The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review
Abstract
1. Introduction
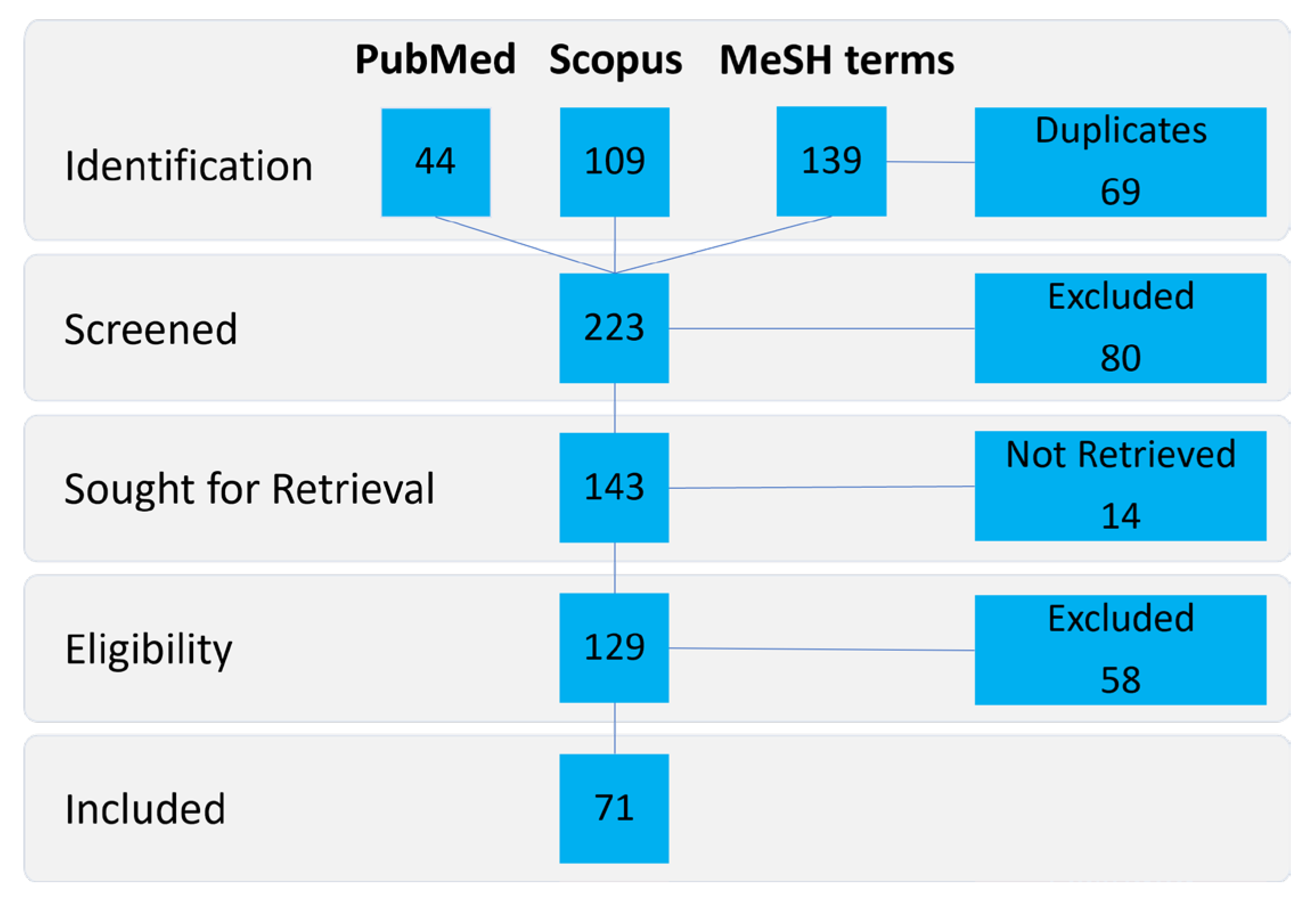
2. Materials and Methods
3. Results
3.1. Location as a Factor
3.2. Implant and Particle Parameters as a Factor
3.2.1. Molecular Weight
3.2.2. Viscosity
3.2.3. Crystallinity
3.3. Duration of Implantation as a Factor
3.4. Additives as a Factor
3.5. Comparisons to Other Biodegradable Materials
4. Discussion
4.1. Location as a Factor
4.2. Implant and Particle Parameters as a Factor
4.3. Duration of Implantation as a Factor
4.4. Additives as a Factor
4.5. Comparisons to Other Biodegradable Materials
4.6. Summary of the Factors
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bos, R.R.; Boering, G.; Rozema, F.R.; Leenslag, J.W. Resorbable poly(L-lactide) plates and screws for the fixation of zygomatic fractures. J. Oral Maxillofac. Surg. 1987, 45, 751–753. [Google Scholar] [CrossRef]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.-K. Biocompatible Polymers and their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.H.; Eaton, L.L.; Cohen, S.R. Current Concepts in the Use of Bellafill. Plast. Reconstr. Surg. 2015, 136, 171S–179S. [Google Scholar] [CrossRef]
- Ray, S.; Adelnia, H.; Ta, H.T. Collagen and the effect of poly-l-lactic acid based materials on its synthesis. Biomater. Sci. 2021, 9, 5714–5731. [Google Scholar] [CrossRef]
- Walton, M.; Cotton, N.J. Long-term in vivo degradation of poly-L-lactide (PLLA) in bone. J. Biomater. Appl. 2007, 21, 395–411. [Google Scholar] [CrossRef]
- Bos, R.R.; Rozema, F.R.; Boering, G.; Nijenhuis, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W. Degradation of and tissue reaction to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in rats. Biomaterials 1991, 12, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, H.; Luo, Q.; Chen, J.; Zhao, N.; Gao, W.; Pu, Y.; He, B.; Xie, J. In vivo inducing collagen regeneration of biodegradable polymer microspheres. Regen. Biomater. 2021, 8, rbab042. [Google Scholar] [CrossRef] [PubMed]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Stiles, C.E.; Hayes, C.L. Tissue response to single-polymer fibers of varying diameters: Evaluation of fibrous encapsulation and macrophage density. J. Biomed. Mater. Res. 2000, 52, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: All immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef]
- Schulze, M.; Fobker, M.; Puetzler, J.; Hillebrand, J.; Niemann, S.; Schulte, E.; Kurzynski, J.; Gosheger, G.; Hasselmann, J. Mechanical and microbiological testing concept for activatable anti-infective biopolymer implant coatings. Biomater. Adv. 2022, 138, 212917. [Google Scholar] [CrossRef]
- Puetzler, J.; Hasselmann, J.; Nonhoff, M.; Fobker, M.; Niemann, S.; Theil, C.; Gosheger, G.; Schulze, M. On-Demand Release of Anti-Infective Silver from a Novel Implant Coating Using High-Energy Focused Shock Waves. Pharmaceutics 2023, 15, 2179. [Google Scholar] [CrossRef]
- Schulze, M.; Nonhoff, M.; Hasselmann, J.; Fobker, M.; Niemann, S.; Theil, C.; Gosheger, G.; Puetzler, J. Shock Wave-Activated Silver-Loaded Biopolymer Implant Coating Eliminates Staphylococcus epidermidis on the Surface and in the Surrounding of Implants. Pharmaceutics 2023, 15, 2670. [Google Scholar] [CrossRef]
- Akagi, H.; Iwata, M.; Ichinohe, T.; Amimoto, H.; Hayashi, Y.; Kannno, N.; Ochi, H.; Fujita, Y.; Harada, Y.; Tagawa, M.; et al. Hydroxyapatite/poly-L-lactide acid screws have better biocompatibility and femoral burr hole closure than does poly-L-lactide acid alone. J. Biomater. Appl. 2014, 28, 954–962. [Google Scholar] [CrossRef]
