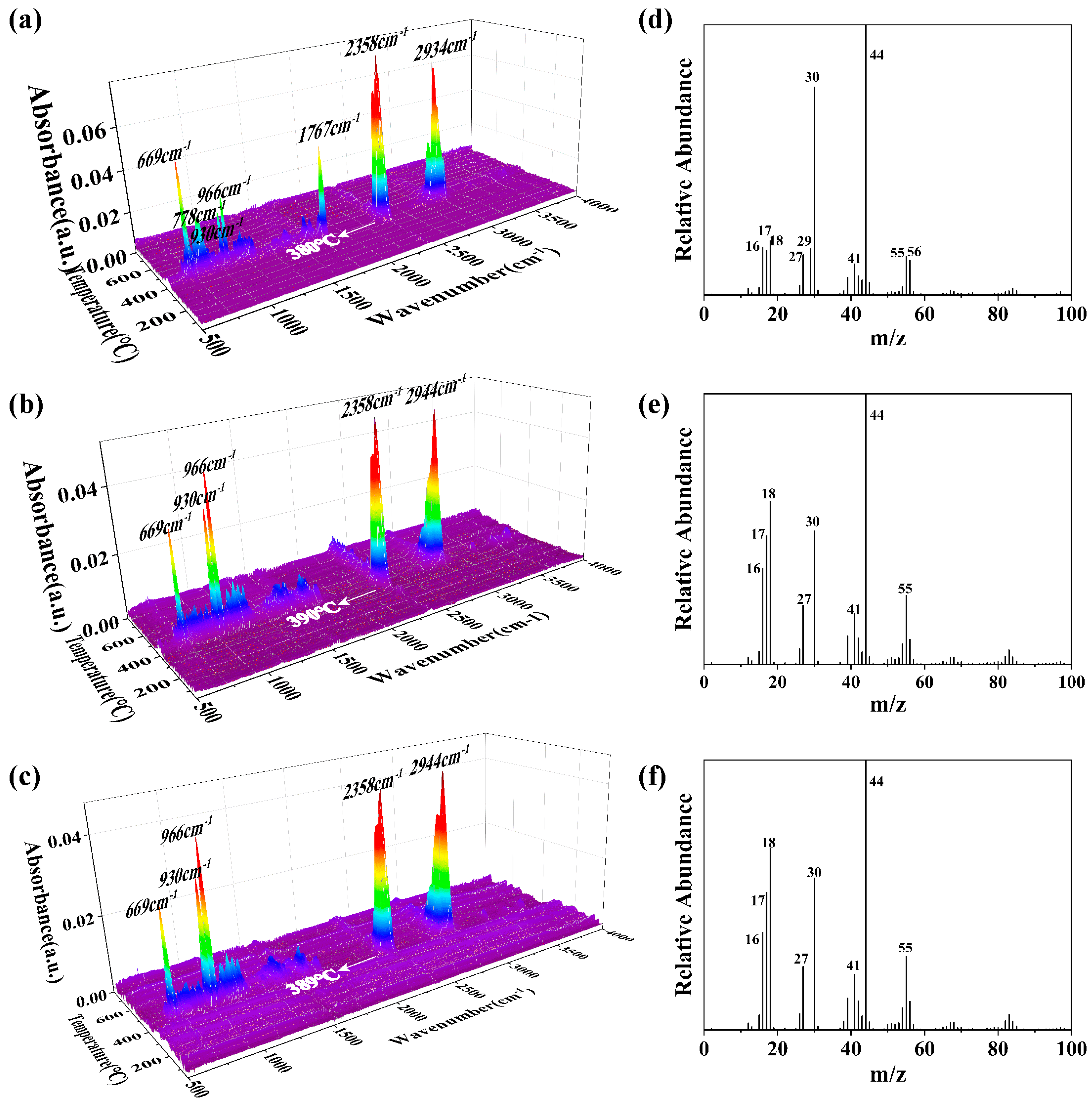
3.5.1. TG-IR-MS Analysis
3.5.2. Raman Analysis
3.5.3. SEM of Residual Carbon
3.5.4. FTIR of Char Residual
3.6. Mechanical Properties and Durability
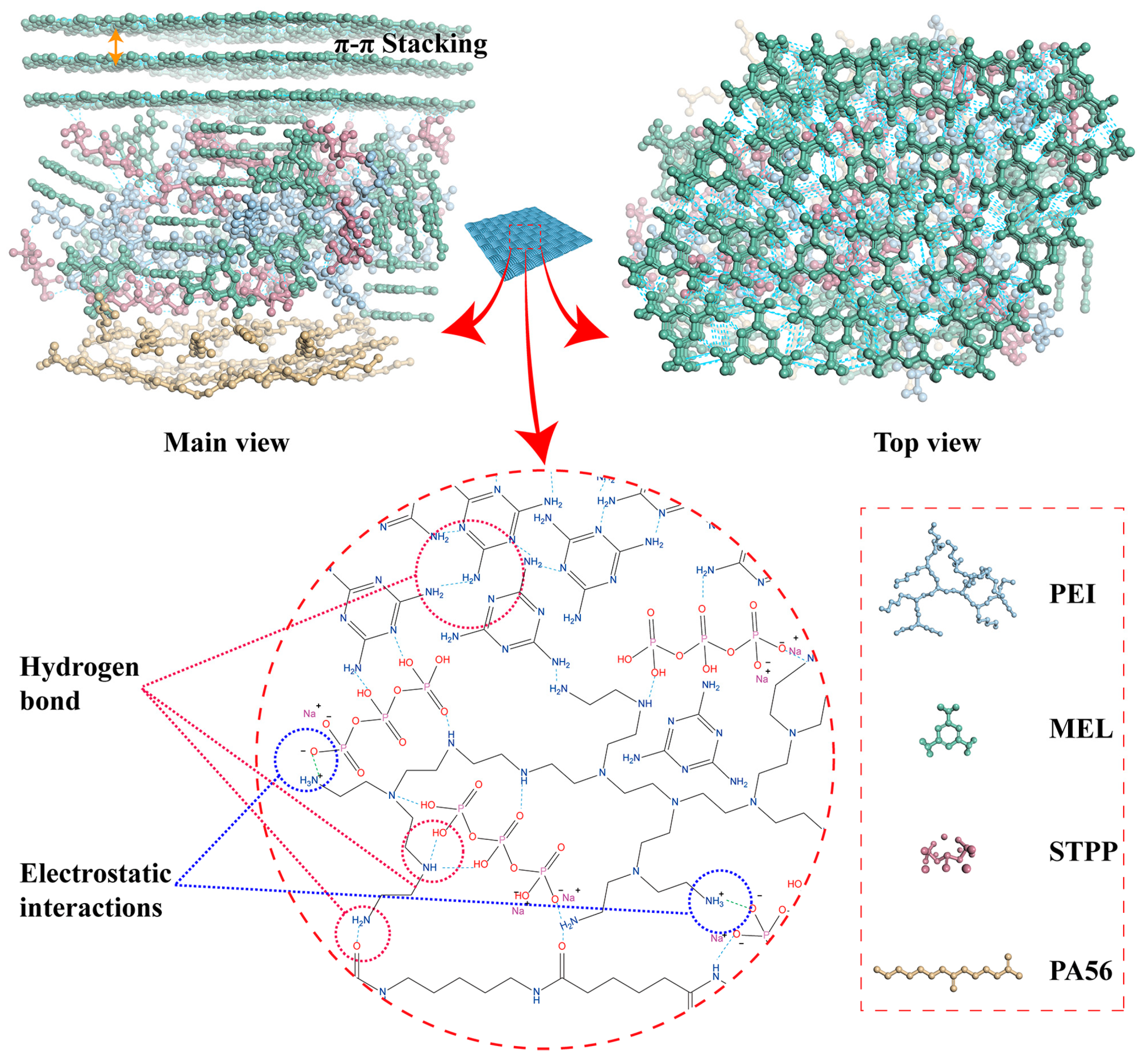
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, M. Aliphatic polyamides. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 487–511. [Google Scholar]
- Doagou-Rad, S.; Islam, A.; Merca, T.D. An application-oriented roadmap to select polymeric nanocomposites for advanced applications: A review. Polym. Compos. 2020, 41, 1153–1189. [Google Scholar] [CrossRef]
- Kausar, A. Trends in graphene reinforced polyamide nanocomposite for functional application: A review. Polym. Plast. Technol. Mater. 2019, 58, 917–933. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; Liu, X.; Wang, X.; Ma, B.; He, Y. Flame-retardant polyamide 56 with high fire safety and good thermal performance. Polym. Adv. Technol. 2022, 33, 2807–2819. [Google Scholar] [CrossRef]
- Jin, W.-J.; Dong, S.; Guan, J.-P.; Cheng, X.-W.; Qin, C.-X.; Chen, G.-Q. Multifunctional and sustainable DOPO-derivative coating for flame-retardant, antibacterial and UV-protective of polyamide 56 protective biomaterials. Surf. Interfaces 2023, 42, 103513. [Google Scholar] [CrossRef]
- Wen, O.Y.; Tohir, M.Z.M.; Yeaw, T.C.S.; Razak, M.A.; Zainuddin, H.S.; Hamid, M.R.A. Fire-resistant and flame-retardant surface finishing of polymers and textiles: A state-of-the-art review. Prog. Org. Coat. 2023, 175, 107330. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of ageing on the fire behaviour of flame-retarded polymers: A review. Polym. Int. 2015, 64, 313–328. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, G.-X.; Zhang, F.-X. Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose 2015, 22, 2787–2796. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Wang, X.; Song, L.; Hu, Y. A novel green phosphorus-containing flame retardant finishing on polysaccharide-modified polyamide 66 fabric for improving hydrophilicity and durability. Int. J. Biol. Macromol. 2023, 239, 124252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, X.; Zhang, S.; Li, H.; Feng, Q.; Sun, J.; Zhao, Q. Improving the Fire Performance of Nylon 6,6 Fabric by Chemical Grafting with Acrylamide. Ind. Eng. Chem. Res. 2013, 52, 2290–2296. [Google Scholar] [CrossRef]
