Montmorillonite/Poly(Pyrrole) for Low-Cost Supercapacitor Electrode Hybrid Materials
Abstract
1. Introduction
| Materials/Synthesis Method (C): Chemical (EC): Electrochemical | Electrolyte | Potential Range (V) | Specific Capacitance (F g−1) | Number of Electrodes in Cell | Ref. | ||
|---|---|---|---|---|---|---|---|
| 0.02 V·s−1 | 0.05 V·s−1 | 0.1 V·s−1 | |||||
| PPy (EC) | 0.1 M TBA-ClO4 | 0.0–0.7 | 50 | 35 | 30 | Two (S) | [20] |
| PVF (EC) | 0.1 M TBA-ClO4 | 0.0–0.7 | 25 | 20 | 15 | Two (S) | [20] |
| PPy/PVF (EC) | 0.1 M TBA-ClO4 | 0.0–0.7 | 380 | 350 | 260 | Two (S) | [20] |
| PMnO2 (EC) | 1 M Na2SO4 | 0.0–1.0 | 70 | 60 | 50 | Two (s) | [21] |
| PGM (EC) | 1 M Na2SO4 | 0.0–1.0 | 180 | 100 | 50 | Two (s) | [21] |
| PGM/PMnO2 (EC) | 1 M Na2SO4 | 0.0–1.0 | 480 | 320 | 200 | Two (s) | [21] |
| PPy (C) | 1 M KOH | 0.0–0.9 | 280 | 150 | 100 | Three | [22] |
| PPy/Ni (C) | 1 M KOH | 0.0–0.9 | 380 | 220 | 105 | Three | [22] |
| CoMnO2 (C) | 1.0 M KOH | 0.0–0.5 | 350 | 300 | 300 | Three | [23] |
| CoMnO2/VGCF (C) | 1.0 M KOH | 0.0–0.5 | 450 | 400 | 320 | Three | [23] |
| PPy/MnO2 (C) | 1.0 M Na2SO4 | 0.3–0.9 | 40 | 20 | 15 | Three | [7] |
| CNT/MnO2 (C) | 1.0 M Na2SO4 | 0.3–0.9 | 150 | 125 | 100 | Three | [7] |
| CNT/PPy/MnO2 (C) | 1.0 M Na2SO4 | 0.3–0.9 | 285 | 270 | 250 | Three | [7] |
| PPy/GO (C) | 3.0 M LiCl | 0.0–0.6 | 280 | 265 | 250 | Three | [24] |
| NMC (C) | 1.0 M H2SO4 | −1.0–0.0 | 180 | 170 | ~155 | Three | [14] |
| MMT | 1.0 M H2SO4 | 65 | 26 | 15 | Three | This work | |
| PPy(Cl) | 1.0 M H2SO4 | 196 | 126 | 88 | Three | This work | |
| MMT/PPy(Cl) | 1.0 M H2SO4 | 465 | 225 | 130 | Three | This work | |
2. Experimental Section
2.1. Products, Materials and Analysis Techniques
2.2. Synthesis Method
3. Results and Discussion
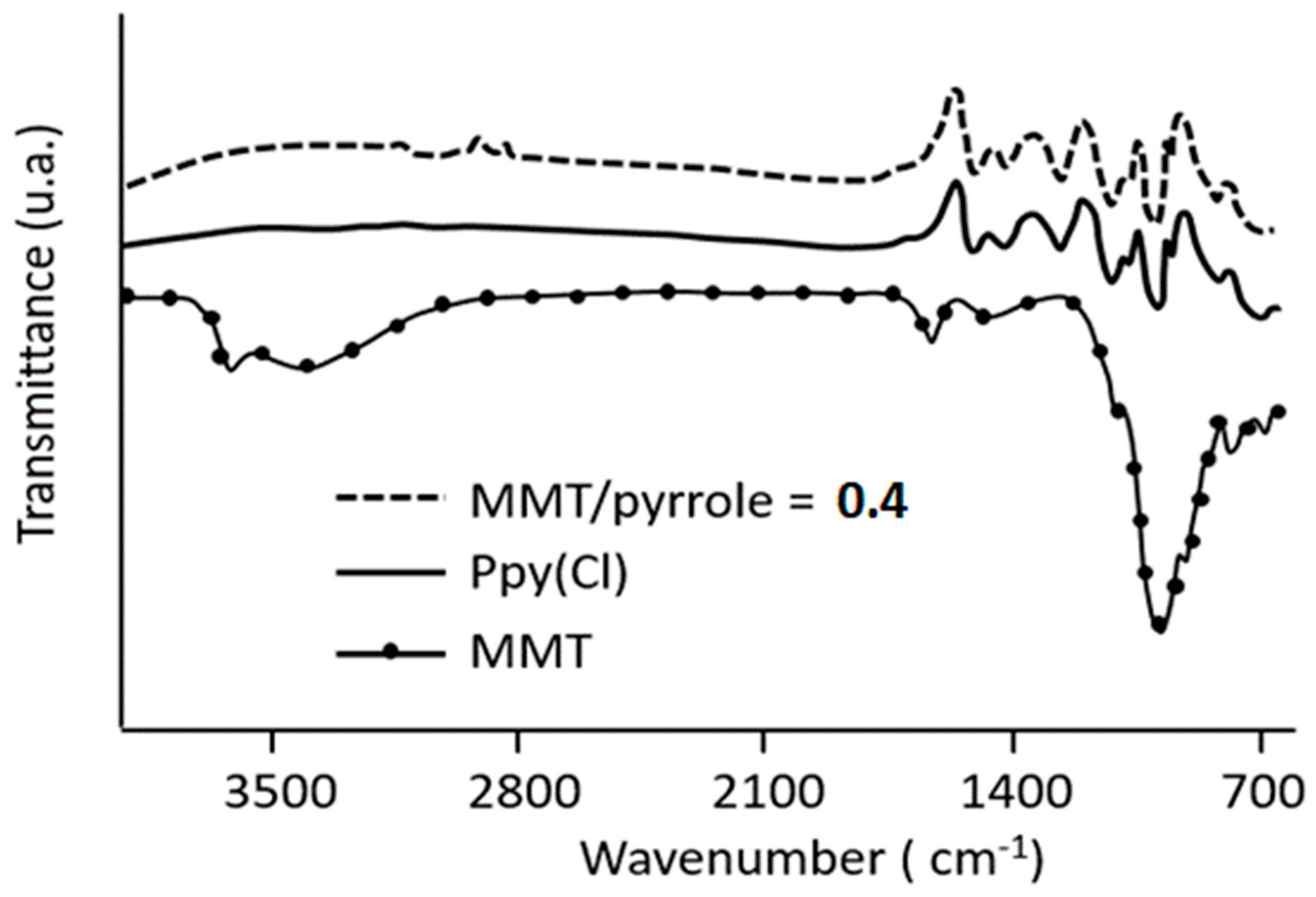
3.1. FTIR Analysis
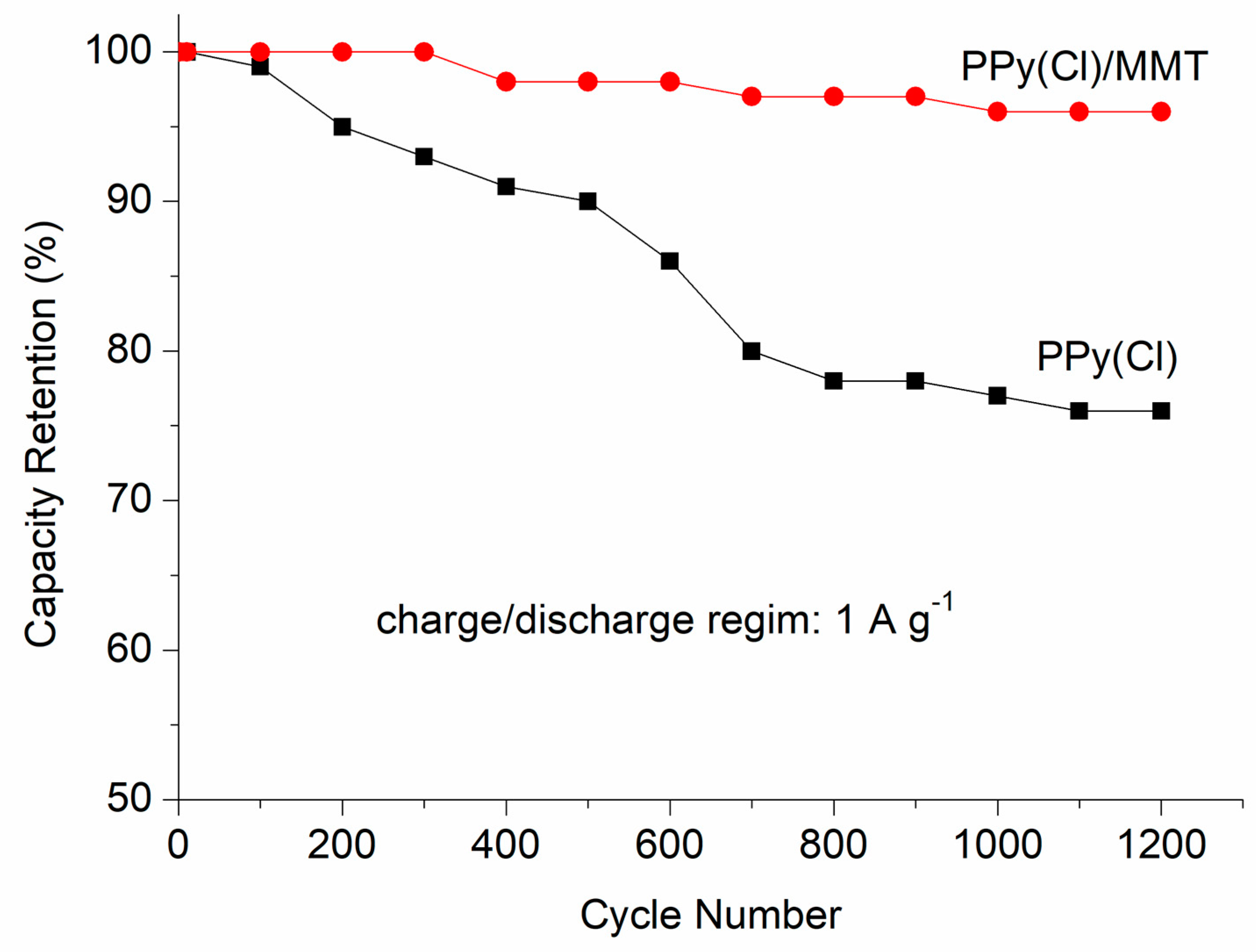
3.2. Electrochemical Measurements
3.2.1. Influence of Mass Ratio MMT/Pyrrole
3.2.2. Influence of the Temperature on the Electrochemical Performance
3.2.3. Effect of pH on the Performance of the Conductive Composite Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Santino, L.M.; Lu, Y.; Acharya, S.; Bloom, L.; Cotton, D.; Wayne, A.; D’Arcy, J.M. Enhancing Cycling Stability of Aqueous Polyaniline Electrochemical Capacitors. ACS Appl. Mater. Interfaces 2016, 8, 29452–29460. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, C.; Song, X.; Liu, J.; Zhao, L.; Zhang, P.; Gao, L. Engineering the volumetric effect of Polypyrrole for auto-deformable supercapacitor. Chem. Eng. J. 2019, 374, 59–67. [Google Scholar] [CrossRef]
