Harnessing Enhanced Flame Retardancy in Rigid Polyurethane Composite Foams through Hemp Seed Oil-Derived Natural Fillers
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of Bio-Based Polyol
2.2.1. Synthesis of Epoxidized Hemp Seed Oil (EHSO)
2.2.2. Synthesis of Hemp Seed Polyols (HSPOs)
2.3. Preparation of HSO-Based RPUF Composite
2.4. Characterization Methods
3. Results and Discussion
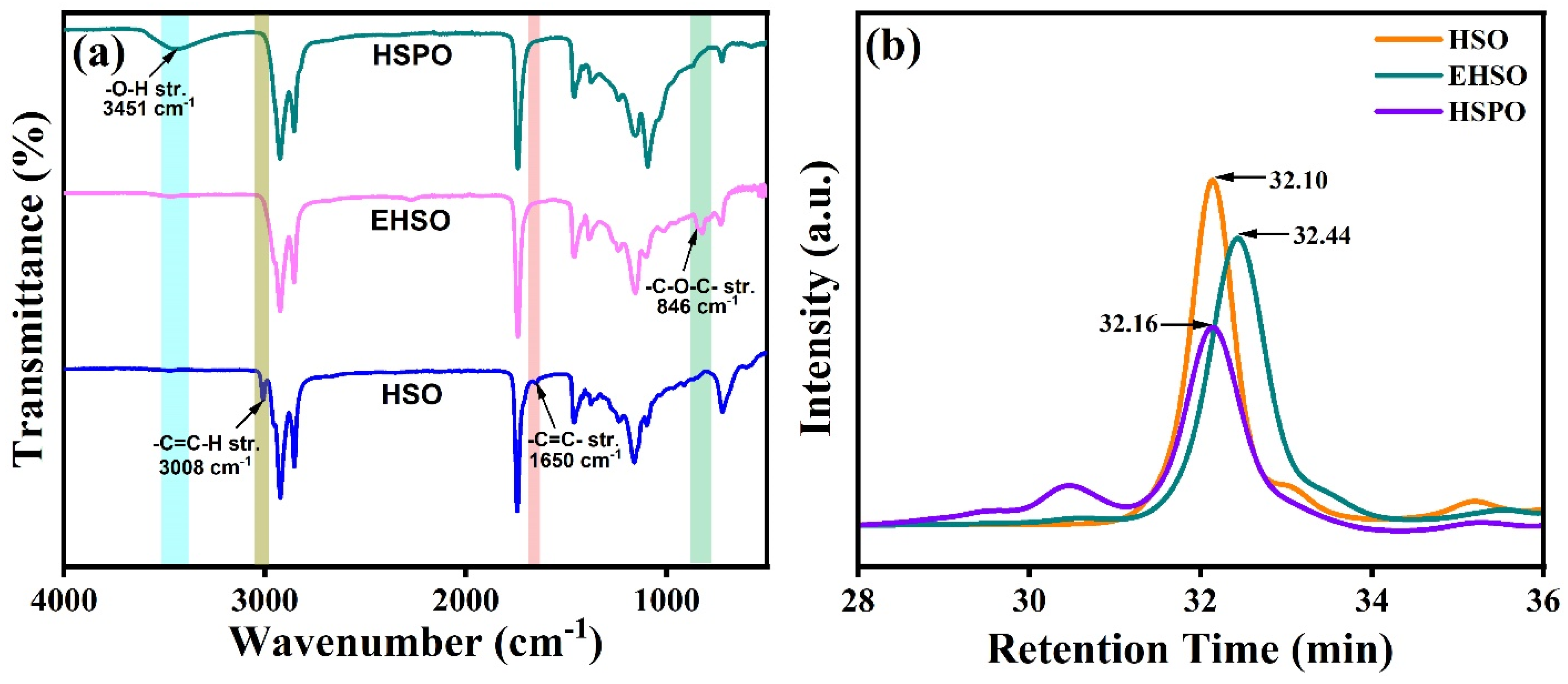
3.1. Characterization of HSO, EHSO, and HSPO
3.2. Properties of HSO-Based RPUF Composite
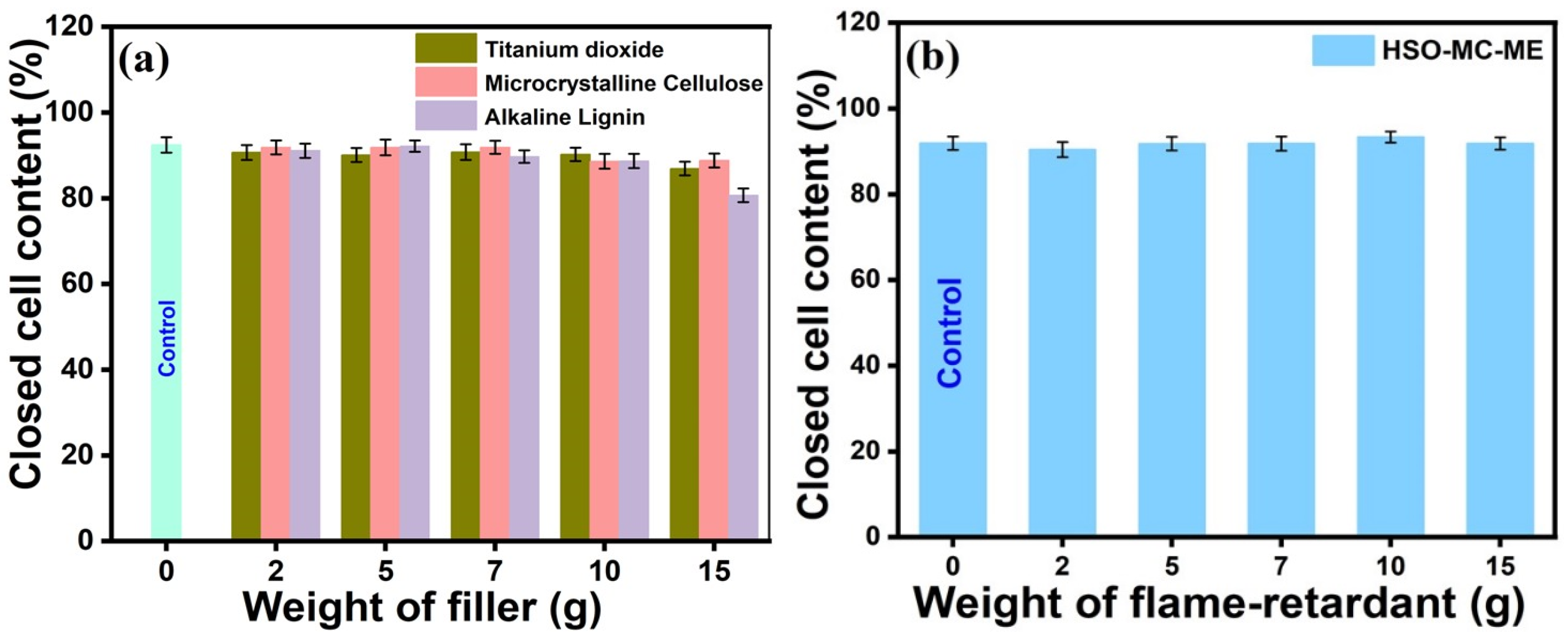
3.2.1. Apparent Density and Closed-Cell Content
3.2.2. Compressive Strength
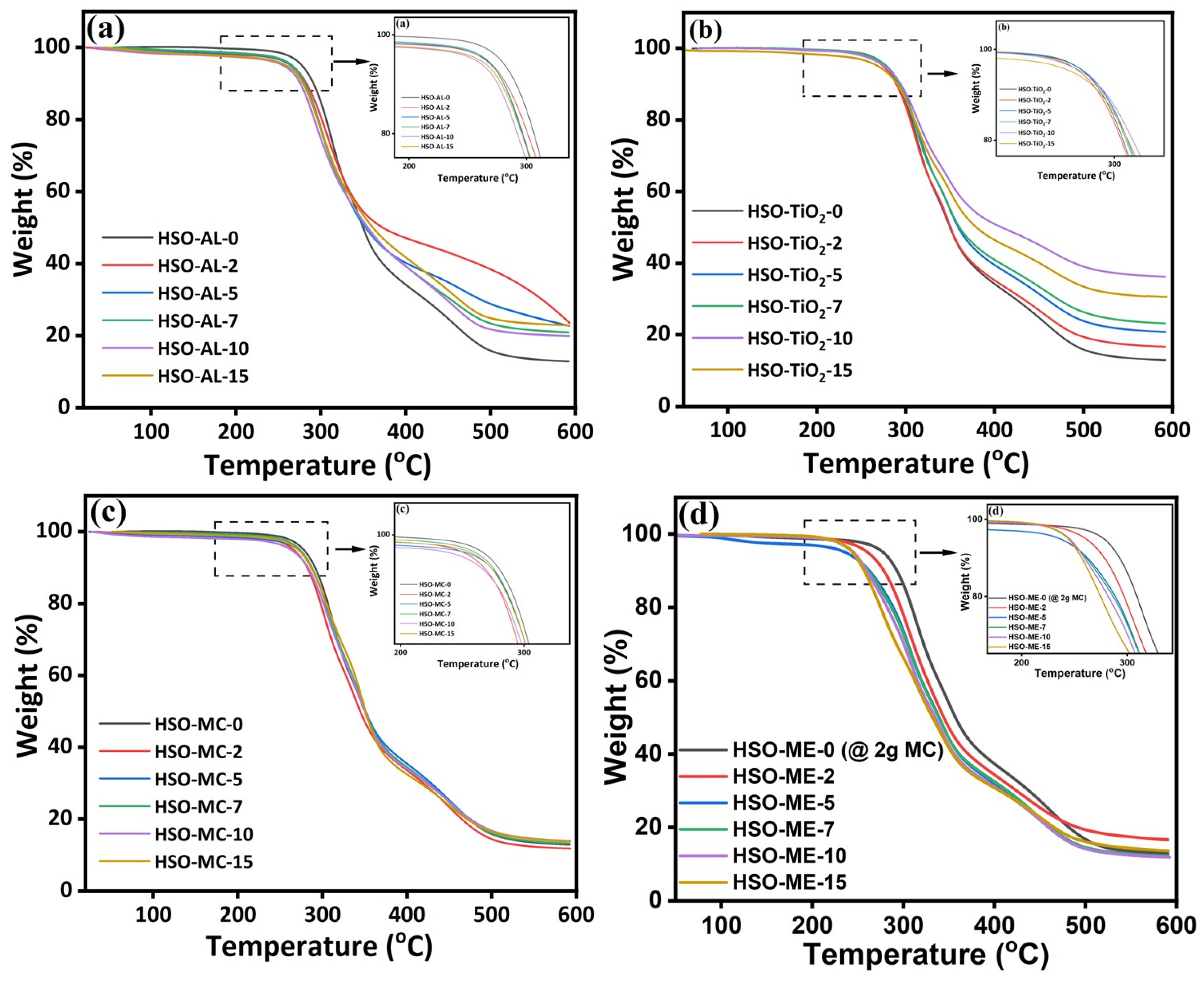
3.2.3. Thermogravimetric Analysis
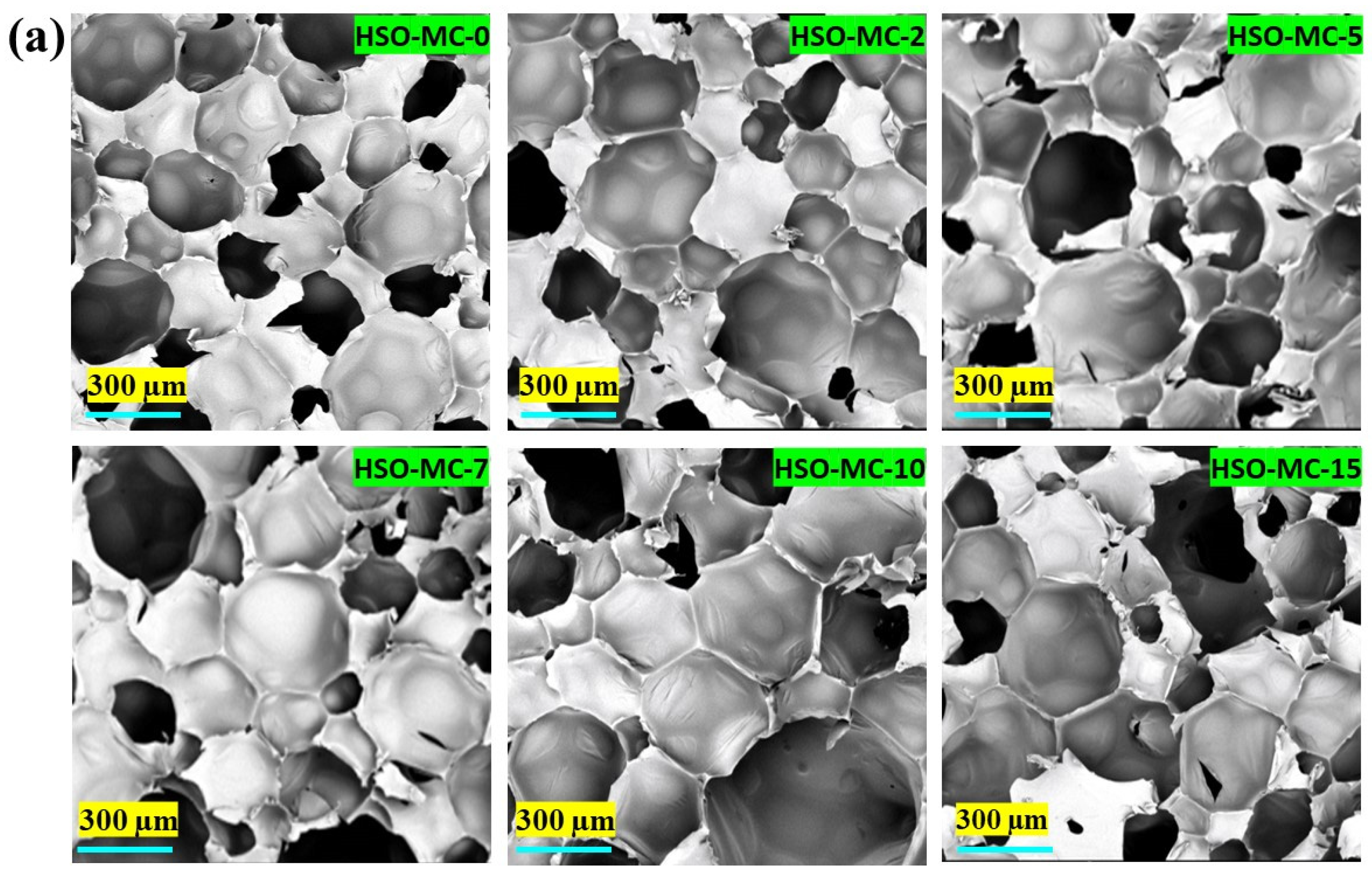
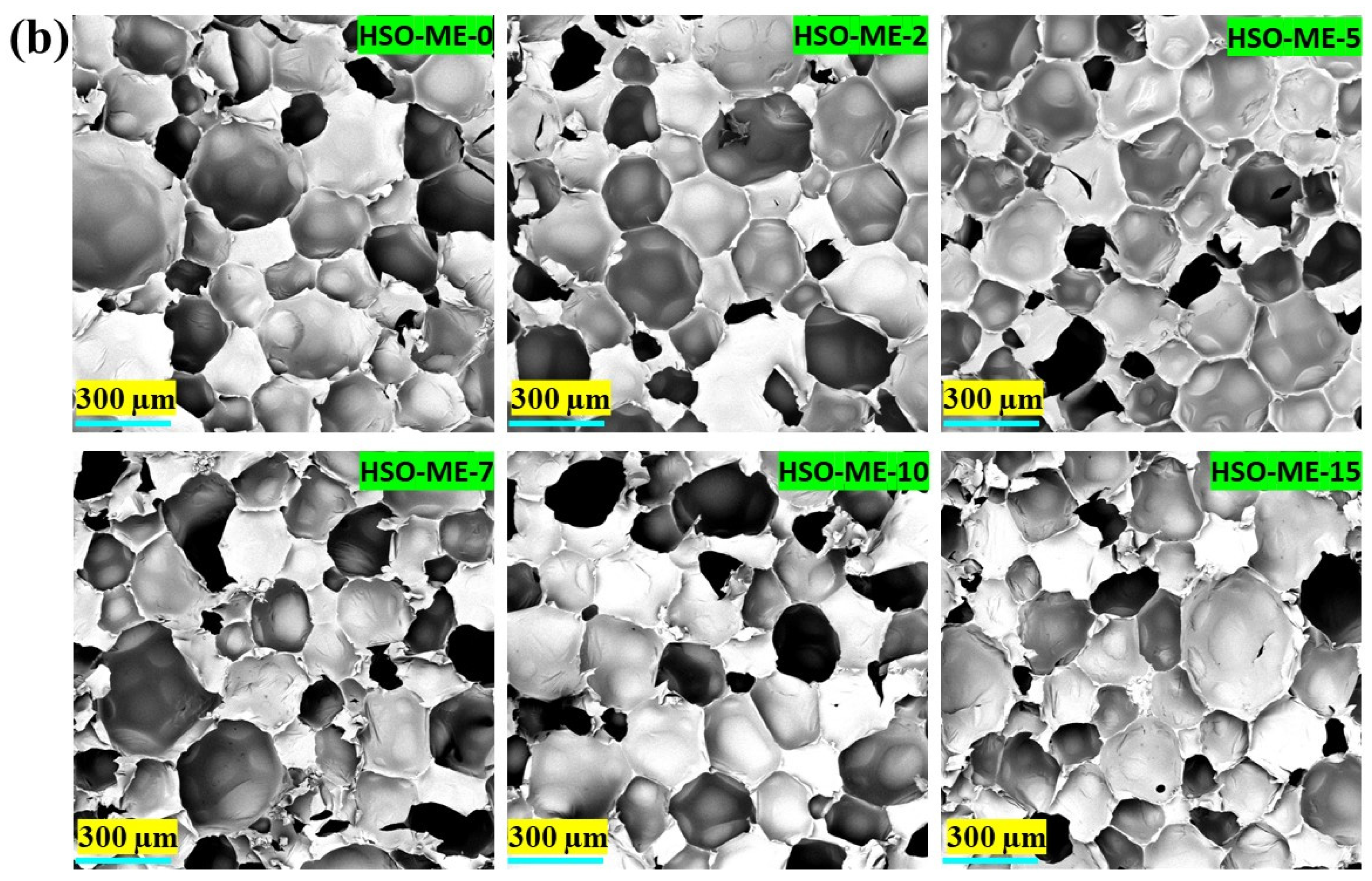
3.2.4. Scanning Electron Microscopy
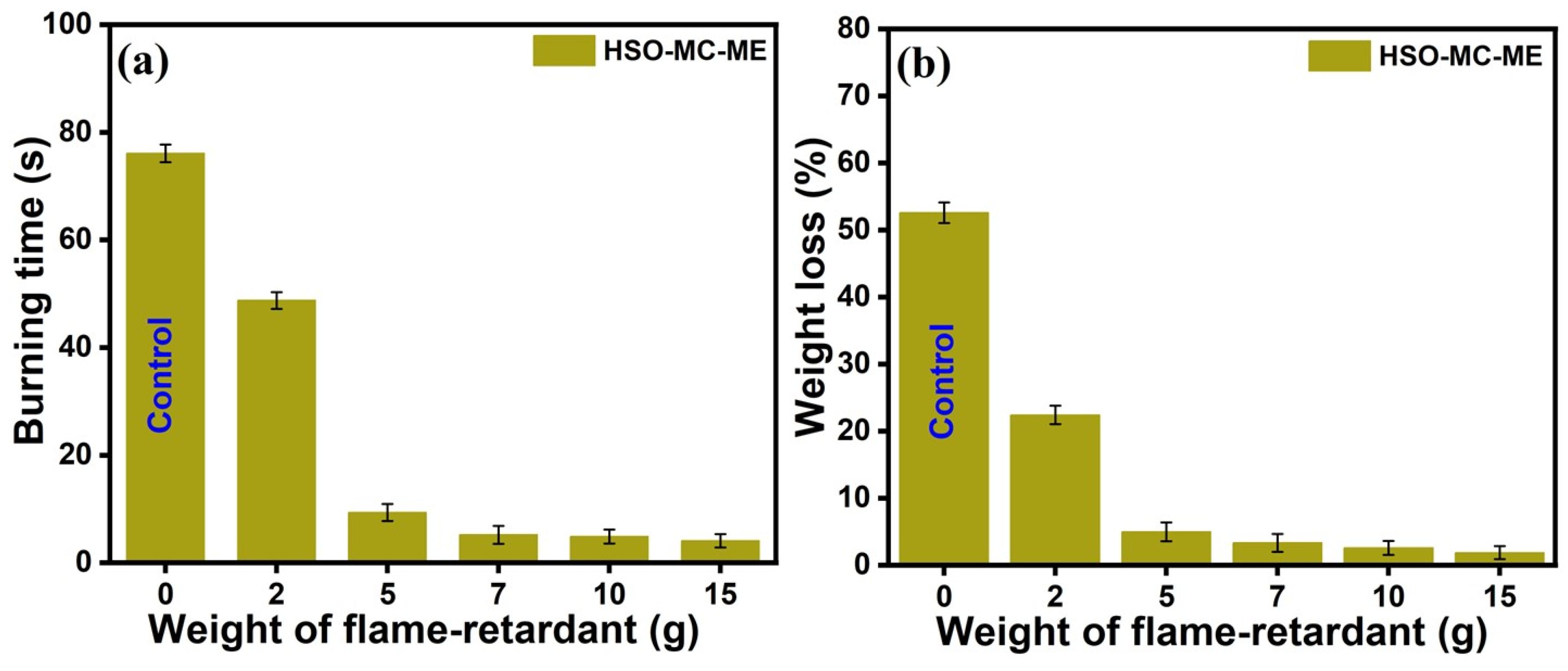
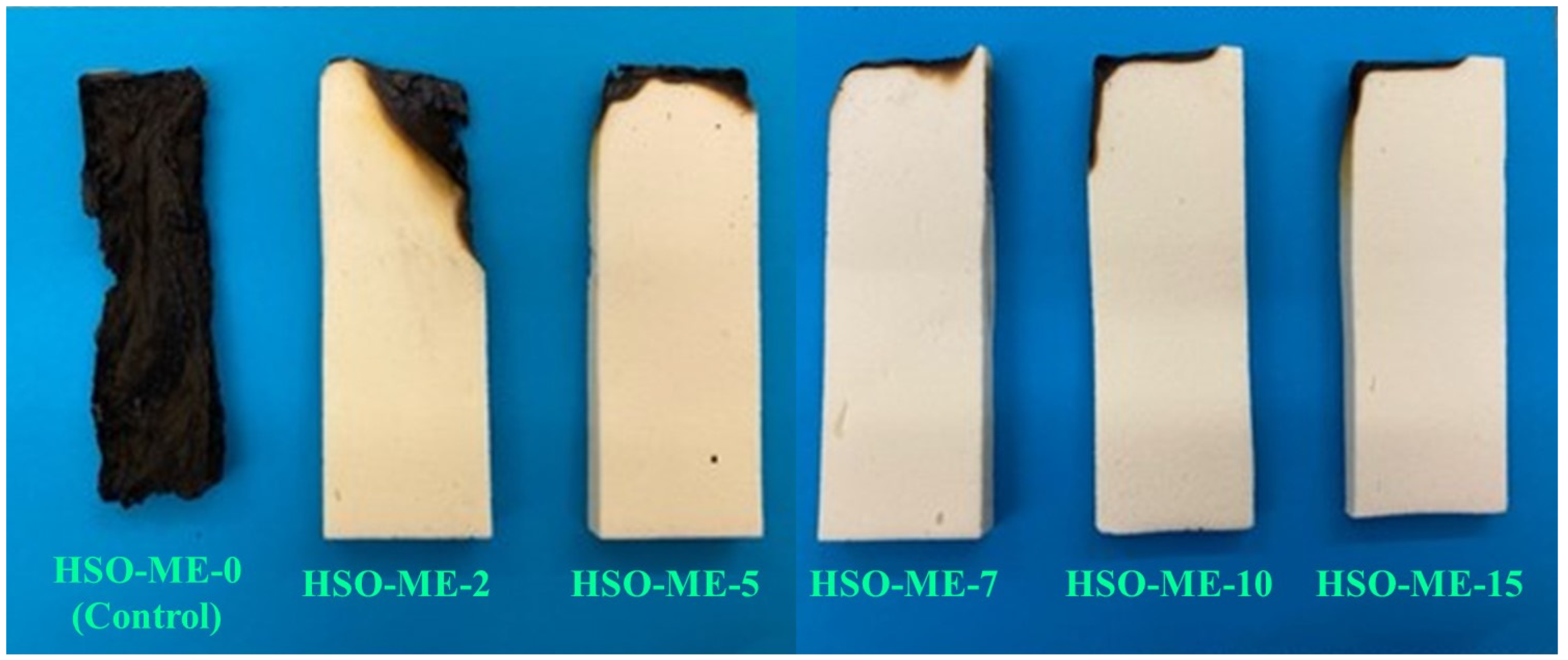
3.2.5. Horizontal Burning Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carré, C.; Ecochard, Y.; Caillol, S.; Averous, L. From the synthesis of biobased cyclic carbonate to polyhydroxyurethanes: A promising route towards renewable non-isocyanate polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Szycher, M. (Ed.) Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited: Shawbury, UK, 2005; ISBN 1859574912. [Google Scholar]
