The Use of an Advanced Intelligent–Responsive Polymer for the Study of Dynamic Water–Carbon Dioxide Alternating Displacement
Abstract
1. Introduction
2. Experimental Methods
2.1. Experimental Materials and Synthesis Methods
2.2. Synthesis and Preparation of Polymers
2.2.1. Infrared Spectroscopy
2.2.2. 1H Magnetic Nuclear Resonance Spectroscopy
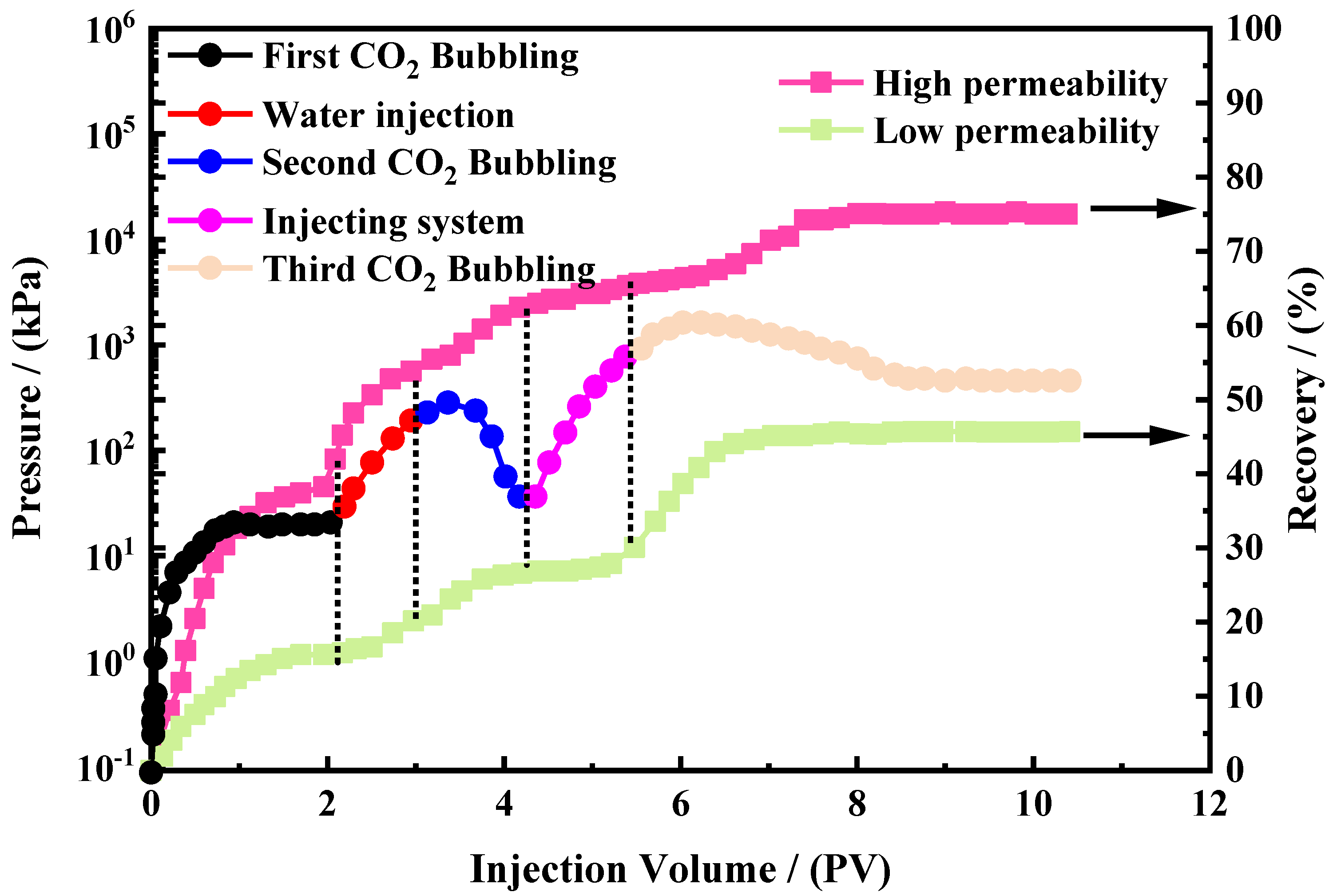
2.2.3. Evaluation of Blocking Performance
- η represents the blocking efficiency, %.