- Tsafack, M.J.; Levalois-Gruetzmacher, J. Flame retardancy of cotton textiles by plasma-induced graft-polymerization (PIGP). Surf. Coat. Technol. 2006, 201, 2599–2610. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Gu, Y.; Zeng, Q. Flame retardant polyacrylonitrile fabrics prepared by organic-inorganic hybrid silica coating via sol-gel technique. Prog. Org. Coat. 2017, 112, 225–233. [Google Scholar] [CrossRef]
- Hu, W. The melting point of chain polymers. J. Chem. Phys. 2000, 113, 3901–3908. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Peng, B.; Ren, Y.; Cheng, B.; Ding, C.; Su, X.; He, J.; Lin, S. Flame retardant cellulosic fabrics via layer-by-layer self-assembly double coating with egg white protein and phytic acid. J. Clean. Prod. 2020, 243, 118641. [Google Scholar] [CrossRef]
- Zheng, X.-T.; Dong, Y.-Q.; Liu, X.-D.; Xu, Y.-L.; Jian, R.-K. Fully bio-based flame-retardant cotton fabrics via layer-by-layer self assembly of laccase and phytic acid. J. Clean. Prod. 2022, 350, 131525. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Deshmukh, A.; Suárez-Villagrán, M.Y.; Das, R.; Pal, V.; Dey, S.; Miller, J.H., Jr.; Machado, L.D.; Kumbhakar, P.; Tiwary, C.S. 2D Hexagonal Boron Nitride-Coated Cotton Fabric with Self-Extinguishing Property. ACS Appl. Mater. Interfaces 2020, 12, 45274–45280. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, J.; Zhang, J.S.; Gu, X.; Bourbigot, S.; Li, H.; Tang, W.; Zhang, S. Preparation of a novel intumescent flame retardant based on supramolecular interactions and its application in polyamide 11. ACS Appl. Mater. Interfaces 2017, 9, 24964–24975. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yi, D.; Feng, H.; Hou, B.; Hao, J. Influence of the Crystal Structure of Melamine Trimetaphosphate 2D Supramolecules on the Properties of Polyamide 6. ACS Appl. Mater. Interfaces 2023, 15, 12393–12402. [Google Scholar] [CrossRef] [PubMed]
- Maryin, P.V.; Tran, T.-H.; Frolova, A.A.; Buldakov, M.A.; Choinzonov, E.L.; Kozelskaya, A.I.; Rutkowski, S.; Tverdokhlebov, S.I. Electrospun Poly-L-Lactic Acid Scaffolds Surface-Modified via Reactive Magnetron Sputtering Using Different Mixing Ratios of Nitrogen and Xenon. Polymers 2023, 15, 2969. [Google Scholar] [CrossRef]
- König, A.; Fehrenbacher, U.; Kroke, E.; Hirth, T. Thermal Decomposition Behavior of the Flame Retardant Melamine in Slabstock Flexible Polyurethane Foams. J. Fire Sci. 2009, 27, 187–211. [Google Scholar] [CrossRef]