- Bayer, O.; Muller, E.; Petersen, S.; Piepenbrink, H.F.; Windemuth, E. New types of highly elastic substances, Vulcollans. Rubber Chem. Technol. 1950, 23, 812–835. [Google Scholar] [CrossRef]
- Seymour, R.B.; Kauffman, G.B. Polyurethanes: A class of modern versatile materials. J. Chem. Educ. 1992, 69, 909. [Google Scholar] [CrossRef]
- Król, P. Linear Polyurethanes: Synthesis Methods, Chemical Structures, Properties and Applications; VSP: Leiden, The Netherlands, 2008. [Google Scholar]
- Woods, G. Flexible Polyurethane Foams: Chemistry and Technology; Applied Science Publishers: London, UK, 1982. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2013. [Google Scholar]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Huang, X.; De Hoop Cornelis, F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High bio-content polyurethane (PU) foam made from bio-polyol and cellulose nanocrystals (CNCs) via microwave liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Singh, I.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancement in plant oil derived polyol-based polyurethane foam for future perspective: A review. Eur. J. Lipid Sci. Technol. 2020, 122, 1900225. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans DA, C.; Martin, D.J.; Annamalai, P.K. Hybrid polyether-palm oil polyester polyol based rigid polyurethane foam reinforced with cellulose nanocrystal. Ind. Crops Prod. 2018, 112, 378–388. [Google Scholar] [CrossRef]