- Vishnuvardhan, T.K.; Basavaraja, C.; Raghavendra, S.C. Synthesis, characterization and a.c. conductivity of polypyrrole/Y2O3 composites. Bull. Mater. Sci. 2006, 29, 77–83. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Ko, J.M.; Kim, D.Y.; Kim, B.C.; Wallace, G.G. Performance evaluation of CNT/polypyrrole/MnO2 composite electrodes for electrochemical capacitors. Electrochim. Acta 2007, 52, 7377–7385. [Google Scholar] [CrossRef]
- Molahalli, V.; Bhat, V.S.; Shetty, A.; Hundekal, D.; Toghan, A.; Hegde, G. ZnO doped SnO2 nano flower decorated on graphene oxide/polypyrrole nanotubes for symmetric supercapacitor applications. J. Energy Storage 2023, 69, 107953. [Google Scholar] [CrossRef]
- Arvas, M.B. Hydrothermal synthesis of polypyrrole/dye-functionalized carbon cloth electrode for wide potential window supercapacitor. Synth. Met. 2023, 293, 117275. [Google Scholar] [CrossRef]
- Ramasamy, V.; Sathishpriya, T.; Thenpandiyan, E.; Suresh, G.; Sagadevan, S. Short communication A facile and eco-friendly synthesis of Mn-doped CaCO3/PMMA nanocomposite for highly efficient supercapacitor in energy storage applications. Inorg. Chem. Commun. 2023, 155, 111062. [Google Scholar] [CrossRef]
- Xiao, S.; Bi, F.; Zhao, L.; Wang, L.; Gai, G. Design and synthesis of H-TiO2/MnO2 core–shell nanotube arrays with high capacitance and cycling stability for supercapacitors. J. Mater. Sci. 2017, 52, 7744–7753. [Google Scholar] [CrossRef]
- Al-hamyd, M.A.; Al-asadi, A.S.; Al-mudhaffer, M.F. Physica E: Low-dimensional Systems and Nanostructures Preparation and characterization of zinc–aluminum layered doubled hydroxide/graphene nanosheets composite for supercapacitor electrode. Phys. E Low-Dimens. Syst. Nanostructures 2022, 136, 115005. [Google Scholar] [CrossRef]
- Mazeikiene, R.; Malinauskas, A. Kinetics of the electrochemical degradation of polypyrrole. Polym. Degrad. Stab. 2002, 75, 255–258. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, Z.; Ying, Z.; Liu, X.; Wan, H. Activated nitrogen-doped porous carbon ensemble on montmorillonite for high-performance supercapacitors. J. Alloys Compd. 2018, 743, 44–51. [Google Scholar] [CrossRef]
