A Review of Polymer-Based Environment-Induced Nanogenerators: Power Generation Performance and Polymer Material Manipulations
Abstract
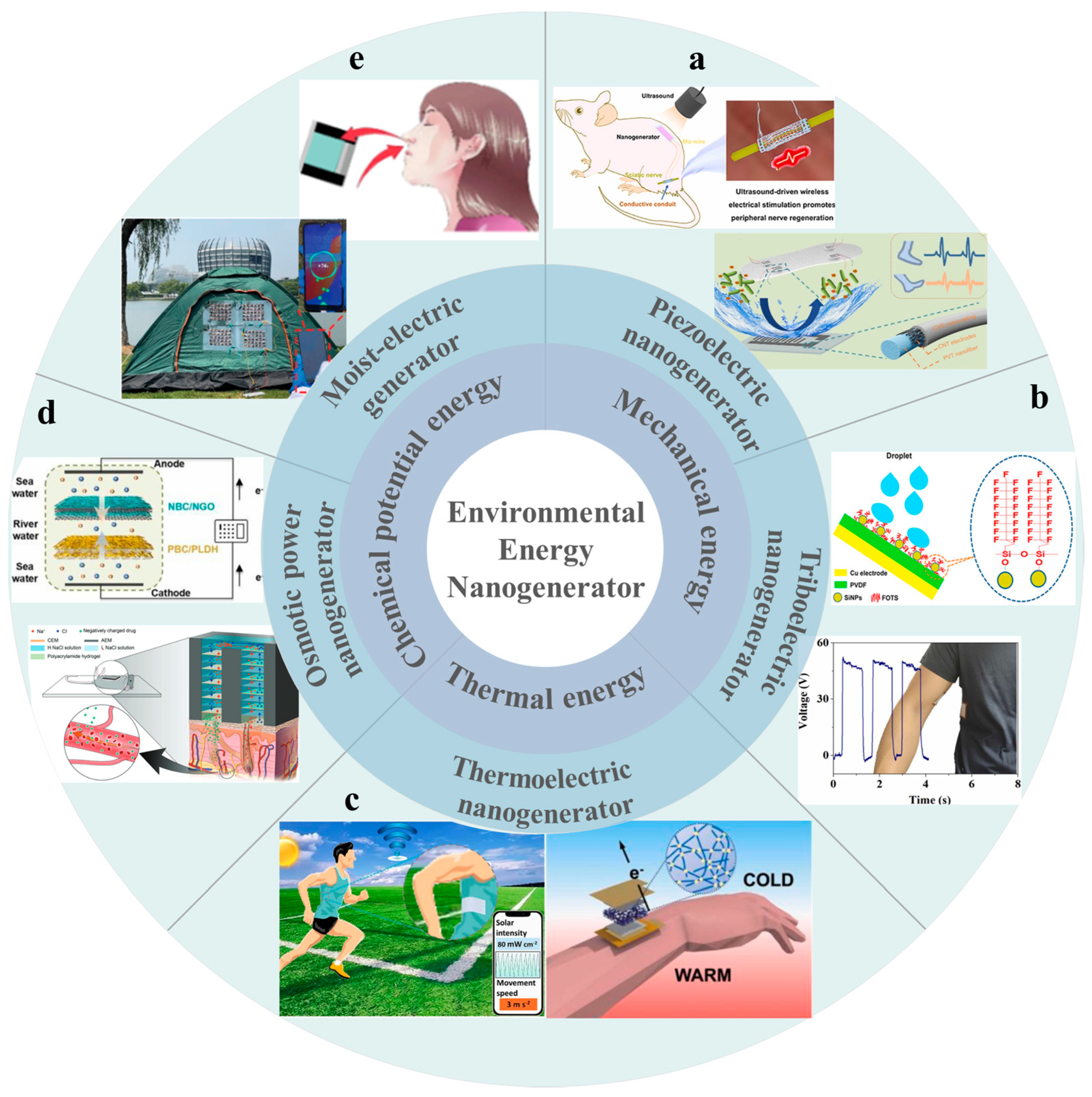
1. Introduction
2. Working Principle of Polymer-Based Nanogenerators Using Environmental Energy
2.1. Piezoelectric Nanogenerator
2.2. Triboelectric Nanogenerator
2.3. Thermoelectric Nanogenerator
2.4. Osmotic Power Nanogenerators Using Salinity Difference
2.5. Moist-Electric Nanogenerators
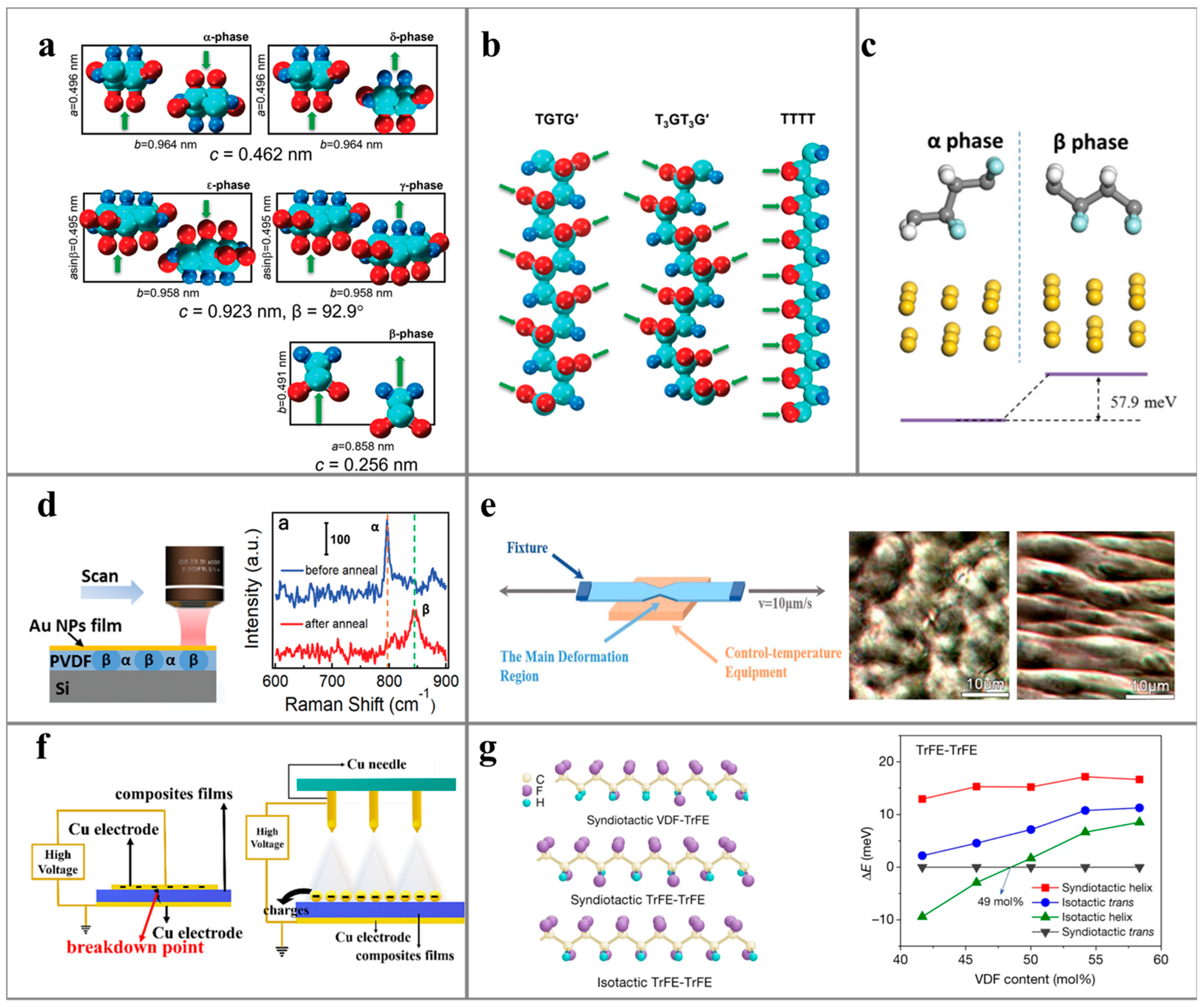
3. Dielectric Polymers for Environment-Induced Power Generation
3.1. Dielectric Polymers for PENG
3.2. Dielectric Polymers for TriboENG
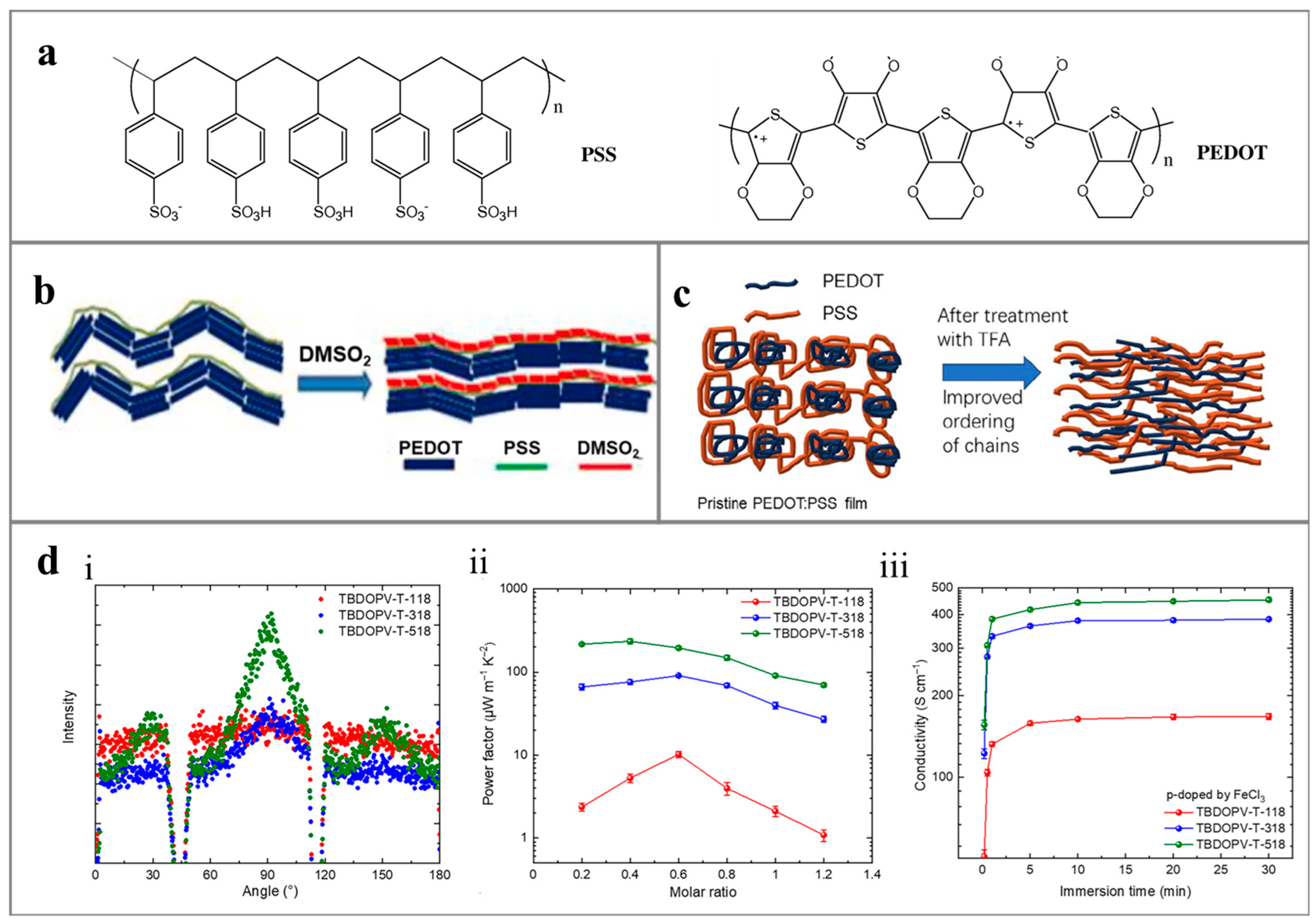
4. Conductive Polymers for Environment-Induced Power Generation
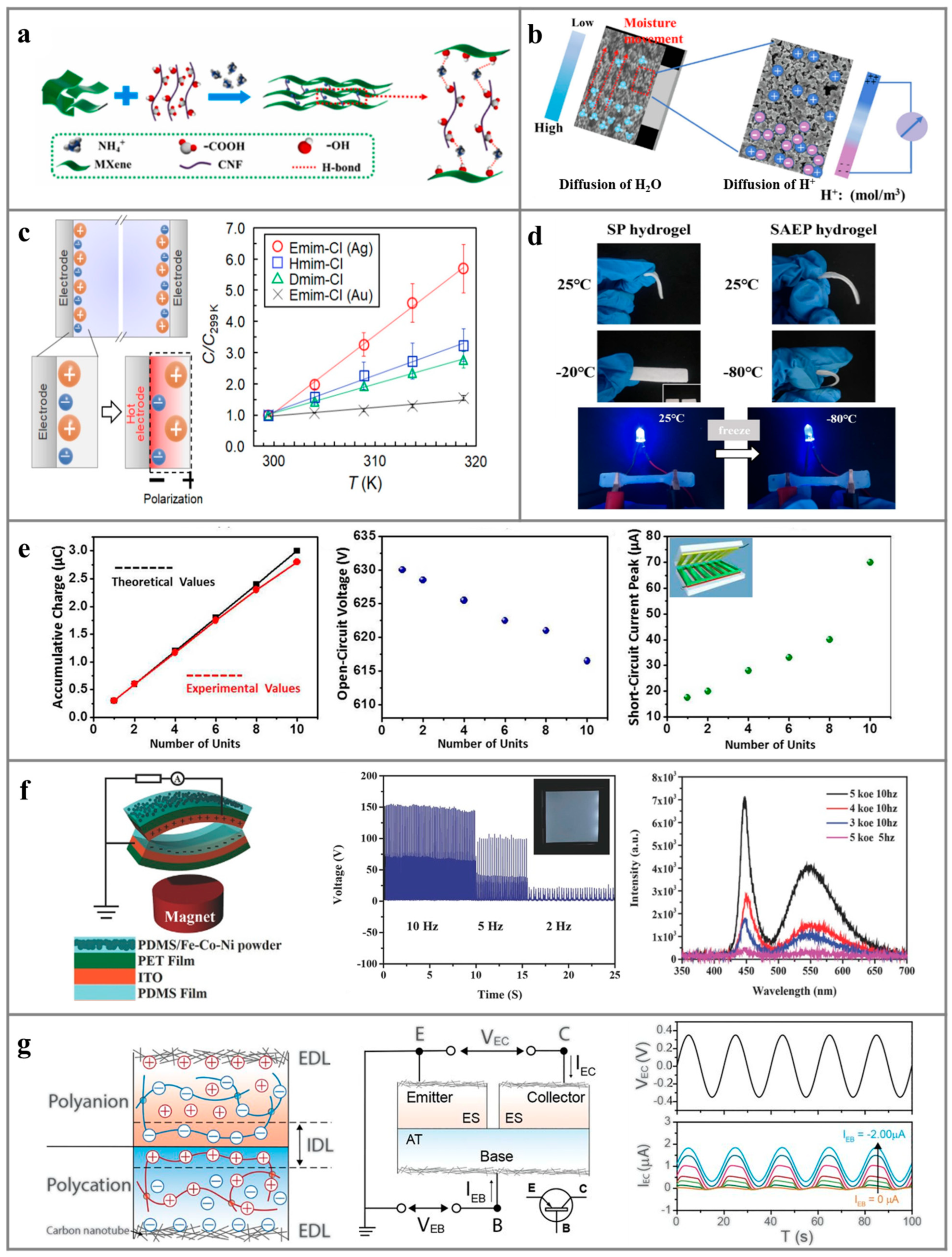
5. Ionic Hydrogels for Environment-Induced Power Generation
6. Natural Polymers for Environment-Induced Power Generation
7. Performance Enhancement Methods of Polymer-Based Nanogenerators
7.1. Improvement of Electrical Conductivity and Thermal Conductivity
7.2. Reduction in Ion Transport Resistance
7.3. Utilization of Composite Polymers
8. Conclusions and Future Prospects
Funding
Conflicts of Interest
References
- Strezov, V.; Cho, H.H. Environmental impact assessment from direct emissions of Australian thermal power generation technologies. J. Clean. Prod. 2020, 270, 122515. [Google Scholar] [CrossRef]