- Asawa, Y.; Sakamoto, T.; Komura, M.; Watanabe, M.; Nishizawa, S.; Takazawa, Y.; Takato, T.; Hoshi, K. Early stage foreign body reaction against biodegradable polymer scaffolds affects tissue regeneration during the autologous transplantation of tissue-engineered cartilage in the canine model. Cell Transplant. 2012, 21, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kirigakubo, M.; Kobayashi, M.; Tabata, T.; Shimura, K.; Ikada, Y. Study on the efficacy of biodegradable poly(L-lactide) mesh for supporting transplanted particulate cancellous bone and marrow: Experiment involving subcutaneous implantation in dogs. Biomaterials 1993, 14, 729–736. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; van Diest, P.J.; Smit, T.H.; Berkhof, H.; Burger, E.H.; Wuisman, P.I.J.M. Four-year follow-up of poly-L-lactic Acid cages for lumbar interbody fusion in goats. J. Long. Term. Eff. Med. Implants 2005, 15, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.T.C.; Walboomers, X.F.; von den Hoff, J.W.; Maltha, J.C.; Jansen, J.A. Soft-tissue response to silicone and poly-L-lactic acid implants with a periodic or random surface micropattern. J. Biomed. Mater. Res. 2002, 61, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.T.C.; Walboomers, X.F.; von den Hoff, J.W.; Maltha, J.C.; Jansen, J.A. The effect of bone anchoring and micro-grooves on the soft tissue reaction to implants. Biomaterials 2002, 23, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Tan, R.P.; Chan, A.H.P.; Lee, B.S.L.; Santos, M.; Hung, J.; Liao, Y.; Bilek, M.M.M.; Fei, J.; Wise, S.G.; et al. Immobilized Macrophage Colony-Stimulating Factor (M-CSF) Regulates the Foreign Body Response to Implanted Materials. ACS Biomater. Sci. Eng. 2020, 6, 995–1007. [Google Scholar] [CrossRef]
- Fujihara, Y.; Takato, T.; Hoshi, K. Immunological response to tissue-engineered cartilage derived from auricular chondrocytes and a PLLA scaffold in transgenic mice. Biomaterials 2010, 31, 1227–1234. [Google Scholar] [CrossRef]
- Kang, S.-W.; Cho, E.R.; Jeon, O.; Kim, B.-S. The effect of microsphere degradation rate on the efficacy of polymeric microspheres as bulking agents: An 18-month follow-up study. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 253–259. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Kang, S.M.; Gu, Y.J.; Zhang, X.R.; Yon, D.K.; Shin, B.H.; Ham, J.R.; Lee, W.K.; Jeong, J.G.; Kwon, H.J.; et al. Bio-characteristics and Efficacy Analysis of Biodegradable Poly Dioxanone Dermal Filler in a Mouse Model and Humans. In Vivo 2023, 37, 1093–1102. [Google Scholar] [CrossRef]
- Shumaker, P.R.; England, L.J.; Dover, J.S.; Ross, E.V.; Harford, R.; Derienzo, D.; Bogle, M.; Uebelhoer, N.; Jacoby, M.; Pope, K. Effect of monopolar radiofrequency treatment over soft-tissue fillers in an animal model: Part 2. Lasers Surg. Med. 2006, 38, 211–217. [Google Scholar] [CrossRef]
- Majola, A.; Vainionpää, S.; Vihtonen, K.; Vasenius, J.; Törmälä, P.; Rokkanen, P. Intramedullary fixation of cortical bone osteotomies with self-reinforced polylactic rods in rabbits. Int. Orthop. 1992, 16, 101–108. [Google Scholar] [CrossRef]
- Matsusue, Y.; Yamamuro, T.; Oka, M.; Shikinami, Y.; Hyon, S.H.; Ikada, Y. In vitro and in vivo studies on bioabsorbable ultra-high-strength poly(L-lactide) rods. J. Biomed. Mater. Res. 1992, 26, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Morizane, K.; Goto, K.; Kawai, T.; Fujibayashi, S.; Otsuki, B.; Shimizu, T.; Matsuda, S. Bone Coverage around Hydroxyapatite/Poly(L-Lactide) Composite Is Determined According to Depth from Cortical Bone Surface in Rabbits. Materials 2021, 14, 1458. [Google Scholar] [CrossRef]
- Sun, L.; Sun, X.; Ruan, W.; Che, G.; Zhu, F.; Liu, C.; Wan, M. Mechanism of remodeling and local effects in vivo of a new injectable cosmetic filler. Sci. Rep. 2023, 13, 9599. [Google Scholar] [CrossRef]
- Isotalo, T.; Halasz, A.; Talja, M.; Tammela, T.L.; Paasimaa, S.; Törmälä, P. Tissue Biocompatibility of a New Caprolactone-Coated Self-Reinforced Self-Expandable Poly-L-Lactic Acid Bioabsorbable Urethral Stent. J. Endourol. 1999, 13, 525–530. [Google Scholar] [CrossRef]
- Koskikare, K.; Hirvensalo, E.; Pätiälä, H.; Rokkanen, P.; Pohjonen, T.; Törmälä, P.; Lob, G. Intraosseous plating with absorbable self-reinforced poly-L-lactide plates in the fixation of distal femoral osteotomies on rabbits. J. Biomed. Mater. Res. 1996, 30, 417–421. [Google Scholar] [CrossRef]
- Manninen, M.J. Self-reinforced poly-l-lactide screws in the fixation of cortical bone osteotomies in rabbits. J. Mater. Sci Mater. Med. 1993, 4, 179–185. [Google Scholar] [CrossRef]