- Maroufi, P.; Najafi Moghadam, P.; Vahabi, H. New nitrogen-rich flame retardant based on conductive poly(aniline-co-melamine). React. Funct. Polym. 2020, 150, 104548. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Xu, W.; Liu, Y.C.; Xia, J.K.; Wu, Q.X.; Xu, W.J. Preparation and characterization of flame-retardant melamine cyanurate/polyamide 6 nanocomposites by in situ polymerization. J. Appl. Polym. Sci. 2009, 113, 2109–2116. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Luo, L.; Yang, Y.; Wang, J.; Chen, Y.; Yang, Y.; Yuan, Z.; Chen, X. Molecules Featuring the Azaheterocycle Moiety toward the Application of Flame-Retardant Polymers. ACS Chem. Health Saf. 2023, 30, 343–361. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Sha, K.; Xiao, R. Preparation and characterizations of flame retardant melamine cyanurate/polyamide 6 composite fibers via in situ polymerization. Text. Res. J. 2017, 87, 561–569. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Xiao, R. Preparation and characterization of flame-retarded polyamide 66 with melamine cyanurate by in situ polymerization. Macromol. Res. 2017, 25, 779–785. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, J.; Steele, T.; Merkel, O.; Kissel, T. A new synthesis method and degradation of hyper-branched polyethylenimine grafted polycaprolactone block mono-methoxyl poly (ethylene glycol) copolymers (hy-PEI-g-PCL-b-mPEG) as potential DNA delivery vectors. Polymer 2009, 50, 3895–3904. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Y.; Long, J.; Zhao, Q.; Ding, X.; Chen, L.; Wang, Y. Layer-by-layer assembled flame-retardant architecture toward high-performance carbon fiber composite. Chem. Eng. J. 2018, 353, 550–558. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Geng, J.; Yin, Y.; Liang, Q.; Zhu, Z.; Luo, H. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism. Chem. Eng. J. 2019, 361, 1497–1510. [Google Scholar] [CrossRef]
- Cheng, X.; Tang, R.; Yao, F.; Yang, X. Flame retardant coating of wool fabric with phytic acid/polyethyleneimine polyelectrolyte complex. Prog. Org. Coat. 2019, 132, 336–342. [Google Scholar] [CrossRef]
- Shabanian, M.; Khoobi, M.; Hemati, F.; Khonakdar, H.A.; Faghihi, K.; Wagenknecht, U.; Shafiee, A. Effects of polyethyleneimine-functionalized MCM-41 on flame retardancy and thermal stability of polyvinyl alcohol. Particuology 2015, 19, 14–21. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Yang, A.; Chen, P.; Zhao, T.; Liu, F. Polyethyleneimine-Modified Amorphous Silica for the Selective Adsorption of CO2/N2 at High Temperatures. ACS Omega 2021, 6, 35389–35397. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Han, Y.; Du, P.; Fei, Z.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Liu, Q.; Qiao, X. Polyethylenimine (PEI)-impregnated resin adsorbent with high efficiency and capacity for CO 2 capture from flue gas. New J. Chem. 2019, 43, 18345–18354. [Google Scholar] [CrossRef]
