Effect of Chitosan–Riboflavin Bioconjugate on Green Mold Caused by Penicillium digitatum in Lemon Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fungal Isolates
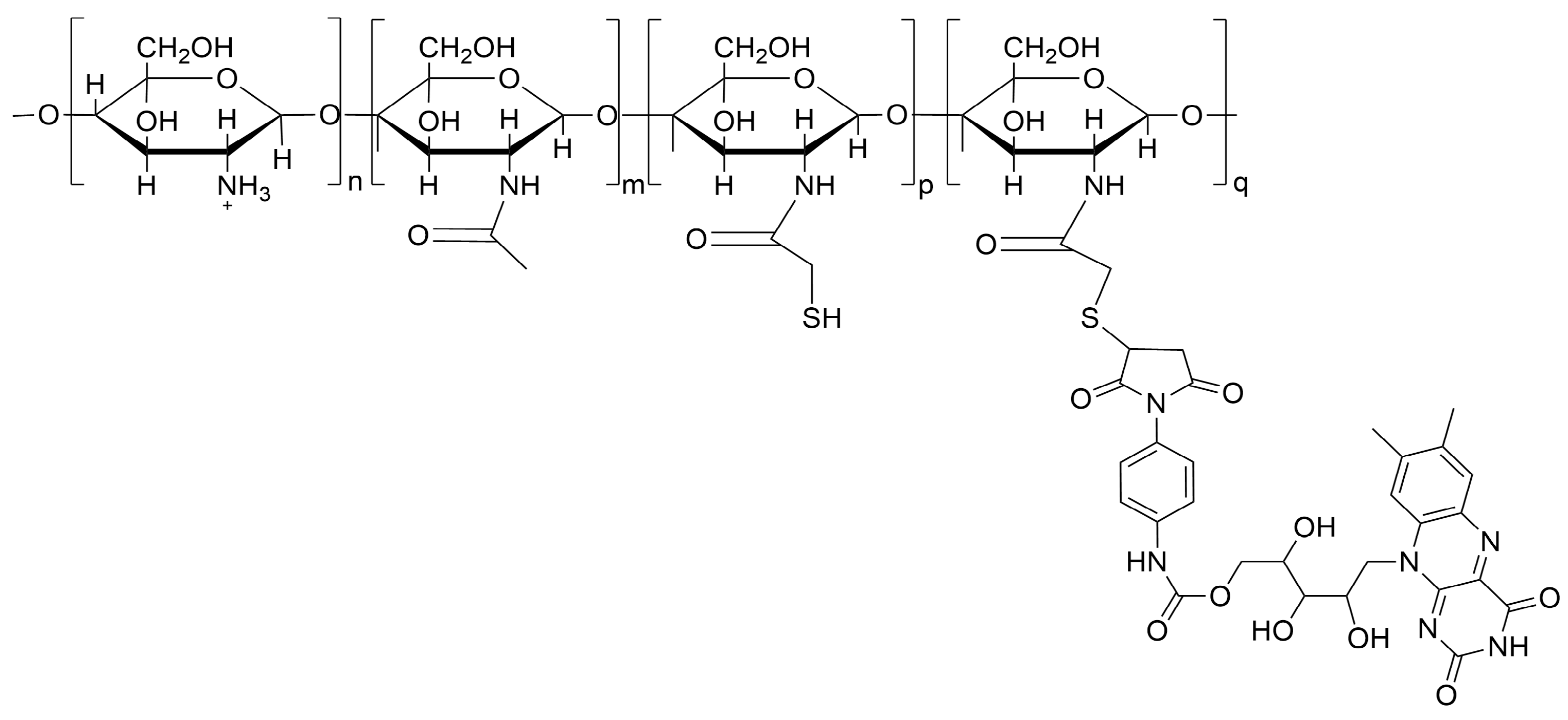
2.3. CH-RF Biofungicide
2.4. In Vitro Tests
2.5. Biotests in Lemon Fruit in Temperate and Cold Storage
2.6. Disease Evaluation
2.7. Statistical Analysis
3. Results
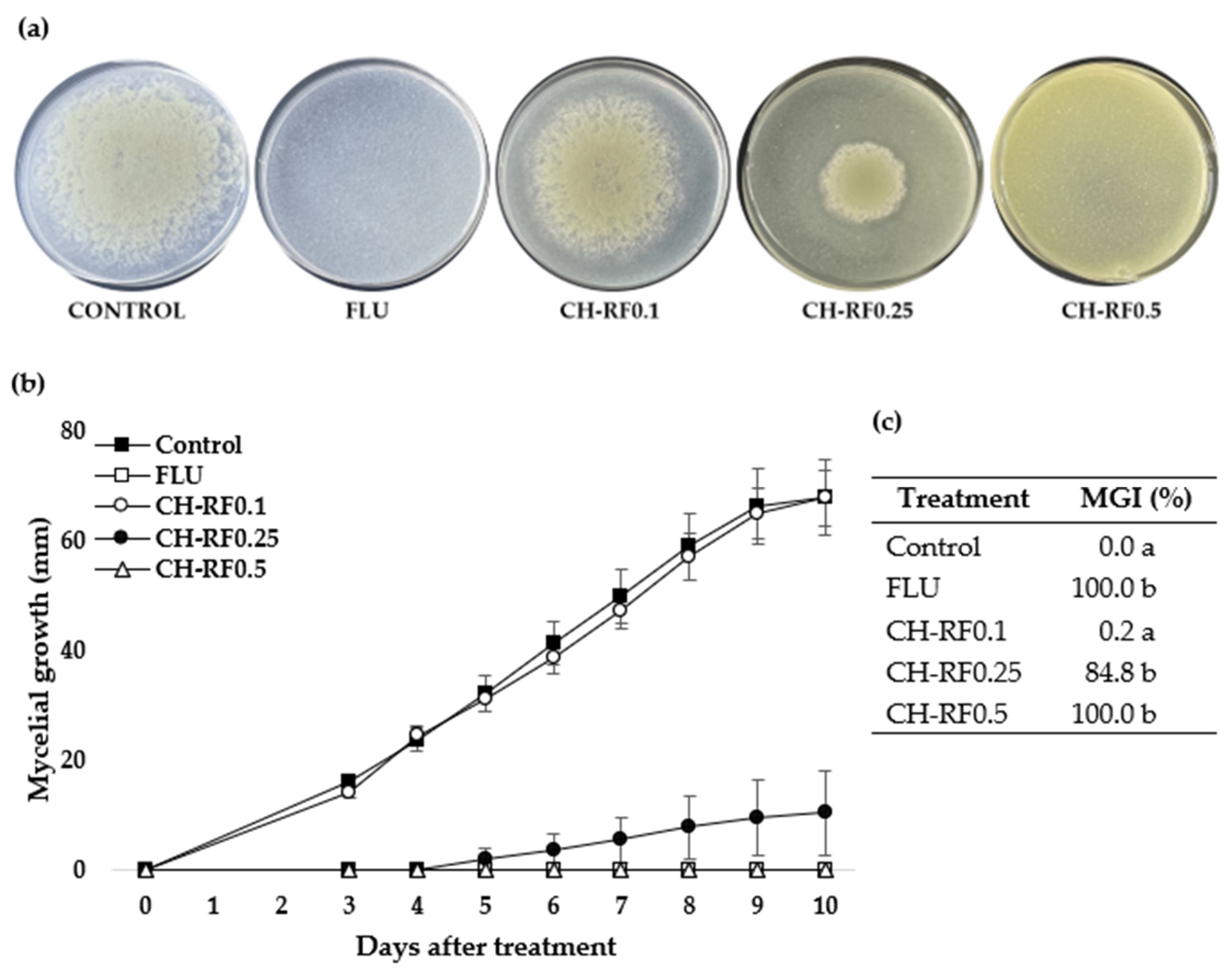
3.1. In Vitro Performance of CH-RF
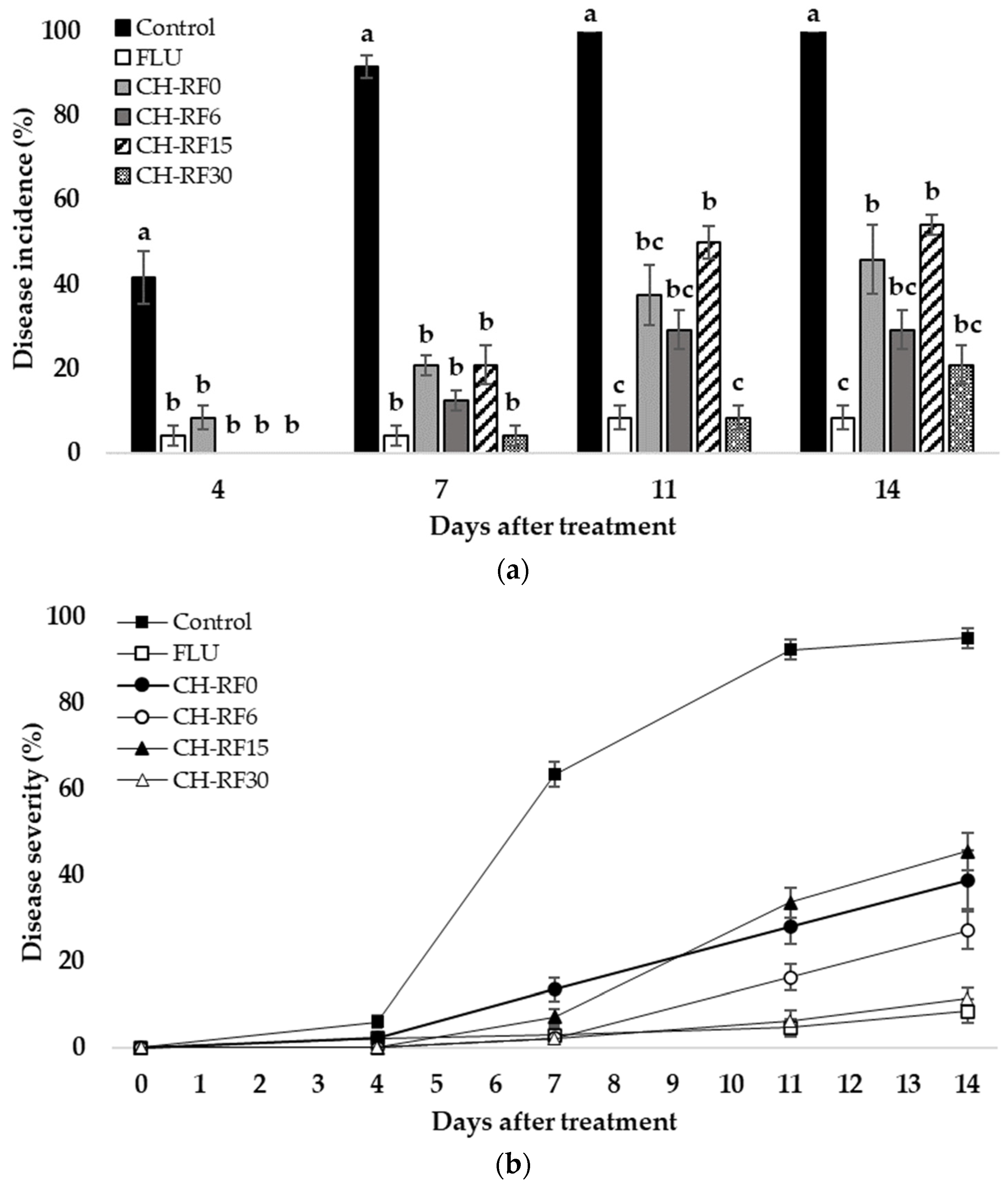
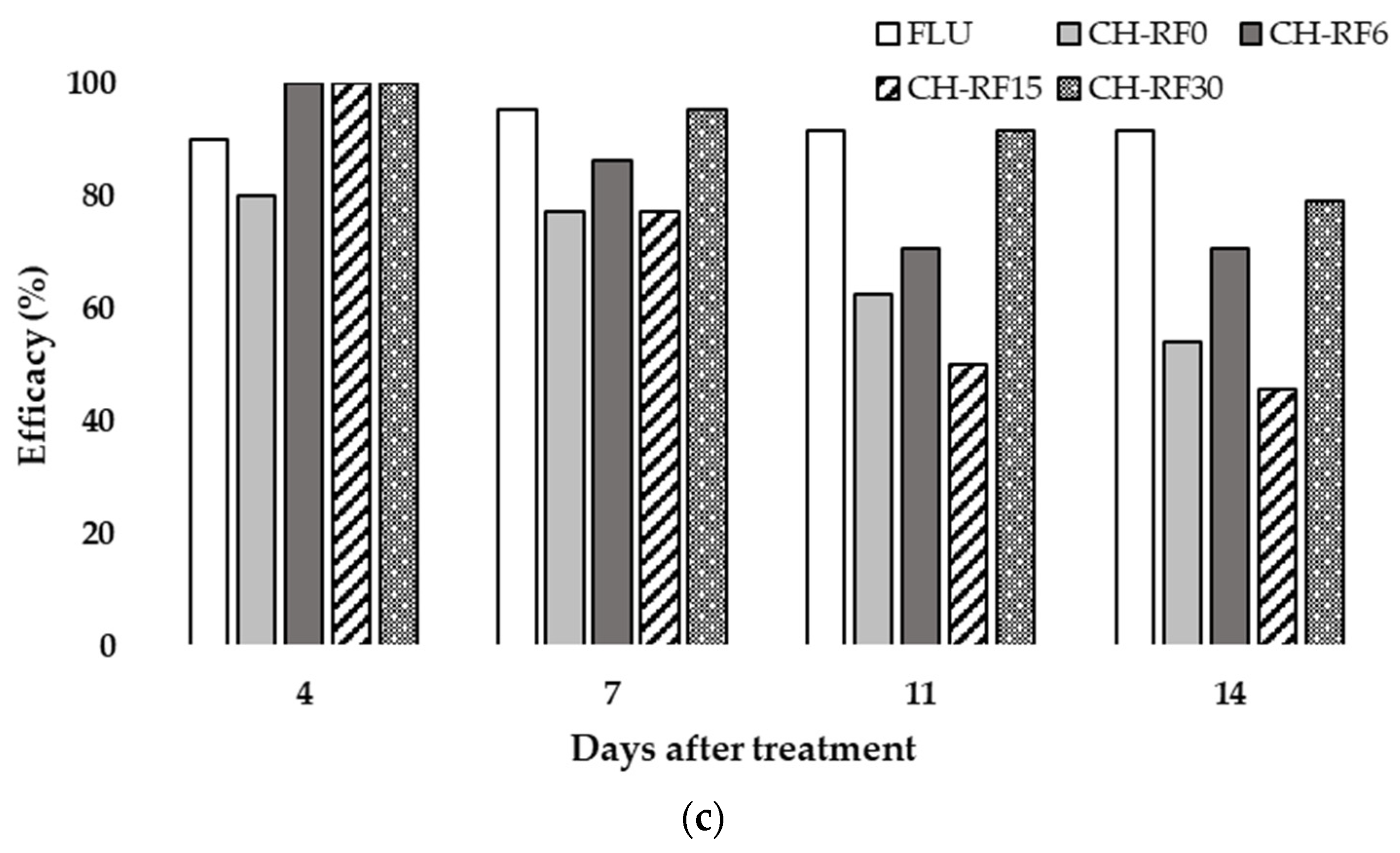
3.2. In Vivo Performance of CH-RF on Lemon Fruit in Temperate Condition
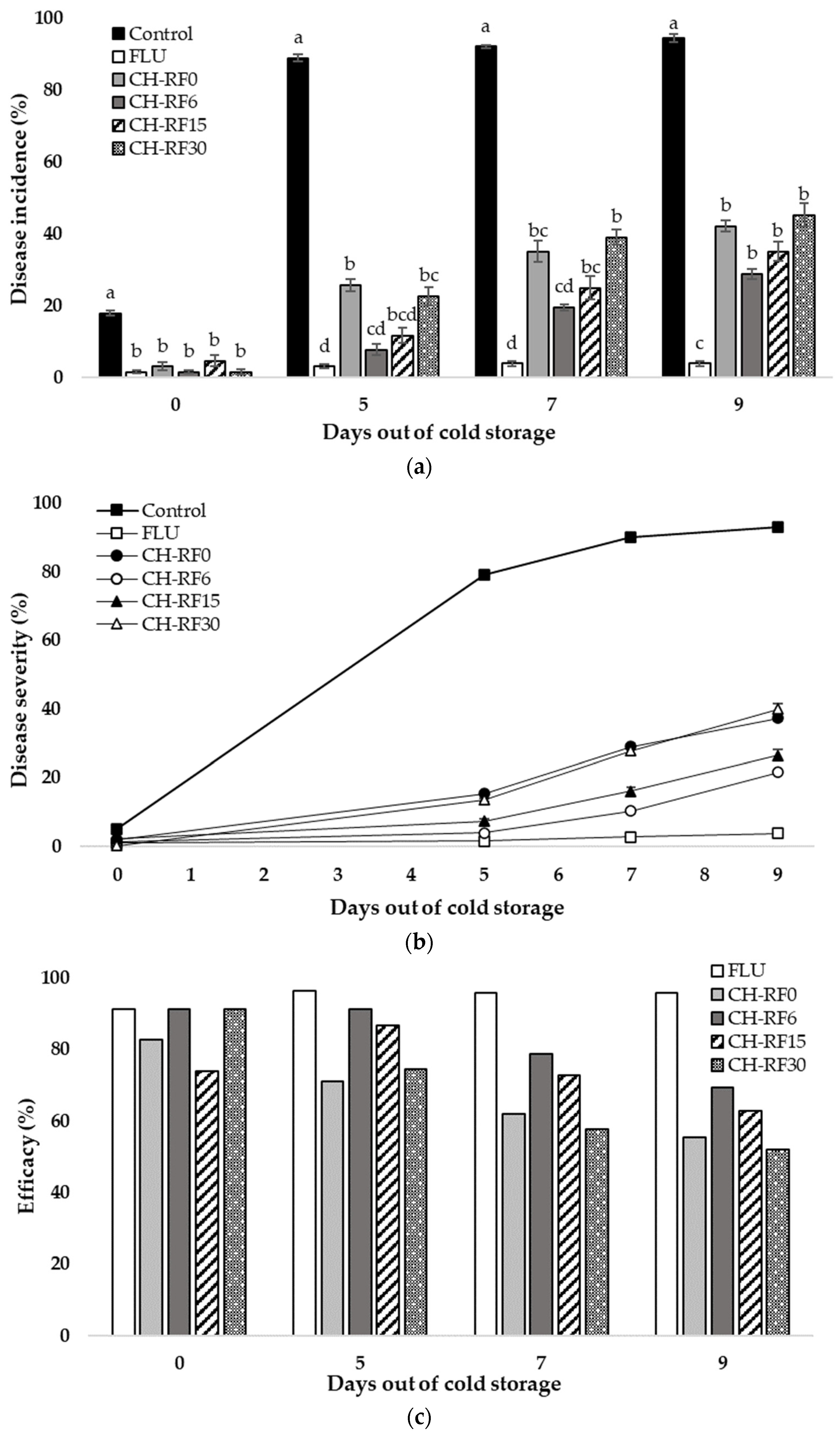
3.3. In Vivo Performance of CH-RF in Lemon Fruit in Cold Storage
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, J.H.; Bazioli, J.M.; de Moraes Pontes, J.G.; Fill, T.P. Penicillium Digitatum Infection Mechanisms in Citrus: What Do We Know so Far? Fungal Biol. 2019, 123, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Palou, L. Penicillium Digitatum, Penicillium Italicum (Green Mold, Blue Mold). In Postharvest Decay; Elsevier: Amsterdam, The Netherlands, 2014; pp. 45–102. ISBN 978-0-12-411552-1. [Google Scholar]
- Ladaniya, M. Postharvest Disease Management with Fungicides. In Citrus Fruit; Elsevier: Amsterdam, The Netherlands, 2023; pp. 563–594. ISBN 978-0-323-99306-7. [Google Scholar]