- ΔP1 represents the stabilized differential pressure value during the first injection of CO2, kPa.
- ΔP2 represents the breakthrough pressure difference value during the second injection of CO2, kPa.
3. Results and Discussion
3.1. Infrared and Nuclear Magnetic Analyses
3.1.1. Infrared Spectral Characterization
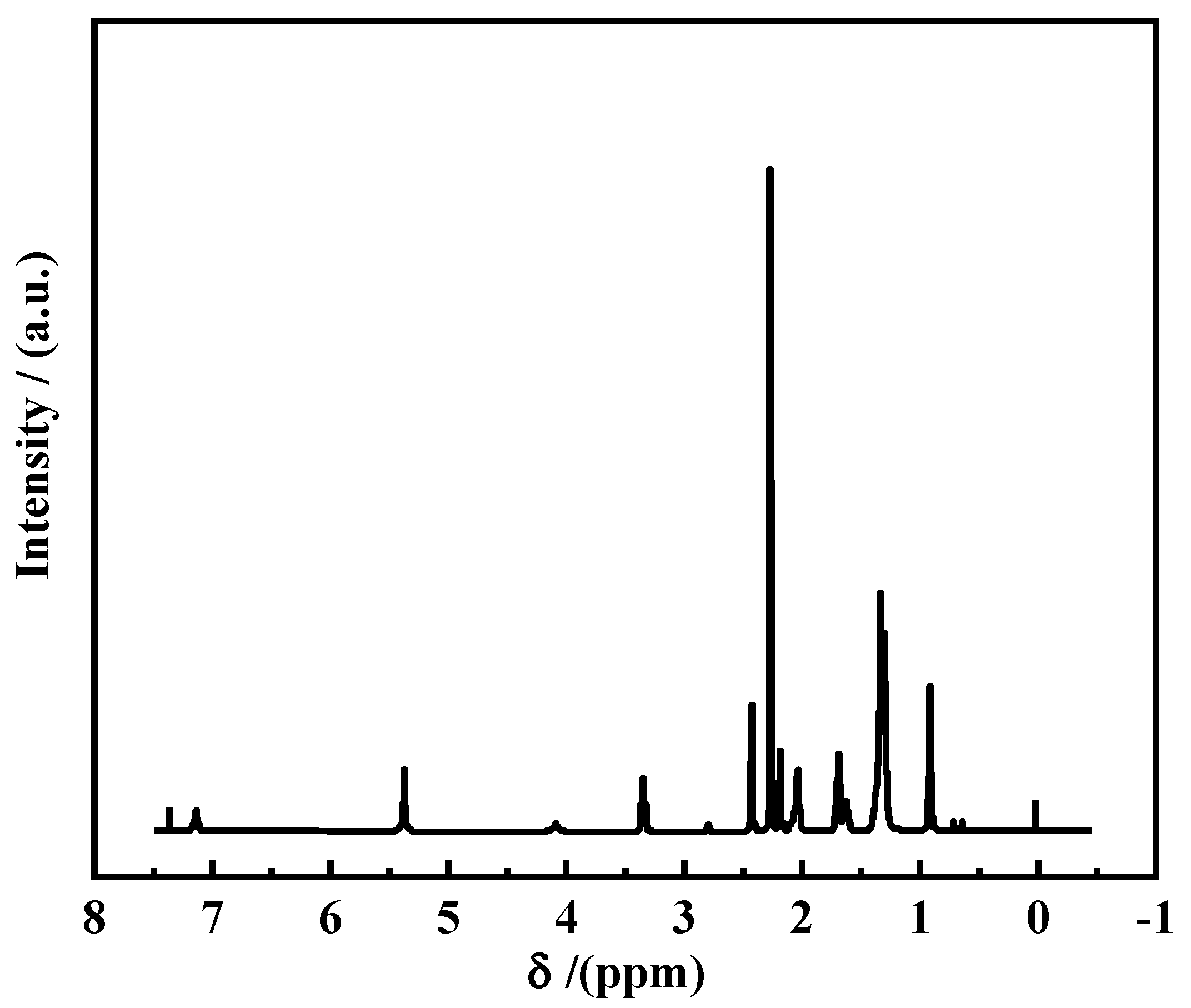
3.1.2. NMR Spectral Analysis
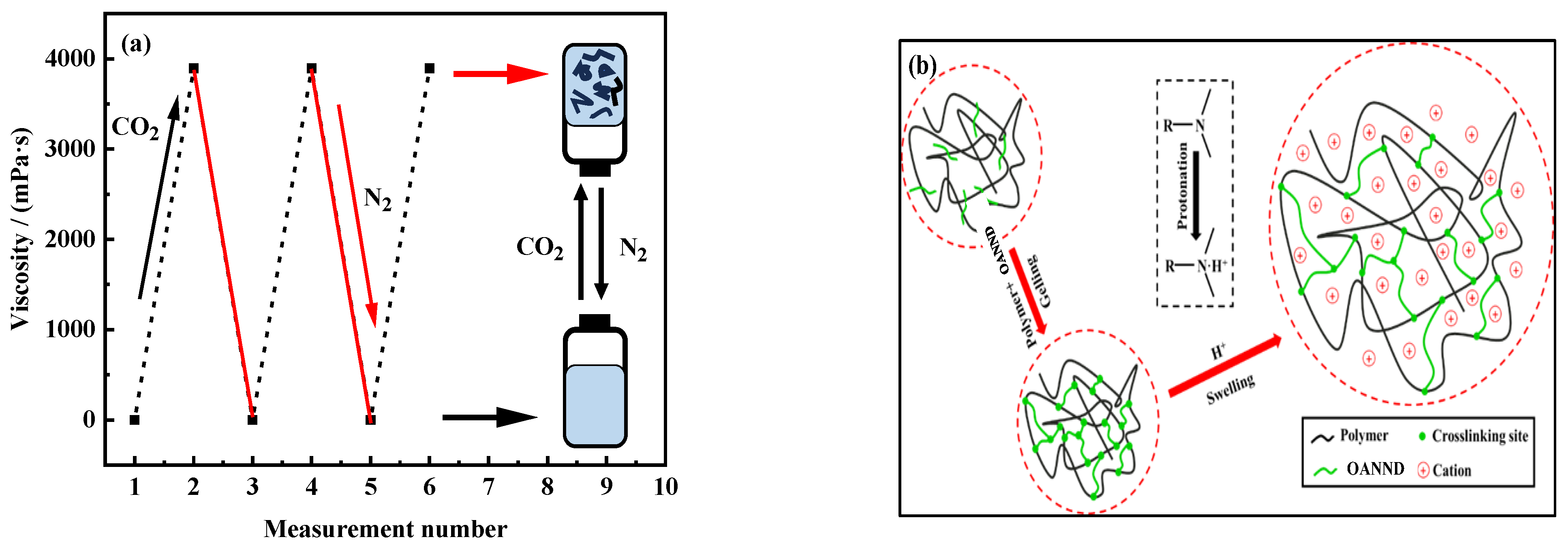
3.2. Analysis of Reversible Responsive Behavior
3.3. The Response of CO2 to Polymer Rheology Analysis
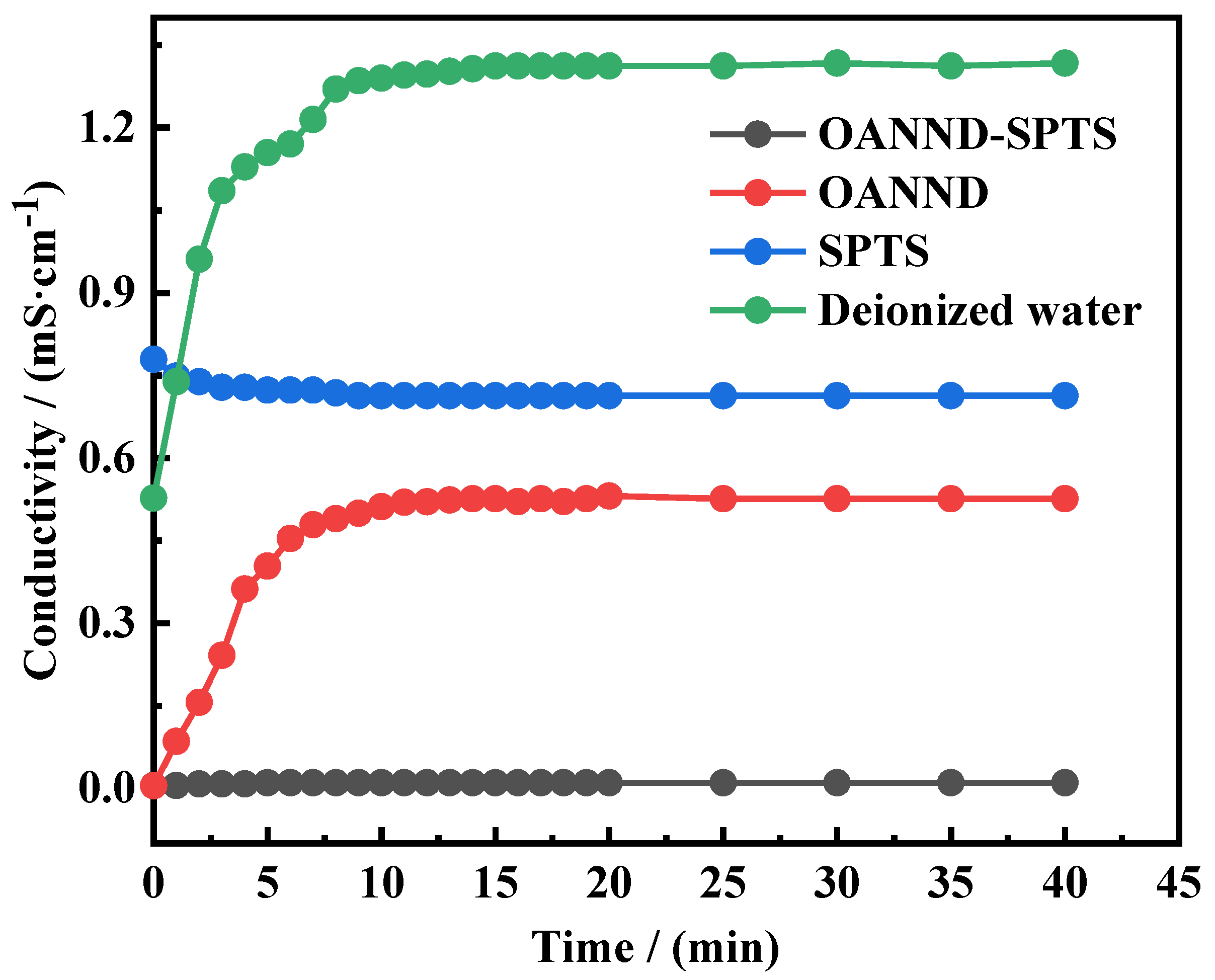
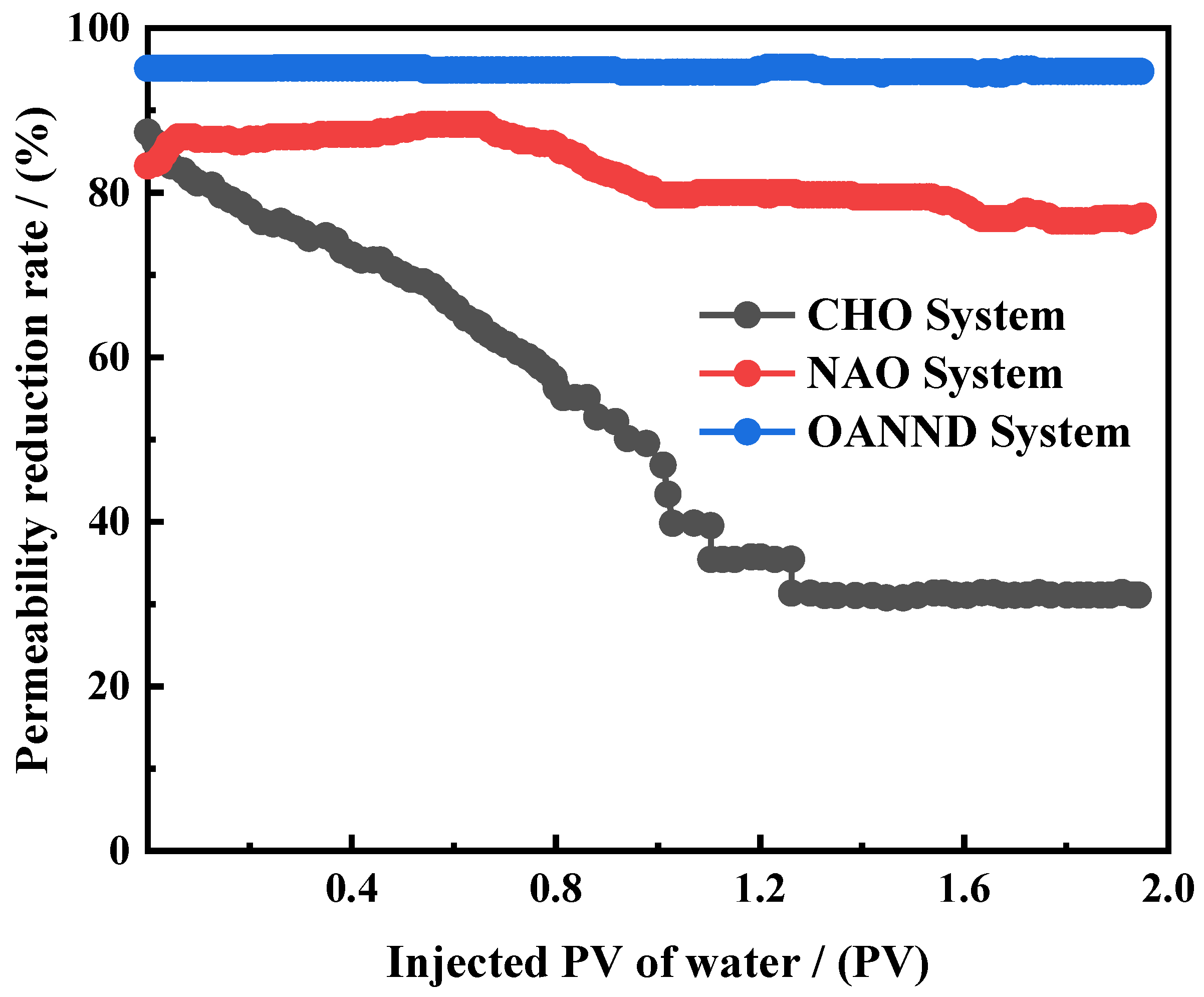
3.4. Sealing Performance Analysis
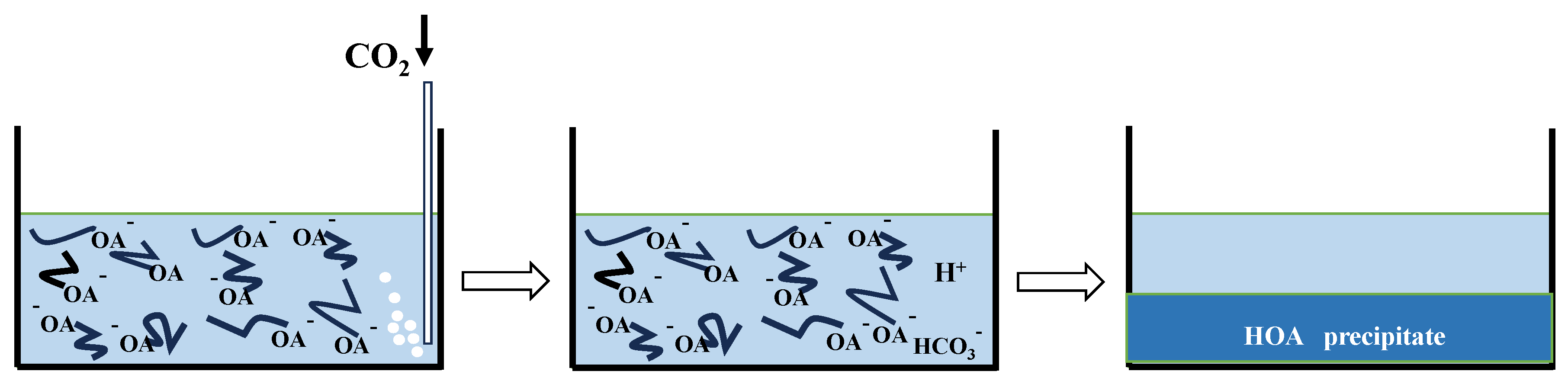
3.5. Double-Tube Oil Repulsion Experiment and Action Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, D.F.; Taber, J.J. Carbon dioxide flooding. J. Pet. Technol. 1992, 44, 396–400. [Google Scholar] [CrossRef]
- Holm, L.; Josendal, V. Mechanisms of oil displacement by carbon dioxide. J. Pet. Technol. 1974, 26, 1427–1438. [Google Scholar] [CrossRef]
- Tafty, M.F.; Masihi, M.; Momeni, A. Recovery improvement using water and gas injection scenarios. Pet. Sci. Technol. 2011, 29, 290–300. [Google Scholar] [CrossRef]
- You, Q.; Wang, H.; Zhang, Y.; Liu, Y.; Fang, J.; Dai, C. Experimental study on spontaneous imbibition of recycled fracturing flow-back fluid to enhance oil recovery in low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2018, 166, 375–380. [Google Scholar] [CrossRef]