- Petrović, Z.S. Polyurethanes from vegetable oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Gharib, J.; Pang, S.; Holland, D. Synthesis and characterisation of polyurethane made from pyrolysis bio-oil of pine wood. Eur. Polym. J. 2020, 133, 109725. [Google Scholar] [CrossRef]
- Acuña, P.; Zhang, J.; Yin, G.Z.; Liu, X.Q.; Wang, D.Y. Bio-based rigid polyurethane foam from castor oil with excellent flame retardancy and high insulation capacity via cooperation with carbon-based materials. J. Mater. Sci. 2021, 56, 2684–2701. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H.; Oh, K.W. Bio-based polyurethane foams with castor oil-based multifunctional polyols for improved compressive properties. Polymers 2021, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.F.; dos Santos, J.C.; de Freitas, V.A.A.; Pereira, R.B.D.; Panzera, T.H.; Scarpa, F. Castor-oil biobased foam: The effect of the composition on the physical and mechanical properties via a statistical mixture design. RSC Sustain. 2024, 2, 975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jeon, H.K.; Malsam, J.; Herrington, R.; Macosko, C.W. Substituting soybean oil-based polyol into polyurethane flexible foams. Polymer 2007, 48, 6656–6667. [Google Scholar] [CrossRef]
- Fang, Z.; Qiu, C.; Ji, D.; Yang, Z.; Zhu, N.; Meng, J.; Hu, X.; Guo, K. Development of high-performance biodegradable rigid polyurethane foams using full modified soy-based polyols. J. Agric. Food Chem. 2019, 67, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, M.F.; Wendt, B.L. Design and formulation of soybean oil-derived flexible polyurethane foams and their underlying polymer structure/property relationships. Polymer 2013, 54, 2511–2520. [Google Scholar] [CrossRef]
- He, W.; Kang, P.; Fang, Z.; Hao, J.; Wu, H.; Zhu, Y.; Guo, K. Flow reactor synthesis of bio-based polyol from soybean oil for the production of rigid polyurethane foam. Ind. Eng. Chem. Res. 2020, 59, 17513–17519. [Google Scholar] [CrossRef]
- Riyapan, D.; Saetung, A.; Saetung, N. A novel rigid PU foam based on modified used palm oil as sound-absorbing material. J. Polym. Environ. 2019, 27, 1693–1708. [Google Scholar] [CrossRef]
- Fourati, Y.; Hassen, R.B.; Bayramoğlu, G.; Boufi, S. A one-step route synthesis of polyurethane network from epoxidized rapeseed oil. Prog. Org. Coat. 2017, 105, 48–55. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Malewska, E.; Ryszkowska, J.; Kurańska, M.; Gloc, M.; Leszczyński, M.K.; Prociak, A. Vegetable fillers and rapeseed oil-based polyol as natural raw materials for the production of rigid polyurethane foams. Materials 2021, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Aydoğmuş, E.; Kamişli, F. New commercial polyurethane synthesized with biopolyol obtained from canola oil: Optimization, characterization, and thermophysical properties. J. Mol. Struct. 2022, 1256, 132495. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, S.J.; Feng, G.D.; Jia, P.Y.; Ren, X.L.; Zhang, M.; Zhou, Y.H. Properties of rigid polyurethane foam modified by tung oil-based polyol and flame-retardant particles. Polymers 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Cherney, J.H.; Small, E. Industrial hemp in North America: Production, politics and potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by n-hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Li, R.; Zhang, P.; Liu, T.; Muhunthan, B.; Xin, J.; Zhang, J. Use of hempseed-oil-derived polyacid and rosin-derived anhydride acid as cocuring agents for epoxy materials. ACS Sustain. Chem. Eng. 2018, 6, 4016–4025. [Google Scholar] [CrossRef]
- Jariwala, S.; Desai, Y.N.; Sahu, P.; Gupta, R.K. Hemp Seed Oil Derived Rigid Polyurethane Foams and Their Underlying Flame Retardancy Properties. J. Polym. Environ. 2024, 1–13. [Google Scholar] [CrossRef]