- Kellerman, M.; Liebenberg, E.; Njombolwana, N.; Erasmus, A.; Fourie, P.H. Postharvest Dip, Drench and Wax Coating Application of Pyrimethanil on Citrus Fruit: Residue Loading and Green Mould Control. Crop Prot. 2018, 103, 115–129. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, H.; Zhang, X.; Muhayimana, S.; Liu, X.; Xue, Y.; Huang, Q. Comparative Cytotoxic Effects of Five Commonly Used Triazole Alcohol Fungicides on Human Cells of Different Tissue Types. J. Environ. Sci. Health Part B 2020, 55, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lafon, P.-A.; Salvador-Prince, L.; Gines, A.R.; Trousse, F.; Torrent, J.; Prevostel, C.; Crozet, C.; Liu, J.; Perrier, V. Prenatal Exposure to Low Doses of Fungicides Corrupts Neurogenesis in Neonates. Environ. Res. 2021, 195, 110829. [Google Scholar] [CrossRef]
- Oiki, S.; Yaguchi, T.; Urayama, S.; Hagiwara, D. Wide Distribution of Resistance to the Fungicides Fludioxonil and Iprodione in Penicillium Species. PLoS ONE 2022, 17, e0262521. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan Oligosaccharide: Biological Activities and Potential Therapeutic Applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Hua, C.; Li, Y.; Wang, X.; Kai, K.; Su, M.; Shi, W.; Zhang, D.; Liu, Y. The Effect of Low and High Molecular Weight Chitosan on the Control of Gray Mold (Botrytis Cinerea) on Kiwifruit and Host Response. Sci. Hortic. 2019, 246, 700–709. [Google Scholar] [CrossRef]