- Manninen, M.J.; Pivrinta, U.; Ptil, H.; Rokkanen, P.; Taurio, R.; Tamminmki, M.; Trml, P. Shear strength of cancellous bone after osteotomy fixed with absorbable self-reinforced polyglycolic acid and poly-L-lactic acid rods. J. Mater. Sci Mater. Med. 1992, 3, 245–251. [Google Scholar] [CrossRef]
- Morizane, K.; Shikinami, Y.; Fujibayashi, S.; Goto, K.; Otsuki, B.; Kawai, T.; Shimizu, T.; Matsuda, S. Implantable composite devices of unsintered hydroxyapatite and poly-l-lactide with dispersive marbling morphology to enhance in vivo bioactivity and bioresorbability. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.; Böstman, O.; Tynninen, O.; Laitinen, O. Long-term tissue response to bioabsorbable poly-L-lactide and metallic screws: An experimental study. Bone 2006, 39, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.; Salminen, S.; Laitinen, O.; Tynninen, O.; Böstman, O. Tissue response to polyglycolide, polydioxanone, polylevolactide, and metallic pins in cancellous bone: An experimental study on rabbits. J. Orthop. Res. 2006, 24, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.K.; Salminen, S.T.; Tynninen, O.; Böstman, O.M.; Laitinen, O. Tissue restoration after implantation of polyglycolide, polydioxanone, polylevolactide, and metallic pins in cortical bone: An experimental study in rabbits. Calcif. Tissue Int. 2010, 87, 90–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puumanen, K.A.; Böhling, T.O.; Pihlajamäki, H.K.; Törmälä, P.O.; Waris, T.H.; Ashammakhi, N.A. Prefabricating bone in muscle by using free tibial periosteal grafts and self-reinforced polyglycolide and poly-L-lactide pins. Eur. J. Plast. Surg. 2001, 24, 19–24. [Google Scholar] [CrossRef]
- Korpela, A.; Aarnio, P.; Sariola, H.; Törmälä, P.; Harjula, A. Comparison of tissue reactions in the tracheal mucosa surrounding a bioabsorbable and silicone airway stents. Ann. Thorac. Surg. 1998, 66, 1772–1776. [Google Scholar] [CrossRef]
- Kunz, E.; Weckbach, A.; Rein, S. Resorbierbare Osteosynthesestifte. Eine experimentelle Untersuchung zur Biomechanik und zum Abbauverhalten verschiedener Stifte aus Polyglycolid und Poly(-L-Lactid). Unfallchirurgie 1995, 21, 1–7. [Google Scholar] [CrossRef]
- Päivärinta, U.; Böstman, O.; Majola, A.; Toivonen, T.; Törmälä, P.; Rokkanen, P. Intraosseous cellular response to biodegradable fracture fixation screws made of polyglycolide or polylactide. Arch. Orthop. Trauma. Surg. 1993, 112, 71–74. [Google Scholar] [CrossRef]
- Bergsma, J.E.; Rozema, F.R.; Bos, R.; Boering, G.; de Bruijn, W.C.; Pennings, A.J. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polylactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef]
- Chang, P.-C.; Liu, B.-Y.; Liu, C.-M.; Chou, H.-H.; Ho, M.-H.; Liu, H.-C.; Wang, D.-M.; Hou, L.-T. Bone tissue engineering with novel rhBMP2-PLLA composite scaffolds. J. Biomed. Mater. Res. A 2007, 81, 771–780. [Google Scholar] [CrossRef]
- Marascalco, P.J.; Blair, H.C.; Nieponice, A.; Robinson, L.J.; Kameneva, M.V. Intravenous injections of soluble drag-reducing polymers reduce foreign body reaction to implants. ASAIO J. 2009, 55, 503–508. [Google Scholar] [CrossRef]
- Schakenraad, J.M.; Oosterbaan, J.A.; Nieuwenhuis, P.; Molenaar, I.; Olijslager, J.; Potman, W.; Eenink, M.J.; Feijen, J. Biodegradable hollow fibres for the controlled release of drugs. Biomaterials 1988, 9, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Handolin, L.; Partio, E.K.; Arnala, I.; Pajarinen, J.; Pätiälä, H.; Rokkanen, P. The effect of low-intensity pulsed ultrasound on bone healing in SR-PLLA rod fixed experimental distal femur osteotomy in rat. J. Mater. Sci. Mater. Med. 2007, 18, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Hooper, K.A.; Nickolas, T.L.; Yurkow, E.J.; Kohn, J.; Laskin, D.L. Characterization of the inflammatory response to biomaterials using a rodent air pouch model. J. Biomed. Mater. Res. 2000, 50, 365–374. [Google Scholar] [CrossRef]
- Doval Neto, J.; Marques, R.F.C.; Motta, A.C.; Duek, E.A.D.R.; de Oliveira, G.J.P.L.; Marcantonio, C. Analysis of the biocompatibility of a biocelulose and a poly L- lactic acid membrane. Braz. J. Oral Sci. 2021, 21, e220616. [Google Scholar] [CrossRef]