- Jlassi, K.; Singh, A.; Aswal, D.K.; Losno, R.; Benna-Zayani, M.; Chehimi, M.M. Novel, ternary clay/polypyrrole/silver hybrid materials through in situ photopolymerization. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 193–199. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Electrochemical energy storage in montmorillonite K10 clay based composite as supercapacitor using ionic liquid electrolyte. J. Colloid Interface Sci. 2016, 464, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gierszewska, M.; Jakubowska, E.; Olewnik-Kruszkowska, E. Effect of chemical crosslinking on properties of chitosan-montmorillonite composites. Polym. Test. 2019, 77, 105872. [Google Scholar] [CrossRef]
- Ge, W.; Ma, Q.; Ai, Z.; Wang, W.; Jia, F.; Song, S. Applied Clay Science Three-dimensional reduced graphene oxide/montmorillonite nanosheet aerogels as electrode material for supercapacitor application. Appl. Clay Sci. 2021, 206, 106022. [Google Scholar] [CrossRef]
- Luo, X.; Hsu, F.; Gan, Y.; Pao, C.; Lee, M.; Wang, C.; Lin, J.; Chen, C.; Wu, K.; Chuang, W. Intercalation of Fe-montmorillonite for developing nacre-inspired flexible all-solid-state supercapacitor with circular economy approach. Chin. J. Phys. 2023, 84, 405–413. [Google Scholar] [CrossRef]
- Tian, W.; Mao, X.; Brown, P.; Rutledge, G.C.; Hatton, T.A. Electrochemically Nanostructured Polyvinylferrocene/Polypyrrole Hybrids with Synergy for Energy Storage. Adv. Funct. Mater. 2015, 25, 4803–4813. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Mohd Azahari, M.N.; Azman, N.H.N.; Sulaiman, Y. Ultrahigh specific energy of layer by layer polypyrrole/graphene oxide/multi-walled carbon nanotube| polypyrrole/manganese oxide composite for supercapacitor. J. Energy Storage 2020, 28, 101219. [Google Scholar] [CrossRef]
- Vijeth, H.; Ashokkumar, S.P.; Yesappa, L.; Vandana, M.; Devendrappa, H. Nickel oxide nanoparticle incorporated polypyrrole nanocomposite for supercapacitor application. AIP Conf. Proc. 2020, 2244, 3–7. [Google Scholar]
- Kim, S.H.; Kim, Y.I.; Park, J.H.; Ko, J.M. Cobalt-manganese oxide/carbon-nanofiber composite electrodes for supercapacitors. Int. J. Electrochem. Sci. 2009, 4, 1489–1496. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, T.; Du, X.; Wang, J.; Hu, J.; Wei, L.P. High performance asymmetric supercapacitor based on polypyrrole/graphene composite and its derived nitrogen-doped carbon nano-sheets. J. Power Sources 2017, 346, 120–127. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Sanchez, R.M.T. Applied Clay Science Effect of modi fi ed montmorillonite on biodegradable PHB nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- Mejri, C.; Oueslati, W.; Ben, A.; Amara, H. Applied Surface Science Advances Structure and reactivity assessment of dioctahedral montmorillonite during provoked variable sequential cation exchange process via XRD modelling approach. Appl. Surf. Sci. Adv. 2023, 15, 100403. [Google Scholar] [CrossRef]
- Boudjema, J.M.F.S.; Vispe, E.; Choukchou-Braham, A.; Mayoral, J.A.; Bachir, R. Preparation and characterization of activated montmorillonite clay supported 11-molybdo vanadophosphoric acid for cyclohexene oxidation. RSC Adv. 2015, 5, 6853–6863. [Google Scholar] [CrossRef]
- Khatti, T.; Naderi-manesh, H.; Mehdi, S. Materials Science & Engineering B Polypyrrole-Coated Polycaprolactone-Gelatin Conductive Nano fi bers: Fabrication and Characterization. Mater. Sci. Eng. B 2019, 250, 114440. [Google Scholar]
- Varesano, A.; Dall’Acqua, L.; Tonin, C. A study on the electrical conductivity decay of polypyrrole coated wool textiles. Polym. Degrad. Stab. 2005, 89, 125–132. [Google Scholar] [CrossRef]
- Farbod, M.; Mobini, N. Physical properties, thermal stability, and glass transition temperature of multi-walled carbon nanotube/polypyrrole nanocomposites. Compos. Interfaces 2014, 21, 737–747. [Google Scholar] [CrossRef]
- Belabed, C.; Haine, N.; Benabdelghani, Z.; Bellal, B.; Trari, M. Photocatalytic hydrogen evolution on the hetero-system polypyrrol/TiO2 under visible light. Int. J. Hydrogen Energy 2014, 39, 17533–17539. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Liu, Y.; Wang, L.; Gao, C. Enhanced thermoelectric properties of polyaniline/polypyrrole/carbon nanotube ternary composites by treatment with a secondary dopant using ferric chloride. J. Mater. Chem. C 2020, 8, 528–535. [Google Scholar] [CrossRef]
- Borralleras, P.; Segura, I.; Aranda, M.A.G.; Aguado, A. Influence of the polymer structure of polycarboxylate-based superplasticizers on the intercalation behaviour in montmorillonite clays. Constr. Build. Mater. 2019, 220, 285–296. [Google Scholar] [CrossRef]
- Yeh, J.M.; Chin, C.P.; Chang, S. Enhanced corrosion protection coatings prepared from soluble electronically conductive polypyrrole-clay nanocomposite materials. J. Appl. Polym. Sci. 2003, 88, 3264–3272. [Google Scholar] [CrossRef]