- Cao, K.; Guo, Y.; Zhang, M.; Arrington, C.B.; Long, T.E.; Odle, R.R.; Liu, G. Mechanically strong, thermally stable, and flame retardant poly (ether imide) terminated with phosphonium bromide. Macromolecules 2019, 52, 7361–7368. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Chen, H.; Ding, C.; Xuan, Y.; Pan, M.; Mei, C. A branched polyelectrolyte complex enables efficient flame retardant and excellent robustness for wood/polymer composites. Polymers 2020, 12, 2438. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.; Ma, T.; Zhang, S.; Liu, Z.; Pan, M. Densification and polyelectrolyte assisted fabricating laminated wood composites for outstanding flame retardancy and mechanical properties. Constr. Build. Mater. 2023, 393, 132041. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.; Yang, Y.; Liu, Y.; Ding, D.; Chen, Y.; Zhang, G. Synthesis and application of a novel high durable cotton flame retardant rich in PN covalent bonds and ammonium phosphate groups. Chem. Eng. J. 2023, 454, 140422. [Google Scholar] [CrossRef]
- Korotkov, A.S. Melamine/monoammonium phosphate complex as the polyphosphate substitute in flame retardant coatings. J. Fire Sci. 2016, 34, 89–103. [Google Scholar] [CrossRef]
- Yang, W.; Yang, F.; Yang, R.; Wang, B. Ammonium polyphosphate/melamine cyanurate synergetic flame retardant system for use in papermaking. BioResources 2016, 11, 2308–2318. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Y.; Xu, D.; Yang, J.; Wang, X.; Luo, T.; Zhang, Z. Hydrolysis Mechanism of Water-Soluble Ammonium Polyphosphate Affected by Zinc Ions. ACS Omega 2023, 8, 17573–17582. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A.; Henclik, A.; Kulczycka, J.; Cholewa, M. Comparative evaluation of sodium tripolyphosphate production technologies with the use of a complex quality method. Pol. J. Chem. Technol. 2020, 22, 48–54. [Google Scholar] [CrossRef]
- Yang, S.; Dai, L.; Mao, L.; Liu, J.; Yuan, F.; Li, Z.; Gao, Y. Effect of sodium tripolyphosphate incorporation on physical, structural, morphological and stability characteristics of zein and gliadin nanoparticles. Int. J. Biol. Macromol. 2019, 136, 653–660. [Google Scholar] [CrossRef] [PubMed]
- GB/T 5455-2014; Textiles-Burning Behaviour-Determination of Damaged Length, Afterglow Time and Afterflame Time of Vertically Oriented Specimens. Standards Press of China: Beijing, China, 2014.