- Nordström, P.; Pihlajamäki, H.; Toivonen, T.; Törmälä, P.; Rokkanen, P. Tissue response to polyglycolide and polylactide pins in cancellous bone. Arch. Orthop. Trauma Surg. 1998, 117, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, N.M.; Cheng, H.; Hill, P.S.; Guerreiro, J.D.T.; Dang, T.T.; Ma, M.; Watson, S.; Hwang, N.S.; Langer, R.; Anderson, D.G. Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. Biomacromolecules 2012, 13, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Weir, N.A.; Buchanan, F.J.; Orr, J.F.; Dickson, G.R. Degradation of poly-L-lactide. Part 1: In vitro and in vivo physiological temperature degradation. Proc. Inst. Mech. Eng. H 2004, 218, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Pistner, H.; Gutwald, R.; Ordung, R.; Reuther, J.; Mühling, J. Poly(L-lactide): A long-term degradation study in vivo. I. Biological results. Biomaterials 1993, 14, 671–677. [Google Scholar] [CrossRef]
- Peltoniemi, H.H.; Tulamo, R.M.; Toivonen, T.; Hallikainen, D.; Törmälä, P.; Waris, T. Biodegradable semirigid plate and miniscrew fixation compared with rigid titanium fixation in experimental calvarial osteotomy. J. Neurosurg. 1999, 90, 910–917. [Google Scholar] [CrossRef]
- Peltoniemi, H.H.; Hallikainen, D.; Toivonen, T.; Helevirta, P.; Waris, T. SR-PLLA and SR-PGA miniscrews: Biodegradation and tissue reactions in the calvarium and dura mater. J. Cranio-Maxillofac. Surg. 1999, 27, 42–50. [Google Scholar] [CrossRef]
- Peltoniemi, H.H.; Tulamo, R.M.; Pihlajamäki, H.K.; Kallioinen, M.; Pohjonen, T.; Törmälä, P.; Rokkanen, P.U.; Waris, T. Consolidation of craniotomy lines after resorbable polylactide and titanium plating: A comparative experimental study in sheep. Plast. Reconstr. Surg. 1998, 101, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, H.H.; Tulamo, R.-M.; Toivonen, T.; Pihlajamäki, H.K.; Pohjonen, T.; Törmälä, P.; Waris, T. Intraosseous Plating. J. Craniofacial Surg. 1998, 9, 171–176. [Google Scholar] [CrossRef]
- Suuronen, R.; Pohjonen, T.; Hietanen, J.; Lindqvist, C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J. Oral Maxillofac. Surg. 1998, 56, 604–614; discussion 614–615. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.; Alitalo, I.; Toivonen, T.; Vasenius, J.; Trml, P.; Vainionp, S. Tissue response to a braided poly-L-lactide implant in an experimental reconstruction of anterior cruciate ligament. J. Mater. Sci. Mater. Med. 1993, 4, 547–554. [Google Scholar] [CrossRef]
- Saadai, P.; Nout, Y.S.; Encinas, J.; Wang, A.; Downing, T.L.; Beattie, M.S.; Bresnahan, J.C.; Li, S.; Farmer, D.L. Prenatal repair of myelomeningocele with aligned nanofibrous scaffolds-a pilot study in sheep. J. Pediatr. Surg. 2011, 46, 2279–2283. [Google Scholar] [CrossRef]
- Landes, C.; Ballon, A.; Ghanaati, S.; Tran, A.; Sader, R. Treatment of malar and midfacial fractures with osteoconductive forged unsintered hydroxyapatite and poly-L-lactide composite internal fixation devices. J. Oral Maxillofac. Surg. 2014, 72, 1328–1338. [Google Scholar] [CrossRef]
- Oishi, K.; Arai, H.; Kuroki, H.; Fujioka, T.; Tomita, M.; Tasaki, D.; Oi, K.; Nagaoka, E.; Fujiwara, T.; Takeshita, M.; et al. A prospective randomized controlled study to assess the effectiveness of super FIXSORB WAVE® for sternal stabilization after sternotomy. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 665–673. [Google Scholar] [CrossRef]
- Stein, T.; Mehling, A.P.; Ulmer, M.; Reck, C.; Efe, T.; Hoffmann, R.; Jäger, A.; Welsch, F. MRI graduation of osseous reaction and drill hole consolidation after arthroscopic Bankart repair with PLLA anchors and the clinical relevance. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.E.; Varin, J. Poly-L-lactic acid rod fixation results in foot surgery. J. Foot Ankle Surg. 1998, 37, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Handolin, L.; Kiljunen, V.; Arnala, I.; Kiuru, M.J.; Pajarinen, J.; Partio, E.K.; Rokkanen, P. Effect of ultrasound therapy on bone healing of lateral malleolar fractures of the ankle joint fixed with bioabsorbable screws. J. Orthop. Sci. 2005, 10, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.; Mehling, A.P.; Reck, C.; Buckup, J.; Efe, T.; Hoffmann, R.; Jäger, A.; Welsch, F. MRI assessment of the structural labrum integrity after Bankart repair using knotless bio-anchors. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Micari, A.; Cioppa, A.; Vadalà, G.; Schmidt, A.; Sievert, H.; Rubino, P.; Angelini, A.; Scheinert, D.; Biamino, G. Evaluation of the biodegradable peripheral Igaki-Tamai stent in the treatment of de novo lesions in the superficial femoral artery: The GAIA study. JACC Cardiovasc. Interv. 2014, 7, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.A. Role of massage in preventing formation of papules and nodules after injecting poly-L-lactic acid. JAMA Facial Plast. Surg. 2014, 16, 457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veizi, E.; Alkan, H.; Çay, N.; Şahin, A.; Çepni, Ş.; Tecimel, O.; Fırat, A. Clinical and radiological comparison of bioactive glass and poly-L-lactic acid/hydroxyapatite bioabsorbable interference screws for tibial graft fixation in anterior cruciate ligament reconstruction. Orthop. Traumatol. Surg. Res. 2022, 108, 103247. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, T.; Matsusue, Y.; Uchida, A.; Shimada, K.; Shimozaki, E.; Kitaoka, K. Bioabsorbable osteosynthetic implants of ultra high strength poly-L-lactide. A clinical study. Int. Orthop. 1994, 18, 332–340. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, J.H. Clinical courses and degradation patterns of absorbable plates in facial bone fracture patients. Arch. Craniofac. Surg. 2019, 20, 297–303. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, J.W.; Kim, S.B.; Park, J.H.; Chung, K.S.; Ha, J.K.; Kim, J.G.; Kim, W.J. Comparison of Poly-L-Lactic Acid and Poly-L-Lactic Acid/Hydroxyapatite Bioabsorbable Screws for Tibial Fixation in ACL Reconstruction: Clinical and Magnetic Resonance Imaging Results. Clin. Orthop. Surg. 2017, 9, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.; Laflamme, Y.G.; Gagnon, S.; Canet, F.; Rouleau, D.M. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J. Shoulder Elb. Surg. 2011, 20, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Böstman, O.M. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: A three- to nine-year follow-up study. J. Bone Joint Surg. Br. 1998, 80, 333–338. [Google Scholar] [CrossRef]
- Pelto-Vasenius, K.; Hirvensalo, E.; Vasenius, J.; Partio, E.K.; Böstman, O.; Rokkanen, P. Redisplacement after ankle osteosynthesis with absorbable implants. Arch. Orthop. Trauma. Surg. 1998, 117, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Ramsingh, V.; Prasad, N.; Lewis, M. Pre-tibial reaction to biointerference screw in anterior cruciate ligament reconstruction. Knee 2014, 21, 91–94. [Google Scholar] [CrossRef]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; De Bruijn, W.C. Foreign body reactions to resorbable poly(l-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- Hayashi, M.; Muramatsu, H.; Sato, M.; Tomizuka, Y.; Inoue, M.; Yoshimoto, S. Surgical treatment of facial fracture by using unsintered hydroxyapatite particles/poly l-lactide composite device (OSTEOTRANS MX(®)): A clinical study on 17 cases. J. Craniomaxillofac. Surg. 2013, 41, 783–788. [Google Scholar] [CrossRef]
- Sinisaari, I.; Pätiälä, H.; Böstman, O.; Mäkelä, E.A.; Hirvensalo, E.; Partio, E.K.; Törmälä, P.; Rokkanen, P. Wound infections associated with absorbable or metallic devices used in the fixation of fractures, arthrodeses and osteotomies. Eur. J. Orthop. Surg. Traumatol. 1995, 5, 41–43. [Google Scholar] [CrossRef]
- Tams, J.; Rozema, F.R.; Bos, R.R.; Roodenburg, J.L.; Nikkels, P.G.; Vermey, A. Poly(L-lactide) bone plates and screws for internal fixation of mandibular swing osteotomies. Int. J. Oral Maxillofac. Surg. 1996, 25, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Illi, O.E.; Gitzelmann, C.A.; Gasser, B.; Misteli, F.; Ruedi, M. Five years of experience with biodegradable implants in paediatric surgery. J. Mater. Sci. Mater. Med. 1994, 5, 417–423. [Google Scholar] [CrossRef]