- Herrera-Défaz, M.; Fuentealba, D.; Dibona-Villanueva, L.; Schwantes, D.; Jiménez, B.; Ipinza, B.; Latorre, B.; Valdés-Gómez, H.; Fermaud, M. Biocontrol of Botrytis Cinerea on Grape Berries in Chile: Use of Registered Biofungicides and a New Chitosan-Based Fungicide. Horticulturae 2023, 9, 746. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, L.; Zhou, Y.; Ming, J.; Yao, S.; Zeng, K. Wound Healing in Citrus Fruit Is Promoted by Chitosan and Pichia Membranaefaciens as a Resistance Mechanism against Colletotrichum Gloeosporioides. Postharvest Biol. Technol. 2018, 145, 134–143. [Google Scholar] [CrossRef]
- García-Bramasco, C.A.; Blancas-Benitez, F.J.; Montaño-Leyva, B.; Medrano-Castellón, L.M.; Gutierrez-Martinez, P.; González-Estrada, R.R. Influence of Marine Yeast Debaryomyces Hansenii on Antifungal and Physicochemical Properties of Chitosan-Based Films. J. Fungi 2022, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, F.; Lu, Y.; Deng, J. Combination of Chitosan and Salicylic Acid to Control Postharvest Green Mold Caused by Penicillium Digitatum in Grapefruit Fruit. Sci. Hortic. 2018, 233, 54–60. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Pisuchpen, S.; Leelasuphakul, W. Effect of Bacillus Subtilis and Chitosan Applications on Green Mold (Penicilium Digitatum Sacc.) Decay in Citrus Fruit. Postharvest Biol. Technol. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of Postharvest Application of Chitosan Combined with Clove Oil against Citrus Green Mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Da Silva, Y.C.R.; Alves, R.M.; Da Silva, B.M.P.; Bron, I.U.; Cia, P. Chitosan and Hot Water Treatments Reduce Postharvest Green Mould in ‘Murcott’ Tangor. J. Phytopathol. 2020, 168, 542–550. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y. Photodynamic Therapy for the Treatment of Fungal Infections. Infect. Drug Resist. 2022, 15, 3251–3266. [Google Scholar] [CrossRef]
- Seididamyeh, M.; Netzel, M.E.; Mereddy, R.; Harmer, J.R.; Sultanbawa, Y. Photodynamic Inactivation of Botrytis Cinerea Spores by Curcumin—Effect of Treatment Factors and Characterization of Photo-Generated Reactive Oxygen Species. Food Bioprocess Technol. 2023, 17, 670–685. [Google Scholar] [CrossRef]
- Song, L.; Zhang, F.; Yu, J.; Wei, C.; Han, Q.; Meng, X. Antifungal Effect and Possible Mechanism of Curcumin Mediated Photodynamic Technology against Penicillium Expansum. Postharvest Biol. Technol. 2020, 167, 111234. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Volentini, S.I.; Rapisarda, V.A. UVA Photoactivation of Harmol Enhances Its Antifungal Activity against the Phytopathogens Penicillium Digitatum and Botrytis Cinerea. Front. Microbiol. 2017, 8, 347. [Google Scholar] [CrossRef]
- Li, X.; Sheng, L.; Sbodio, A.O.; Zhang, Z.; Sun, G.; Blanco-Ulate, B.; Wang, L. Photodynamic Control of Fungicide-Resistant Penicillium Digitatum by Vitamin K3 Water-Soluble Analogue. Food Control 2022, 135, 108807. [Google Scholar] [CrossRef]