- Yuan, B.; Wood, D.A.; Yu, W. Stimulation and hydraulic fracturing technology in natural gas reservoirs: Theory and case studies (2012–2015). J. Nat. Gas Sci. Eng. 2015, 26, 1414–1421. [Google Scholar] [CrossRef]
- Zhao, F.; Hao, H.; Hou, J.; Hou, L.; Song, Z. CO2 mobility control and sweep efficiency improvement using starch gel or ethylenediamine in ultra-low permeability oil layers with different types of heterogeneity. J. Pet. Sci. Eng. 2015, 133, 52–65. [Google Scholar] [CrossRef]
- Orr, F.M.; Heller, J.P.; Taber, J.J. Carbon dioxide flooding for enhanced oil recovery: Promise and problems. J. Am. Oil Chem. Soc. 1982, 59, 810–817. [Google Scholar] [CrossRef]
- Aycaguer, A.-C.; Lev-On, M.; Winer, A.M. Reducing carbon dioxide emissions with enhanced oil recovery projects: A life cycle assessment approach. Energy Fuels 2001, 15, 303–308. [Google Scholar] [CrossRef]
- Holm, L. CO2 flooding: Its time has come. J. Pet. Technol. 1982, 34, 2739–2745. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Pascual, M. Water-alternating-gas pilot in the largest oil field in argentina: Chihuido de la Sierra Negra, Neuquen Basin. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 17–19 May 2017. [Google Scholar]
- Feng, B.J.; Du, X.J.; Cai, Y. Pilot Test of Water Alternating Gas Injection in Heterogeneous Thick Reservoir of Positive Rhythm Sedimentation of Daqing Oil Field. Adv. Technol. Ser. 1997, 5, 41–48. [Google Scholar]