- GB/T 5454-1997; Textiles-Burning Behaviour-Oxygen Index Method. Standards Press of China: Beijing, China, 1997.
- ISO 5660-1; Reaction-to-Fire Tests. Heat Release, Smoke Production and Mass Loss Rate. Part 1, Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). International Organization for Standardization: Geneva, Switzerland, 2015.
- ASTM D5035; Standard Test Method for Breaking Strength and Elongation of Textile Fabrics. ASTM International: Conshohocken, PA, USA, 2011.
- ASTM D1388; Standard Test Method for Stiffness of Fabrics. ASTM International: Conshohocken, PA, USA, 2014.
- GB/T 8629-2017; Textiles-Domestic Washing and Drying Procedures for Textile Testing. Standards Press of China: Beijing, China, 2017.
- Ziebarth, J.D.; Wang, Y. Understanding the protonation behavior of linear polyethylenimine in solutions through Monte Carlo simulations. Biomacromolecules 2010, 11, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.A.; Murray, S.; Holder, K.M.; Nolen, C.R.; Grunlan, J.C. Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in single step. Macromol. Mater. Eng. 2014, 299, 1180–1187. [Google Scholar] [CrossRef]
- Frueh, J.; Reiter, G.; Keller, J.; Möhwald, H.; He, Q.; Krastev, R. Effect of Linear Elongation of PDMS-Supported Polyelectrolyte Multilayer Determined by Attenuated Total Reflectance IR Radiation. J. Phys. Chem. B 2013, 117, 2918–2925. [Google Scholar] [CrossRef] [PubMed]
- Kipnusu, W.K.; Zhuravlev, E.; Schick, C.; Kremer, F. Homogeneous nucleation in polyamide 66, a two-stage process as revealed by combined nanocalorimetry and IR spectroscopy. Colloid Polym. Sci. 2022, 300, 1247–1255. [Google Scholar] [CrossRef]
- Liu, W.; Shi, R.; Ge, X.; Huang, H.; Chen, X.; Mu, M. A bio-based flame retardant coating used for polyamide 66 fabric. Prog. Org. Coat. 2021, 156, 106271. [Google Scholar] [CrossRef]
- Devallencourt, C.; Saiter, J.; Fafet, A.; Ubrich, E. Thermogravimetry/Fourier transform infrared coupling investigations to study the thermal stability of melamine formaldehyde resin. Thermochim. Acta 1995, 259, 143–151. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R. Characterization and photocatalytic activity of N-doped TiO2 prepared by thermal decomposition of Ti–melamine complex. Appl. Catal. B 2007, 74, 307–312. [Google Scholar] [CrossRef]
- Kang, H.; Wang, Z.; Hao, X.; Liu, R. Thermal induced crystalline transition of bio-based polyamide 56. Polymer 2022, 242, 124540. [Google Scholar] [CrossRef]
- Xun, Z.; He, G.; Huang, Y.; Yu, R. Effect of SiO2 particles on the crystallization and formation of hydrogen bonding in biobased polyamide 56. ACS Appl. Polym. Mater. 2022, 4, 4949–4960. [Google Scholar] [CrossRef]