- Dibona-Villanueva, L.; Fuentealba, D. Novel Chitosan-Riboflavin Conjugate with Visible Light-Enhanced Antifungal Properties against Penicillium Digitatum. J. Agric. Food Chem. 2021, 69, 945–954. [Google Scholar] [CrossRef]
- Jiménez Jiménez, B. Evaluación de un Biofungicida Fotoactivo en Hongos Patógenos de Frutales. Master’s Thesis, Pontificia Universidad Católica de Chile, Santiago, Chile, 2021. [Google Scholar]
- Pérez-Alfonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The Effects of Essential Oils Carvacrol and Thymol on Growth of Penicillium Digitatum and P. Italicum Involved in Lemon Decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative Management Approaches of Citrus Diseases Caused by Penicillium Digitatum (Green Mold) and Penicillium Italicum (Blue Mold). Front. Plant Sci. 2022, 12, 833328. [Google Scholar] [CrossRef]
- Khalil Bagy, H.M.M.; Ibtesam, B.F.M.; Abou-Zaid, E.A.A.; Sabah, B.M.; Nashwa, S.M.A. Control of Green Mold Disease Using Chitosan and Its Effect on Orange Properties during Cold Storage. Arch. Phytopathol. Plant Prot. 2021, 54, 570–585. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Admane, N.; Genovese, F.; Di Renzo, G.C. Advance in Citrus Postharvest Management: Diseases, Cold Storage and Quality Evaluation. In Citrus Pathology; Gill, H., Garg, H., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3071-0. [Google Scholar]