- Stone, H.L. Vertical, conformance in an alternating water-miscible gas flood. In Proceedings of the 57th Annual Fall Technical Conference and Exhibition of the Society of Petroleum Engineers of AIME, New Orleans, LA, USA, 26–29 September 1982. [Google Scholar]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. Insights into CO2 Capture by Flue Gas Hydrate Formation: Gas Composition Evolution in Systems Containing Gas Hydrates and Gas Mixtures at Stable Pressures. ACS Sustain. Chem. Eng. 2018, 6, 5732–5736. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar]
- Kokkinos, N.C.; Nkagbu, D.C.; Marmanis, D.I.; Dermentzis, K.I.; Maliaris, G. Evolution of Unconventional Hydrocarbons: Past, Present, Future and Environmental Foot Print. J. Eng. Sci. Technol. Rev. 2022, 15, 15–24. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, Y.; Wen, X.; Liu, Y.; Feng, M.; Rui, Z. Development and Applications of CO2-Responsive Gels in CO2 Flooding and Geological Storage. Gels 2023, 9, 936. [Google Scholar] [CrossRef]
- Hao, H.; Hou, J.; Zhao, F.; Song, Z.; Hou, L.; Wang, Z. Gas channeling control during CO2 immiscible flooding in 3D radial flow model with complex fractures and heterogeneity. J. Pet. Sci. Eng. 2016, 146, 890–901. [Google Scholar] [CrossRef]
- Christensen, J.R.; Stenby, E.H.; Skauge, A. Review of WAG field experience. SPE Reserv. Eval. Eng. 2001, 4, 97–106. [Google Scholar] [CrossRef]
- Caudle, B.; Dyes, A. Improving miscible displacement by gas-water injection. Trans. AIME 1958, 213, 281–283. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. A comprehensive review on chemically enhanced water alternating gas/CO2 (CEWAG) injection for enhanced oil recovery. J. Pet. Sci. Eng. 2017, 157, 696–715. [Google Scholar] [CrossRef]