- Jianxin, L.; Min, Z.; Zhiqiang, Z.; Baohua, L.; Liban, C. Thermal decomposition studies of highly alternating CO2-cyclohexene oxide copolymer. e-Polymers 2009, 9, 120. [Google Scholar] [CrossRef][Green Version]
- Chen, D.; Liu, B.; Wang, X.; Li, X.; Xu, X.; He, J.; Yang, R. High flame retardant and heat-resistance, low dielectric benzoxazine resin with phthalimide structure. Polym. Degrad. Stab. 2022, 205, 110150. [Google Scholar] [CrossRef]
- Pallmann, J.; Ren, Y.L.; Mahltig, B.; Huo, T.G. Phosphorylated sodium alginate/APP/DPER intumescent flame retardant used for polypropylene. J. Appl. Polym. Sci. 2019, 136, 47794. [Google Scholar] [CrossRef]
- Pagacz, J.; Leszczynska, A.; Modesti, M.; Boaretti, C.; Roso, M.; Malka, I.; Pielichowski, K. Thermal decomposition studies of bio-resourced polyamides by thermogravimetry and evolved gas analysis. Thermochim. Acta 2015, 612, 40–48. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U.J.C. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Zeng, Y.; Weinell, C.E.; Dam-Johansen, K.; Ring, L.; Kiil, S. Effects of coating ingredients on the thermal properties and morphological structures of hydrocarbon intumescent coating chars. Prog. Org. Coat. 2020, 143, 105626. [Google Scholar] [CrossRef]



| Sample | C (wt%) | N (wt%) | O (wt%) | P (wt%) |
|---|---|---|---|---|
| PA56 | 64.58 | 16.41 | 19.01 | 0 |
| PA56@E/P | 63.77 | 15.18 | 19.91 | 1.14 |
| PA56@E/P/M-4 | 61.95 | 18.09 | 19.96 | 0 |
| Sample | T5% (°C) | Tmax (°C) | Char residue at 800 °C (wt%) |
|---|---|---|---|
| PA56 | 350 | 394 | 1.2 |
| PA56@E/P | 330 | 378 | 7.3 |
| PA56@E/P/M-0.5 | 370 | 423 | 5.0 |
| PA56@E/P/M-1 | 366 | 407 | 4.6 |
| PA56@E/P/M-2 | 364 | 425 | 4.9 |
| PA56@E/P/M-3 | 358 | 410 | 4.1 |
| PA56@E/P/M-4 | 356 | 406 | 4.5 |
| Sample | Tm1 (°C) | ΔHm1 (J/g) | Tm2 (°C) | ΔHm2 (J/g) | Tc (°C) |
|---|---|---|---|---|---|
| PA56 | 229.2 | 12.2 | 253 | 61.8 | 217 |
| PA56@E/P | 230.3 | 10.2 | 250.2 | 44.7 | 202.6 |
| PA56@E/P/M-0.5 | 229.2 | 8.8 | 251.1 | 50.3 | 210.7 |
| PA56@E/P/M-1 | 229.9 | 8.5 | 251.2 | 52.7 | 210.7 |
| PA56@E/P/M-2 | 230.9 | 8.2 | 252.2 | 48.8 | 213.7 |
| PA56@E/P/M-4 | 229.3 | 7.1 | 252 | 55 | 211.8 |
| Sample | Weight Gain (%) | After-Flame Time (s) | After-Glow Time (s) | Dripping | Char Length (cm) | LOI (%) |
|---|---|---|---|---|---|---|
| PA56 | - | 0 | 0 | Yes | 16.3 ± 1.2 | 20.9 |
| PA56@E/P | 10.1 ± 0.2 | 0 | 16.2 ± 3.7 | No | 30 | 21.7 |