- Hayati, A.N.; Evans, D.A.C.; Laycock, B.; Martin, D.J.; Annamalai, P.K. A simple methodology for improving the performance and sustainability of rigid polyurethane foam by incorporating industrial lignin. Ind. Crops Prod. 2018, 117, 149–158. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Mielewczyk-Gryń, A.; Piszczyk, Ł. Bio-based polyurethane composites and hybrid composites containing a new type of bio-polyol and addition of natural and synthetic fibers. Materials 2020, 13, 2028. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Asyraf, M.R.M.; Dayana, D.A.Z.N.; Amelia, J.J.N.; Rani, M.S.A.; Norrrahim, M.N.F.; Nurazzi, N.M.; Aisyah, H.A.; Sharma, S.; et al. Polymer composites filled with metal derivatives: A review of flame retardants. Polymers 2021, 13, 1701. [Google Scholar] [CrossRef] [PubMed]
- Peponi, L.; Puglia, D.; Torre, L.; Valentini, L.; Kenny, J.M. Processing of nanostructured polymers and advanced polymeric based nanocomposites. Mater. Sci. Eng. R Rep. 2014, 85, 1–46. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Soares, J.B. Can we make better polyurethane composite foams with oil sands mature fine tailing? Macromol. Mater. Eng. 2016, 301, 383–389. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications–a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane composite foams in high-performance applications: A review. Polym.-Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Obele, C.M.; Ejimofor, M.I.; Atuanya, C.U.; Ibenta, M.E. Cassava stem cellulose (CSC) Nanocrystal for optimal methylene BlueBio sorption with response surface design. Curr. Res. Green Sustain. Chem. 2021, 4, 100067. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Ohwoavworhua, F.O.; Adelakun, T.A. Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: Characterisation and tableting properties. Indian J. Pharm. Sci. 2010, 72, 295–301. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Melamine, silica, and ionic liquid as a novel flame retardant for rigid polyurethane foams with enhanced flame retardancy and mechanical properties. Polym. Test. 2020, 87, 106511. [Google Scholar] [CrossRef]
- ASTM D 4274; Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D 1622; Standard Test Method for Apparent Density of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D 2856; Standard Test Method for Open-Cell Content of Rigid Cellular Plastics by the Air Pycnometer. ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM D 1621; Standard Test Method for Compressive Properties Of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM D 4986-18; Standard Test Method for Horizontal Burning Characteristics of Cellular Polymeric Materials. ASTM International: West Conshohocken, PA, USA, 2018.