- Fetisov, V.; Gonopolsky, A.M.; Davardoost, H.; Ghanbari, A.R.; Mohammadi, A. H Regulation and impact of VOC and CO2 emissions on low-carbon energy systems resilient to climate change: A case study on an environmental issue in the oil and gas industry. Energy Sci. Eng. 2023, 11, 1516–1535. [Google Scholar] [CrossRef]
- Evensen, D.; Whitmarsh, L.; Devine-Wright, P.; Dickie, J.; Bartie, P.; Foad, C.; Bradshaw, M.; Ryder, S.; Mayer, A.; Varley, A. Growing importance of climate change beliefs for attitudes towards gas. Nat. Clim. Chang. 2023, 13, 240–243. [Google Scholar] [CrossRef]
- Dogan, E.; Luni, T.; Majeed, M.T.; Tzeremes, P. The nexus between global carbon and renewable energy sources: A step towards sustainability. J. Clean. Prod. 2023, 416, 137927. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Yue, M.; Yao, H.; Tian, H.; Sun, X.; Wu, Y.; Huang, Z.; Ban, D.; Zheng, H. Hybridized energy harvesting device based on high-performance triboelectric nanogenerator for smart agriculture applications. Nano Energy 2022, 102, 107681. [Google Scholar] [CrossRef]
- Zi, Y.; Wang, Z.L. Nanogenerators: An emerging technology towards nanoenergy. APL Mater. 2017, 5, 74103. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Jia, X.; Chen, M.; Wang, H.; Ji, G.; Zhou, H.; Fang, Z.; Gao, Z. TENG-based self-powered device—The heart of life. Nano Energy 2024, 119, 109080. [Google Scholar] [CrossRef]
- Zeadally, S.; Shaikh, F.K.; Talpur, A.; Sheng, Q. Design architectures for energy harvesting in the Internet of Things. Renew. Sustain. Energy Rev. 2020, 128, 109901. [Google Scholar] [CrossRef]