- Li, W.; Schechter, D.S. Using polymer alternating gas to maximize CO2 flooding performance. In Proceedings of the SPE Biennial Energy Resources Conference, Port of Spain, Trinidad and Tobago, 9–11 June 2014. [Google Scholar]
- Zhang, Y.; Huang, S.S.; Luo, P. Coupling immiscible CO2 technology and polymer injection to maximize EOR performance for heavy oils. J. Can. Pet. Technol. 2010, 49, 25–33. [Google Scholar] [CrossRef]
- Moradi, A.A.; Hsieh, E.T.; Westerman, I.J. Role of imidization in thermal hydrolysis of polyacrylamides. In Water-Soluble Poly-mers for Petroleum Recovery; Springer: Boston, MA, USA, 1988; pp. 271–278. [Google Scholar]
- Su, X.; Feng, Y. Thermoviscosifying smart polymers for oil and gas production: State of the art. Chemphyschem 2018, 19, 1941–1955. [Google Scholar] [CrossRef]
- Quan, H.; Xie, L.; Su, X.; Feng, Y. The thermoviscosifying behavior of water-soluble polymer based on graft polymerization of pluronic F127 with acrylamide and 2-acrylamido-2-methylpropane sulfonic acid sodium salt. Polymers 2019, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L. Polyether-modified poly (acrylic acid): Synthesis and applications. Ind. Eng. Chem. Res. 1998, 37, 4267–4274. [Google Scholar] [CrossRef]
- Durand, A.; Hourdet, D. Thermoassociative graft copolymers based on poly (N-isopropylacrylamide): Relation between the chemical structure and the rheological properties. Macromol. Chem. Phys. 2000, 201, 858–868. [Google Scholar] [CrossRef]