- Crocker, L.B.; Lee, J.H.; Mital, S.; Mills, G.C.; Schack, S.; Bistrović-Popov, A.; Franck, C.O.; Mela, I.; Kaminski, C.F.; Christie, G.; et al. Tuning Riboflavin Derivatives for Photodynamic Inactivation of Pathogens. Sci. Rep. 2022, 12, 6580. [Google Scholar] [CrossRef] [PubMed]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Bäumler, W. The Role of Singlet Oxygen and Oxygen Concentration in Photodynamic Inactivation of Bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Magaraggia, M.; Fabris, C.; Soncin, M.; Camerin, M.; Tallandini, L.; Coppellotti, O.; Guidolin, L. Photodynamic Inactivation of Microbial Pathogens: Disinfection of Water and Prevention of Water-Borne Diseases. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Böcking, T.; Barrow, K.D.; Netting, A.G.; Chilcott, T.C.; Coster, H.G.L.; Höfer, M. Effects of Singlet Oxygen on Membrane Sterols in the Yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000, 267, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Dibona-Villanueva, L.; Fuentealba, D. Protoporphyrin IX–Chitosan Oligosaccharide Conjugate with Potent Antifungal Photodynamic Activity. J. Agric. Food Chem. 2022, 70, 9276–9282. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St. Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and Principles of Photodynamic Therapy as an Alternative Antifungal Discovery Platform. Front. Microbiol. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ipinza-Concha, B.M.; Dibona-Villanueva, L.; Fuentealba, D.; Pinilla-Quispe, A.; Schwantes, D.; Garzón-Nivia, M.A.; Herrera-Défaz, M.A.; Valdés-Gómez, H.A. Effect of Chitosan–Riboflavin Bioconjugate on Green Mold Caused by Penicillium digitatum in Lemon Fruit. Polymers 2024, 16, 884. https://doi.org/10.3390/polym16070884
Ipinza-Concha BM, Dibona-Villanueva L, Fuentealba D, Pinilla-Quispe A, Schwantes D, Garzón-Nivia MA, Herrera-Défaz MA, Valdés-Gómez HA. Effect of Chitosan–Riboflavin Bioconjugate on Green Mold Caused by Penicillium digitatum in Lemon Fruit. Polymers. 2024; 16(7):884. https://doi.org/10.3390/polym16070884
Chicago/Turabian StyleIpinza-Concha, Brenda M., Luciano Dibona-Villanueva, Denis Fuentealba, Alexander Pinilla-Quispe, Daniel Schwantes, María A. Garzón-Nivia, Mario A. Herrera-Défaz, and Héctor A. Valdés-Gómez. 2024. "Effect of Chitosan–Riboflavin Bioconjugate on Green Mold Caused by Penicillium digitatum in Lemon Fruit" Polymers 16, no. 7: 884. https://doi.org/10.3390/polym16070884
APA StyleIpinza-Concha, B. M., Dibona-Villanueva, L., Fuentealba, D., Pinilla-Quispe, A., Schwantes, D., Garzón-Nivia, M. A., Herrera-Défaz, M. A., & Valdés-Gómez, H. A. (2024). Effect of Chitosan–Riboflavin Bioconjugate on Green Mold Caused by Penicillium digitatum in Lemon Fruit. Polymers, 16(7), 884. https://doi.org/10.3390/polym16070884