- Sena, P.; Manfredini, G.; Barbieri, C.; Mariani, F.; Tosi, G.; Ruozi, B.; Ferretti, M.; Marzona, L.; Palumbo, C. Application of poly-L-lactide screws in flat foot surgery: Histological and radiological aspects of bio-absorption of degradable devices. Histol. Histopathol. 2012, 27, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kawai, H.; Nakano, K.; Kanno, T.; Takabatake, K.; Nagatsuka, H.; Furuki, Y. Feasible Advantage of Bioactive/Bioresorbable Devices Made of Forged Composites of Hydroxyapatite Particles and Poly-L-lactide in Alveolar Bone Augmentation: A Preliminary Study. Int. J. Med. Sci. 2019, 16, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Usami, T.; Takada, N.; Sakai, H.; Endo, S.; Sekiya, I.; Ueki, Y.; Murakami, H.; Kuroyanagi, G. Treatment of patellar fractures using bioresorbable forged composites of raw particulate unsintered hydroxyapatite/poly-L-lactide cannulated screws and nonabsorbable sutures. Injury 2021, 52, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Harper, D. Polylactide. II. Viscosity–molecular weight relationships and unperturbed chain dimensions. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 2593–2599. [Google Scholar] [CrossRef]
- Duranti, F.; Salti, G.; Bovani, B.; Calandra, M.; Rosati, M.L. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol. Surg. 1998, 24, 1317–1325. [Google Scholar] [CrossRef]
- Yuan, F.-L.; Xu, M.-H.; Li, X.; Xinlong, H.; Fang, W.; Dong, J. The Roles of Acidosis in Osteoclast Biology. Front. Physiol. 2016, 7, 222. [Google Scholar] [CrossRef]
- Zan, J.; Qian, G.; Deng, F.; Zhang, J.; Zeng, Z.; Peng, S.; Shuai, C. Dilemma and breakthrough of biodegradable poly-l-lactic acid in bone tissue repair. J. Mater. Res. Technol. 2022, 17, 2369–2387. [Google Scholar] [CrossRef]
- Joiner, A.; Schäfer, F.; Hornby, K.; Long, M.; Evans, M.; Beasley, T.; Abraham, P. Enhanced enamel benefits from a novel fluoride toothpaste. Int. Dent. J. 2009, 59, 244–253. [Google Scholar] [CrossRef]
- Ott, G. Fremdkörpersarkome; Springer: Berlin/Heidelberg, Germany, 1970; ISBN 9783642867736. [Google Scholar]
- Oppenheimer, B.S.; Oppenheimer, E.T.; Stout, A.P.; Willhite, M.; Danishefsky, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer 1958, 11, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Morhenn, V.B.; Lemperle, G.; Gallo, R.L. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol. Surg. 2002, 28, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2020, 11, 607945. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Natarajan, E.; Mohan, S.; Krishnamurithy, G.; Murali, M.R.; Parasuraman, S.; Singh, S.; Kamarul, T. The functionalization of the electrospun PLLA fibrous scaffolds reduces the hydrogen peroxide induced cytokines secretion in vitro. Mater. Today Commun. 2021, 26, 101812. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]

| First Author | Year | Study Type | Organism | Location | Implant | Additive | n | |
|---|---|---|---|---|---|---|---|---|
| Majola A. | 1992 | in vivo | rabbit | femur | rod | n/a | 40 | [29] |
| Yamamuro T. | 1994 | clinical (retrospective) | human | various | various | n/a | 143 | [72] |
| Illi O.E. | 1994 | clinical | human | various | plug/nut | n/a | 32 | [83] |
| Kunz E. | 1995 | in vivo | rabbit | subcutaneous leg /muscle/tibia | rod | n/a | 20 | [43] |
| Koskikare K. | 1996 | in vivo | rabbit | femur | plate | n/a | 20 | [34] |
| Burns A.E. | 1998 | clinical (prospective) | human | metatarsus | rod | n/a | 25 | [66] |
| Pelto-Vasenius K. | 1998 | clinical (retrospective) | human | ankle | pin/screw | n/a | 30 | [77] |
| Weir N.A. | 2004 | in vivo | rat | subcutaneous back | rod | n/a | 12 | [54] |
| Handolin L. | 2005 | clinical (prospective) | human | ankle | screw | n/a | 22 | [67] |
| Handolin L. | 2007 | in vivo | rat | femur | rod | n/a | 32 | [49] |
| Pihlajamäki H.K. | 2010 | in vivo | rabbit | femur | pin | n/a | 80 | [40] |
| Stein T. | 2011 | clinical (prospective) | human | shoulder joint | anchor | n/a | 37 | [68] |
| Saadai P. | 2011 | in vivo | sheep | spine | scaffold | n/a | 2 | [62] |
| Stein T. | 2012 | clinical (prospective) | human | shoulder joint | anchor | n/a | 53 | [65] |