| PA56@E/P/M-0.5 | 6.4 ± 0.1 | 0 | 18.3 ± 5.0 | Yes | 20.4 ± 1.7 | 19.6 |
| PA56@E/P/M-1 | 5.9 ± 0.2 | 0 | 0 | No | 14.7 ± 1.4 | 20.9 |
| PA56@E/P/M-2 | 6.0 ± 0.1 | 0 | 0 | No | 13.8 ± 1.0 | 21.6 |
| PA56@E/P/M-3 | 5.5 ± 0.4 | 0 | 0 | No | 12.9 ± 1.4 | 22.1 |
| PA56@E/P/M-4 | 6.2 ± 0.2 | 0 | 0 | No | 12.3 ± 1.3 | 23.2 |
| Sample | TTI (s) | HRR (kW/m2) | pHRR (kW/m2) | THR (MJ/m2) | TSP (m2/m2) | COy (kg/kg) | CO2y (kg/kg) | MLR (g/(s·m2)) | Residue (%) |
|---|---|---|---|---|---|---|---|---|---|
| PA56 | 22 | 97.4 | 132.5 | 10.9 | 59.0 | 0.0214 | 1.85 | 4.1 | 3.8 |
| PA56@E/P | 15 | 27.2 | 139.9 | 7.7 | 150.1 | 0.0331 | 1.59 | 1.7 | 17.2 |
| PA56@E/P/M-1 | 16 | 70.8 | 105.6 | 9 | 134 | 0.0322 | 1.70 | 3.2 | 7.9 |
| PA56@E/P/M-4 | 113 | 17.2 | 35.6 | 6.8 | 142.6 | 0.0197 | 1.52 | 1.1 | 16.0 |
| Sample | Tensile Strength (N) | Elongation at Break (%) | Bending Length (cm) |
|---|---|---|---|
| PA56 | 450.0 ± 11.4 | 85.0 ± 6.5 | 3.89 ± 0.29 |
| PA56@E/P | 456.2 ± 11.5 | 83.5 ± 3.2 | 4.64 ± 0.14 |
| PA56@E/P/M-4 | 451.6 ± 13.7 | 85.7 ± 5.3 | 3.95 ± 0.25 |
| Number of Washings | After-Flame Time (s) | After-Glow Time (s) | Dropping | Char Length (cm) |
|---|---|---|---|---|
| 1 | 0 | 0 | None | 12.3 ± 1.3 |
| 5 | 0 | 0 | None | 12.9 ± 1.5 |
| 10 | 0 | 0 | None | 13.2 ± 0.9 |
| 20 | 0 | 0 | None | 14.1 ± 1.6 |
| 50 | 0 | 0 | None | 15.2 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Liu, Y.; Gu, D.; Zhu, H.; Wang, M.; Dong, M.; Guo, Y.; Sun, H.; Hao, J.; Hao, X. Three-Dimensional Cross-Linking Network Coating for the Flame Retardant of Bio-Based Polyamide 56 Fabric by Weak Bonds. Polymers 2024, 16, 1044. https://doi.org/10.3390/polym16081044
Cui Y, Liu Y, Gu D, Zhu H, Wang M, Dong M, Guo Y, Sun H, Hao J, Hao X. Three-Dimensional Cross-Linking Network Coating for the Flame Retardant of Bio-Based Polyamide 56 Fabric by Weak Bonds. Polymers. 2024; 16(8):1044. https://doi.org/10.3390/polym16081044
Chicago/Turabian StyleCui, Yunlong, Yu Liu, Dongxu Gu, Hongyu Zhu, Meihui Wang, Mengjie Dong, Yafei Guo, Hongyu Sun, Jianyuan Hao, and Xinmin Hao. 2024. "Three-Dimensional Cross-Linking Network Coating for the Flame Retardant of Bio-Based Polyamide 56 Fabric by Weak Bonds" Polymers 16, no. 8: 1044. https://doi.org/10.3390/polym16081044
APA StyleCui, Y., Liu, Y., Gu, D., Zhu, H., Wang, M., Dong, M., Guo, Y., Sun, H., Hao, J., & Hao, X. (2024). Three-Dimensional Cross-Linking Network Coating for the Flame Retardant of Bio-Based Polyamide 56 Fabric by Weak Bonds. Polymers, 16(8), 1044. https://doi.org/10.3390/polym16081044