- Edwards, H.; Johnson, A.; Lawson, E. Structural determination of substituted mercaptothiadiazoles using FT-Raman and FT-IR spectroscopy. J. Mol. Struct. 1995, 351, 51–63. [Google Scholar] [CrossRef]
- He, X.; Mao, J.; Ma, Q.; Tang, Y. Corrosion inhibition of perimidine derivatives for mild steel in acidic media: Electrochemical and computational studies. J. Mol. Liq. 2018, 269, 260–268. [Google Scholar] [CrossRef]
- Bromberg, L. Novel Family of Thermogelling Materials via C-C Bonding between Poly (acrylic acid) and Poly (ethylene ox-ide)-b-poly (propylene oxide)-b-poly (ethylene oxide). J. Phys. Chem. B 1998, 102, 1956–1963. [Google Scholar] [CrossRef]
- Bromberg, L.; Magner, E. Release of hydrophobic compounds from micellar solutions of hydrophobically modified polyelectro-lytes. Langmuir 1999, 15, 6792–6798. [Google Scholar] [CrossRef]
- Verma, G.; Aswal, V.K.; Hassan, P. pH-Responsive self-assembly in an aqueous mixture of surfactant and hydrophobic amino acid mimic. Soft Matter 2009, 5, 2919–2927. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, J.; Zheng, L.; Wang, S.; Li, X.; Inoue, T. Salt-induced viscoelastic wormlike micelles formed in surface active ionic liquid aqueous solution. J. Colloid Interface Sci. 2008, 319, 338–343. [Google Scholar] [CrossRef]
- Yuan, B.; Su, Y.; Moghanloo, R.G.; Rui, Z.; Wang, W.; Shang, Y. A new analytical multi-linear solution for gas flow toward fractured horizontal wells with different fracture intensity. J. Nat. Gas Sci. Eng. 2015, 23, 227–238. [Google Scholar] [CrossRef]