- Kuila, B.; Nandi, A. Structural hierarchy in melt-processed poly (3-hexyl thiophene)-montmorillonite clay nanocomposites: Novel physical, mechanical, optical, and conductivity properties. J. Phys. Chem. B 2006, 110, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, B.; Xu, L.; Xu, G.; Sun, W.; Yu, S. Simple synthesis of Cu2O/Na-bentonite composites and their excellent photocatalytic properties in treating methyl orange solution. Ceram. Int. 2016, 42, 5979–5984. [Google Scholar] [CrossRef]
- Olad, A.; Rashidzadeh, A.; Amini, M. Preparation of Polypyrrole Nanocomposites with Organophilic and Hydrophilic Montmorillonite and Investigation of Their Corrosion Protection on Iron. Adv. Polym. Technol. 2013, 32, 1–10. [Google Scholar] [CrossRef]
- Guo, C.; Tian, S.; Chen, B.; Liu, H.; Li, J. Constructing α-MnO2@PPy core-shell nanorods towards enhancing electrochemical behaviors in aqueous zinc ion battery. Mater. Lett. 2020, 262, 127180. [Google Scholar] [CrossRef]
- Dzulkurnain, N.A.; Mokhtar, M.; Rashid, J.I.A.; Knight, V.F.; Wan Yunus, W.M.Z.; Ong, K.K.; Mohd Kasim, N.A.; Mohd Noor, S.A. A review on impedimetric and voltammetric analysis based on polypyrrole conducting polymers for electrochemical sensing applications. Polymers 2021, 13, 2728. [Google Scholar] [CrossRef]
- Vilímová, P.; Kulhánková, L.; Peikertová, P.; Mamulová Kutláková, K.; Vallová, S.; Koníčková, H.; Plaček, T.; Tokarský, J. Effect of montmorillonite/polypyrrole ratio and oxidizing agent on structure and electrical conductivity of intercalated nanocomposites. Appl. Clay Sci. 2019, 168, 459–468. [Google Scholar] [CrossRef]
- Gao, J.W.; Li, G.; Yao, Y.F.; Jiang, J.M. Preparation and characterization of montmorillonite/polypyrrole nanocomposites by in-situ chemical polymerizatio. J. Macromol. Sci. Part B Phys. 2011, 50, 1364–1375. [Google Scholar] [CrossRef]
- Churchman, G.J.; Jackson, M.L. Reaction of montmorillonite with acid aqueous solutions: Solute activity control by a secondary phase. Geochim. Cosmochim. Acta 1976, 40, 1251–1259. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Sprik, M.; Cheng, J.; Meijer, E.J.; Wang, R. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 2013, 117, 180–190. [Google Scholar] [CrossRef]