| Morizane K. | 2019 | in vivo | rabbit | knee | rod | hydroxyapatite | 32 | [37] |
| Sukegawa S. | 2019 | clinical | human | facial | screw | hydroxyapatite | 5 | [85] |
| Morizane K. | 2021 | in vivo | rabbit | femur/tibia | pin | hydroxyapatite | 32 | [31] |
| Usami T. | 2021 | clinical | human | patella | screw | hydroxyapatite | 15 | [86] |
| Oishi K. | 2023 | clinical (prospective) | human | sternum | plate | hydroxyapatite | 39 | [64] |
| Zhou S.-Y. | 2023 | in vivo | mouse | subcutaneous back | filler | n/a | 8 | [27] |
| First Author | Year | Study Type | Organism | Location | Implant | Additive | n | Symptoms | |
|---|---|---|---|---|---|---|---|---|---|
| Pistner H. | 1993 | in vivo | rat | subcutaneous back/muscle | rod/cube | n/a | 67 | immune cells, FBGCs, fibrous capsule; 3 Oppenheimer effect sarcomas | [55] |
| Sinisaari I. | 1995 | clinical | human | various | various | n/a | 420 | 1 patient with sinus formation | [81] |
| Tams J. | 1996 | clinical | human | facial | plate | n/a | 4 | 1 patient with immune cells, FBGCs, swelling, osteolysis | [82] |
| Böstman O.M. | 1998 | clinical (retrospective) | human | ankle | pin/screw | n/a | 234 | 1 patient with ankle arthrodesis; osteolysis, cystic extensions, FBGCs | [76] |
| Potapov A. | 2011 | clinical (retrospective) | human | biceps tendon | screw | n/a | 22 | 1 patient with sinus, osteolysis, swelling, tenderness | [75] |
| First Author | Year | Study Type | Organism | Location | Implant | Additive | n | Symptoms | |
|---|---|---|---|---|---|---|---|---|---|
| Bergsma E.J. | 1993 | clinical | human | facial | plate, screw | n/a | 10 | 9 patients with swelling, immune cells, FBGCs, fibrous capsule | [79] |
| Hayashi M. | 2013 | clinical | human | facial | plate, screw | hydroxyapatite | 17 | 2 patients with inflammatory granulation tissue, 1 patient with swelling also | [80] |
| Landes C. | 2014 | clinical (prospective) | human | facial | plate | hydroxyapatite | 29 | 2 patients with swelling | [63] |
| Janjua T.A. | 2014 | clinical (prospective) | human | various | filler | n/a | 91 | 27 patients with massage nodules; 8 patients without massage nodules | [70] |
| Ramsingh V. | 2014 | clinical (retrospective) | human | ACL | screw | tri-calcium phosphate | 268 | 14 patients with swelling, pain, edema, myxoma (cysts) | [78] |
| Lee D.W. | 2017 | clinical (retrospective) | human | ACL | screw | n/a/hydroxyapatite | 86/86 | 66 edema + 11 cysts in PLLA/21 edema + 2 cysts in PLLA-HA | [74] |
| Kim Y.M. | 2019 | clinical (retrospective) | human | facial | plate | hydroxyapatite | 15 | 3 patients with redness, tenderness | [73] |
| Veizi E. | 2022 | clinical (retrospective) | human | ACL | screw | hydroxyapatite | 27 | 9 patients with edema + 2 patients with tibial tunnel cysts | [71] |
| First Author | Year | Study Type | Organism | Location | Implant | Additive | n | Symptoms | |
|---|---|---|---|---|---|---|---|---|---|
| Schakenraad J.M. | 1988 | in vivo | rat | subcutaneous back | fiber | n/a | 78 | immune cells, fibrous capsule | [48] |
| Bos R.R.M. | 1991 | in vivo | rat | subcutaneous back | plate | n/a | 35 | immune cells, fibrous capsule | [6] |
| Matsusue Y. | 1992 | in vivo | rabbit | subcutaneous back/knee | rod | n/a | 70 | fibrous tissue | [30] |
| Manninen M.J. | 1992 | in vivo | rabbit | femur | rod | n/a | 42 | immune cells, FBGCs | [36] |
| Kinoshita Y. | 1993 | in vivo | dog | subcutaneous back | mesh | n/a | 22 | immune cells, FBGCs, fibrous capsule | [20] |
| Laitinen O. | 1993 | in vivo | sheep | ACL | fiber | n/a | 32 | immune cells, FBGCs, fibrous tissue | [61] |
| Manninen M.J. | 1993 | in vivo | rabbit | tibia | screw | n/a | 36 | immune cells, FBGCs | [35] |
| Päivärinta U. | 1993 | in vivo | rabbit | femur | screw | n/a | 25 | immune cells, FBGCs | [44] |
| Bergsma J.E. | 1995 | in vivo | rat | subcutaneous back | capsule | n/a | 20 | immune cells, FBGCs, fibrous capsule | [45] |
| Peltoniemi H.H. | 1998 | in vivo | sheep | cranium | plate | n/a | 9 | immune cells, FBGCs, fibrous capsule | [58] |