| Instrument | Model Number | Manufacturer | Conditions |
|---|---|---|---|
| Conductivity meter | DDS-11A | Jintan Melting Instrument Manufacturing Co., Ltd. Changzhou, China | Normal temperature and pressure |
| Thermostatic heating magnetic stirrer | CJJ78-1 | Jintan Dadi Automation Instrument Factory, Changzhou, China | Normal temperature and pressure |
| Rotary evaporator | R206B | Shanghai Shensheng Technology Co., Ltd. Shanghai, China | Normal temperature and pressure |
| Rotary viscometer | Bookfield DV-III | Ametec Blechfeld China Co., Ltd. Shanghai, China | Normal temperature and pressure |
| Rheometer | HAAKE RS-600 | Thermo Fisher Scientific, Waltham, MA, USA | Normal temperature and pressure |
| Circulating water vacuum pump | SHZ-Ⅲ | Gongyi Yuhua Instrument Co., Ltd. Zhengzhou, China | Normal temperature and pressure |
| Electronic balance | ALC-104 | Mettler Toledo Instruments (Shanghai) Co., Ltd. Shanghai, China | Normal temperature and pressure |
| Polymer Concentration/wt% | N2 Environment | CO2 Environment | ||
|---|---|---|---|---|
| Gelation Time/h | Strength Code | Gelation Time/h | Strength Code | |
| 0.5 | >24 h | A | 1.81 | F-B |
| 1.0 | >24 h | A | 2.03 | H |
| 1.5 | >24 h | A | 2.25 | H |
| 2.0 | >24 h | A | 2.49 | H |
| System | Temperature/°C | Salinity of Formation Water/mg∙L−1 | Sand Pack | Injected Water Volume/PV | Maximum Permeability Reduction Rate/% | Final Permeability Reduction Rate/% | |