- Su, Y.-F.; Kotian, R.R.; Lu, N. Energy harvesting potential of bendable concrete using polymer based piezoelectric generator. Compos. Part B Eng. 2018, 153, 124–129. [Google Scholar] [CrossRef]
- Ahmed, A.; Hassan, I.; ElhKady, M.F.; Radhi, A.; Jeong, C.K.; Selvaganapathy, P.R.; Zu, J.; Ren, S.; Wang, Q.; Kaner, R.B. Integrated Triboelectric Nanogenerators in the Era of the Internet of Things. Adv. Sci. 2019, 6, 1802230. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, B.; Zhang, L.; Yang, W. Nanogenerator as new energy technology for self-powered intelligent transportation system. Nano Energy 2019, 66, 104086. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Abdalla, I.; Yu, J.; Ding, B. Nanofibrous membrane constructed wearable triboelectric nanogenerator for high performance biomechanical energy harvesting. Nano Energy 2017, 36, 341–348. [Google Scholar] [CrossRef]
- Pang, D.; Zhang, A.; Guo, Y.; Wu, J. Energy harvesting analysis of wearable thermoelectric generators integrated with human skin. Energy 2023, 282, 128850. [Google Scholar] [CrossRef]
- Li, J.; Hacker, T.A.; Wei, H.; Long, Y.; Yang, F.; Ni, D.; Rodgers, A.; Cai, W.; Wang, X. Long-term in vivo operation of implanted cardiac nanogenerators in swine. Nano Energy 2021, 90, 106507. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, Q.; Lee, C. Advanced Implantable Biomedical Devices Enabled by Triboelectric Nanogenerators. Nanomaterials 2022, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Francioso, L.; De Pascali, C.; Farella, I.; Martucci, C.; Cretì, P.; Siciliano, P.; Perrone, A. Flexible thermoelectric generator for ambient assisted living wearable biometric sensors. J. Power Sources 2011, 196, 3239–3243. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Q.; Wang, Z.L.; Li, Z. Nanogenerator-Based Self-Powered Sensors for Wearable and Implantable Electronics. Research 2020, 2020, 8710686. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhao, F.; Lei, Y.; Wang, Y.C. Hydrogel-based triboelectric devices for energy-harvesting and wearable sensing applications. Nano Energy 2022, 95, 106988. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Wang, L.Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Song, J.; Zhou, J.; Wang, Z.L. Piezoelectric and Semiconducting Coupled Power Generating Process of a Single ZnO Belt/Wire. A Technology for Harvesting Electricity from the Environment. Nano Lett. 2006, 6, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Li, Q.; Yang, L.; Cao, Y.; Choi, K.H.; Wang, Q.; Zhang, Q.M. Biocompatible and Flexible Hydrogel Diode-Based Mechanical Energy Harvesting. Adv. Mater. Technol. 2017, 2, 1700118. [Google Scholar] [CrossRef]