- Brady, P.V.; Cygan, R.T.; Nagy, K.L. Molecular controls on kaolinite surface charge. J. Colloid Interface Sci. 1996, 183, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ramoa, S.D.A.; Barra, G.M.O.; Merlini, C.; Livi, S.; Soares, B.G.; Pegoretti, A. Novel electrically conductive polyurethane/montmorillonite-polypyrrole nanocomposites. Express Polym. Lett. 2015, 9, 945–958. [Google Scholar] [CrossRef]
- Rapi, S.; Bocchi, V.; Gardini, G.P. Conducting polypyrrole by chemical synthesis in water. Synth. Met. 1988, 24, 217–221. [Google Scholar] [CrossRef]
- Garsuch, A.; Sattler, R.R.; Pickup, P.G. Formation of polypyrrole from 2,5-bis(2-pyrrolyl)pyrrolidine. Chem. Commun. 2004, 4, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Thielemans, W. Interconnected and high cycling stability polypyrrole supercapacitors using cellulose nanocrystals and commonly used inorganic salts as dopants. J. Energy Chem. 2023, 76, 165–174. [Google Scholar] [CrossRef]
- Wang, Y.; Du Pasquier, A.; Li, D.; Atanassova, P.; Sawrey, S.; Oljaca, M. Electrochemical double layer capacitors containing carbon black additives for improved capacitance and cycle life. Carbon 2018, 133, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.-B.; Cai, J.-J.; Luo, Y.-C.; Kang, L. Nano-composite of polypyrrole/modified mesoporous carbon for electrochemical capacitor application. Electrochim. Acta 2010, 55, 8067–8073. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, G.-C.; Wang, R.-Y.; Li, X.-W. Key A novel layered manganese oxide/poly(aniline-co-o-anisidine) nanocomposite and its application for electrochemical supercapacitor. Electrochim. Acta 2010, 55, 5414–5419. [Google Scholar] [CrossRef]
- Abdillah, O.B.; Rus, Y.B.; Ulfa, M.; Dedi; Iskandar, F. Recent progress on reduced graphene oxide and polypyrrole composites for high performance supercapacitors: A review. J. Energy Storage 2023, 74 Pt A, 109300. [Google Scholar]
- Yang, K.; Cho, K.; Kim, S. Effect of carbon black addition on thermal stability and capacitive performances of supercapacitors. Sci. Rep. 2018, 8, 11989. [Google Scholar] [CrossRef] [PubMed]
- Fahim, H.; Sanad, M.M.S.; Ghebache, Z.; Boudieb, N. Effect of polymerization conditions on the physicochemical and supercapacitor applications electrochemical properties of SnO2/polypyrrole composites for supercapacitor applications. J. Mol. Struct. 2022, 1251, 131964. [Google Scholar]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, R.; Sun, X.; Lu, L.; Chen, Y. Constructing two dimensional composite nanosheets with montmorillonite and graphene-like carbon: Towards high-rate-performance PVA based gel polymer electrolytes for quasi-solid-state supercapacitors. Mater. Chem. Phys. 2022, 287, 126333. [Google Scholar] [CrossRef]