|---|---|---|---|---|---|---|---|
| Pore Volume/mL | Initial Permeability/×10−3 μm2 | ||||||
| OANND | 90 | 200,000 | 110 | 1698.5 | 3 | 94.5 | 93.1 |
| 80 | 20,000 | 100 | 19.4 | 2 | 96.5 | 94.2 | |
| 70 | 20,000 | 100 | 59.6 | 2 | 98.7 | 97.9 | |
| 70 | 20,000 | 109 | 120.2 | 2 | 97.9 | 97.2 | |
| NAO | 80 | 20,000 | 98 | 31.6 | 2 | 89.4 | 82.8 |
| CHO | 80 | 20,000 | 105 | 26.1 | 2 | 87.5 | 30.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, J.; Yuan, Y.; Yong, Z.; Yan, Z.; Zhang, J.; Lu, G. The Use of an Advanced Intelligent–Responsive Polymer for the Study of Dynamic Water–Carbon Dioxide Alternating Displacement. Polymers 2024, 16, 1040. https://doi.org/10.3390/polym16081040
Zhang F, Zhang J, Yuan Y, Yong Z, Yan Z, Zhang J, Lu G. The Use of an Advanced Intelligent–Responsive Polymer for the Study of Dynamic Water–Carbon Dioxide Alternating Displacement. Polymers. 2024; 16(8):1040. https://doi.org/10.3390/polym16081040
Chicago/Turabian StyleZhang, Feng, Jingong Zhang, Yidong Yuan, Zishu Yong, Zhuoyue Yan, Jiayuan Zhang, and Guochao Lu. 2024. "The Use of an Advanced Intelligent–Responsive Polymer for the Study of Dynamic Water–Carbon Dioxide Alternating Displacement" Polymers 16, no. 8: 1040. https://doi.org/10.3390/polym16081040
APA StyleZhang, F., Zhang, J., Yuan, Y., Yong, Z., Yan, Z., Zhang, J., & Lu, G. (2024). The Use of an Advanced Intelligent–Responsive Polymer for the Study of Dynamic Water–Carbon Dioxide Alternating Displacement. Polymers, 16(8), 1040. https://doi.org/10.3390/polym16081040