- DiSalvo, F.J. Thermoelectric Cooling and Power Generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Kim, B.; Park, S.; Kim, E. Bisulfate transport in hydrogels for self-healable and transparent thermoelectric harvesting films. Energy Environ. Sci. 2022, 15, 2049–2060. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, H.; Zhang, Z.; Jiang, L.; Qu, L. Direct Power Generation from a Graphene Oxide Film under Moisture. Adv. Mater. 2015, 27, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, P.; Xu, C.; Wang, Q.; Zhang, F.; Yang, K.; Jiang, W.; Feng, J.; Luo, Z. Ultrasound-driven in vivo electrical stimulation based on biodegradable piezoelectric nanogenerators for enhancing and monitoring the nerve tissue repair. Nano Energy 2022, 102, 107707. [Google Scholar] [CrossRef]
- Su, C.; Huang, X.; Zhang, L.; Zhang, Y.; Yu, Z.; Chen, C.; Ye, Y.; Guo, S. Robust superhydrophobic wearable piezoelectric nanogenerators for self-powered body motion sensors. Nano Energy 2023, 107, 108095. [Google Scholar] [CrossRef]
- Vu, D.L.; Le, C.D.; Vo, C.P.; Ahn, K.K. Surface polarity tuning through epitaxial growth on polyvinylidene fluoride membranes for enhanced performance of liquid-solid triboelectric nanogenerator. Compos. Part B Eng. 2021, 223, 109135. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.G.; Zhao, H.X.; Cai, Y.W.; Li, J.Z.; Li, G.Z.; Zhang, X.N.; Wang, B.L.; Han, J.C.; Wang, Y.; et al. Waterproof, breathable and washable triboelectric nanogenerator based on electrospun nanofiber films for wearable electronics. Nano Energy 2021, 90, 106639. [Google Scholar] [CrossRef]