| Peltoniemi H.H. | 1998 | in vivo | sheep | cranium | plate | n/a | 6 | immune cells, FBGCs, fibrous capsule | [59] |
| Suuronen R. | 1998 | in vivo | sheep | facial | plate | n/a | 5 | immune cells, FBGCs | [60] |
| Nordström P. | 1998 | in vivo | rat | femur | pin | n/a | 51 | immune cells, FBGCs, fibrous tissue | [52] |
| Korpela A. | 1998 | in vivo | rabbit | trachea | stent | n/a | 9 | immune cells | [42] |
| Peltoniemi H.H. | 1999 | in vivo | sheep | cranium | screw | n/a | 20 | immune cells, FBGCs, fibrous capsule | [57] |
| Isotalo T. | 1999 | in vivo | rabbit | subcutaneous back | rod | n/a/caprolactone | 15 | immune cells, FBGCs, fibrous tissue | [33] |
| Peltoniemi H.H. | 1999 | in vivo | sheep | cranium | screw | n/a | 10 | immune cells, FBGCs, fibrous capsule | [56] |
| Hooper K.A. | 2000 | in vivo | rat | subcutaneous back | disc | n/a | n/a | immune cells, FBGCs | [50] |
| Puumanen K.A. | 2001 | in vivo | rabbit | back with tibial graft | pin | n/a | 12 | immune cells | [41] |
| Parker J.A.T.C. | 2002 | in vivo | goat | subcutaneous flank | disc | n/a | 6 | immune cells | [22] |
| Parker J.A. | 2002 | in vivo | goat | subcutaneous flank/subperiosteal frontal bone | anchor | n/a | 18 | immune cells, FBGCs, fibrous capsule | [23] |
| van Dijk M. | 2005 | in vivo | goat | vertebra | cage | n/a | 43 | immune cells, FBGCs | [21] |
| Pihlajamäki H. | 2006 | in vivo | rabbit | femur | screw | n/a | 32 | immune cells | [38] |
| Pihlajamäki H. | 2006 | in vivo | rabbit | knee | pin | n/a | 20 | immune cells, fibrous tissue | [39] |
| Shumaker P.R. | 2006 | in vivo | pig | abdomen | filler | n/a | 1 | immune cells, FBGCs | [28] |
| Chang P.C. | 2007 | in vivo | rat | calf | scaffold | rhBMP2 | 20 | immune cells, FBGCs | [46] |
| Kang S.-W. | 2007 | in vivo | mouse | subcutaneous back | microsphere | n/a | 28 | immune cells | [26] |
| Marascalco P.J. | 2009 | in vivo | rat | subcutaneous back | scaffold | n/a | 15 | FBGCs, new collagen | [47] |
| Fujihara Y. | 2010 | in vivo | mouse | subcutaneous back | scaffold | n/a | 3 | immune cells, FBGCs, fibrous tissue | [25] |
| Asawa Y. | 2012 | in vivo | dog | subcutaneous | scaffold | n/a | 6 | immune cells, cytokines | [19] |
| Sena P. | 2012 | clinical | human | foot | screw | n/a | 33 | 13 patients with immune cells, FBGCs | [84] |
| Vacanti N.M. | 2012 | in vivo | rat | subcutaneous back | fiber | n/a/dexamethasone | 36 | immune cells, fibrous capsule | [53] |
| Akagi H. | 2014 | in vivo | dog | femur | screw | hydroxyapatite | 12 | immune cells, fibrous tissue | [18] |
| Werner M. | 2014 | clinical (prospective) | human | artery | stent | n/a | 30 | 4 patients with immune cells, 2 with FBGCs also | [69] |
| Yang N. | 2020 | in vivo | mouse | subcutaneous back | scaffold | PIII + M-CSF | 20 | FBGCs, fibrous capsule, cytokines | [24] |
| Zhang Y. | 2021 | in vivo | rabbit | subcutaneous back | filler | n/a | 21 | immune cells, FBGCs, fibrous capsule | [7] |
| Neto J.D. | 2021 | in vivo | rat | subcutaneous back | membrane | n/a | 15 | immune cells, fibrous tissue, granulation tissue | [51] |
| Sun L. | 2023 | in vivo | rabbit | subcutaneous back | filler | n/a | 12 | immune cells, FBGCs, fibrous tissue | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonhoff, M.; Puetzler, J.; Hasselmann, J.; Fobker, M.; Gosheger, G.; Schulze, M. The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review. Polymers 2024, 16, 817. https://doi.org/10.3390/polym16060817
Nonhoff M, Puetzler J, Hasselmann J, Fobker M, Gosheger G, Schulze M. The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review. Polymers. 2024; 16(6):817. https://doi.org/10.3390/polym16060817
Chicago/Turabian StyleNonhoff, Melanie, Jan Puetzler, Julian Hasselmann, Manfred Fobker, Georg Gosheger, and Martin Schulze. 2024. "The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review" Polymers 16, no. 6: 817. https://doi.org/10.3390/polym16060817
APA StyleNonhoff, M., Puetzler, J., Hasselmann, J., Fobker, M., Gosheger, G., & Schulze, M. (2024). The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review. Polymers, 16(6), 817. https://doi.org/10.3390/polym16060817