- Lerma-García, M.J.; Ramis-Ramos, G.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Authentication of extra virgin olive oils by Fourier-transform infrared spectroscopy. Food Chem. 2010, 118, 78–83. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ionescu, M.; Wan, X.; Radojcic, D.; Petroviƈ, Z.S. Synthesis of a novel limonene based mannich polyol for rigid polyurethane foams. J. Polym. Environ. 2015, 23, 261–268. [Google Scholar] [CrossRef]
- Lee, S.T.; Ramesh, N.S. (Eds.) Polymeric Foams: Mechanisms and Materials; CRC Press: Boca Raton, FL, USA, 2004; Volume 1, p. 360. [Google Scholar]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Properties of rigid polyurethane-polyisocyanurate foams modified with the selected fillers. J. Appl. Polym. Sci. 2010, 115, 2460–2469. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Preparation of flame-retardant rigid polyurethane foams by combining modified melamine–formaldehyde resin and phosphorus flame retardants. ACS Omega 2020, 5, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Mane, J.V.; Chandra, S.; Sharma, S.; Ali, H.; Chavan, V.M.; Manjunath, B.S.; Patel, R.J. Mechanical property evaluation of polyurethane foam under quasi-static and dynamic strain rates-an experimental study. Procedia Eng. 2017, 173, 726–731. [Google Scholar] [CrossRef]
- Coccia, F.; Gryshchuk, L.; Moimare, P.; Bossa, F.d.L.; Santillo, C.; Barak-Kulbak, E.; Verdolotti, L.; Boggioni, L.; Lama, G.C. Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams. Polymers 2021, 13, 2556. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef]
- Zhou, X.; Sain, M.M.; Oksman, K. Semi-rigid biopolyurethane foams based on palm-oil polyol and reinforced with cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2016, 83, 56–62. [Google Scholar] [CrossRef]
- Hawkins, M.C.; O’Toole, B.; Jackovich, D. Cell morphology and mechanical properties of rigid polyurethane foam. J. Cell. Plast. 2005, 41, 267–285. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J. Thermal stabilities of drops of burning thermoplastics under the UL 94 vertical test conditions. J. Hazard. Mater. 2013, 246, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Lorenzetti, A.; Schartel, B. Fire phenomena of rigid polyurethane foams. Polymers 2018, 10, 1166. [Google Scholar] [CrossRef] [PubMed]



| Ingredient | Control | F-1 | F-2 | F-3 | F-4 | F-5 |
|---|---|---|---|---|---|---|
| Hemp polyol | 10 | 10 | 10 | 10 | 10 | 10 |
| SG-522 polyol | 10 | 10 | 10 | 10 | 10 | 10 |
| A-1 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Water | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| T-12 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| B8404 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Diisocyanate (MDI) | 31.29 | 31.29 | 31.29 | 31.29 | 31.29 | 31.29 |
| AL a or TiO2 or MC b (g) | 0 | 2 | 5 | 7 | 10 | 15 |
| AL a or TiO2 or MC b (%) | 0 | 3.65 | 8.66 | 11.72 | 15.94 | 22.15 |
| Ingredients | Control | F-1 | F-2 | F-3 | F-4 | F-5 |
|---|---|---|---|---|---|---|
| Hemp polyol | 10 | 10 | 10 | 10 | 10 | 10 |
| SG-522 polyol | 10 | 10 | 10 | 10 | 10 | 10 |
| A-1 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Water | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| T-12 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| B8404 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Diisocyanate (MDI) | 29.29 | 29.29 | 29.29 | 29.29 | 29.29 | 29.29 |
| MC (g) | 2 | 2 | 2 | 2 | 2 | 2 |
| Melamine (g) | 0 | 2 | 5 | 7 | 10 | 15 |
| Melamine (%) | 0 | 3.65 | 8.66 | 11.72 | 15.94 | 22.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahir, M.; Bodhak, C.; Gupta, R.K. Harnessing Enhanced Flame Retardancy in Rigid Polyurethane Composite Foams through Hemp Seed Oil-Derived Natural Fillers. Polymers 2024, 16, 1584. https://doi.org/10.3390/polym16111584
Ahir M, Bodhak C, Gupta RK. Harnessing Enhanced Flame Retardancy in Rigid Polyurethane Composite Foams through Hemp Seed Oil-Derived Natural Fillers. Polymers. 2024; 16(11):1584. https://doi.org/10.3390/polym16111584
Chicago/Turabian StyleAhir, Mansi, Chandan Bodhak, and Ram K. Gupta. 2024. "Harnessing Enhanced Flame Retardancy in Rigid Polyurethane Composite Foams through Hemp Seed Oil-Derived Natural Fillers" Polymers 16, no. 11: 1584. https://doi.org/10.3390/polym16111584
APA StyleAhir, M., Bodhak, C., & Gupta, R. K. (2024). Harnessing Enhanced Flame Retardancy in Rigid Polyurethane Composite Foams through Hemp Seed Oil-Derived Natural Fillers. Polymers, 16(11), 1584. https://doi.org/10.3390/polym16111584