- Wang, J.G.; Yang, Y.; Huang, Z.H.; Kang, F. Interfacial synthesis of mesoporous MnO2/polyaniline hollow spheres and their application in electrochemical capacitors. J. Power Sources 2012, 204, 236–243. [Google Scholar] [CrossRef]
- Torabi, M.; Soltani, M.; Sadrnezhaad, S.K. Impedance analysis of growth and morphology of electropolymerized polypyrrole nanocomposites. J. New Mater. Electrochem. Syst. 2014, 17, 129–132. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, X.; Gao, X.; Wang, J.; Zhao, N.; Sha, J. Equivalent circuit model recognition of electrochemical impedance spectroscopy via machine learning. J. Electroanal. Chem. 2019, 855, 113627. [Google Scholar] [CrossRef]
- Sanqing, H.; Dejin, B.; Yanfei, X.; Huijuan, L. Facile Construction of Three-Dimensional Architectures of a Nanostructured Polypyrrole on Carbon Nanotube Fibers and Their Effect on Supercapacitor Performance. ACS Appl. Energy Mater. 2023, 6, 856–864. [Google Scholar]
- Hallik, A.; Alumaa, A.; Tamm, J.; Sammelselg, V.; Väärtnõu, M.; Jänes, A.; Lust, E. Analysis of electrochemical impedance of polypyrrole|sulfate and polypyrrole|perchlorate films. Synth. Met. 2006, 156, 488–494. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidouche, F.; Ghebache, Z.; Lepretre, J.-C.; Djelali, N.-E. Montmorillonite/Poly(Pyrrole) for Low-Cost Supercapacitor Electrode Hybrid Materials. Polymers 2024, 16, 919. https://doi.org/10.3390/polym16070919
Hamidouche F, Ghebache Z, Lepretre J-C, Djelali N-E. Montmorillonite/Poly(Pyrrole) for Low-Cost Supercapacitor Electrode Hybrid Materials. Polymers. 2024; 16(7):919. https://doi.org/10.3390/polym16070919
Chicago/Turabian StyleHamidouche, Fahim, Zohra Ghebache, Jean-Claude Lepretre, and Nacer-Eddine Djelali. 2024. "Montmorillonite/Poly(Pyrrole) for Low-Cost Supercapacitor Electrode Hybrid Materials" Polymers 16, no. 7: 919. https://doi.org/10.3390/polym16070919
APA StyleHamidouche, F., Ghebache, Z., Lepretre, J.-C., & Djelali, N.-E. (2024). Montmorillonite/Poly(Pyrrole) for Low-Cost Supercapacitor Electrode Hybrid Materials. Polymers, 16(7), 919. https://doi.org/10.3390/polym16070919