- Yang, H.; Ahmed Khan, S.; Li, N.; Fang, R.; Huang, Z.; Zhang, H. Thermogalvanic gel patch for self-powered human motion recognition enabled by photo-thermal-electric conversion. Chem. Eng. J. 2023, 473, 145247. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Chen, J.X.M.; Sun, Y.C.; Aghababaei, O.; Saadatnia, Z.; Naguib, H.E. 3D printing of conductive polymer aerogel thermoelectric generator with tertiary doping. Nano Energy 2023, 117, 108909. [Google Scholar] [CrossRef]
- Kwon, S.-R.; Nam, S.H.; Park, C.Y.; Baek, S.; Jang, J.; Che, X.; Kwak, S.H.; Choi, Y.R.; Park, N.R.; Choi, J.Y.; et al. Electrodeless Reverse Electrodialysis Patches as an Ionic Power Source for Active Transdermal Drug Delivery. Adv. Funct. Mater. 2018, 28, 1705952. [Google Scholar] [CrossRef]
- Sheng, N.; Zhang, M.; Song, Q.; Zhang, H.; Chen, S.; Wang, H.; Zhang, K. Enhanced salinity gradient energy harvesting with oppositely charged bacterial cellulose-based composite membranes. Nano Energy 2022, 101, 107548. [Google Scholar] [CrossRef]
- Yan, H.; Liu, Z.; Qi, R. Development and mechanism investigation of TiO2/Co hydrogel microgenerator utilizing humidity gradient. Energy Convers. Manag. 2023, 291, 117256. [Google Scholar] [CrossRef]
- Wen, X.; Sun, Z.; Xie, X.; Zhou, Q.; Liu, H.; Wang, L.; Qin, X.; Tan, S.C. High-Performance Fully Stretchable Moist-Electric Generator. Adv. Funct. Mater. 2023, 2311128. [Google Scholar] [CrossRef]
- Tao, J.; Wang, Y.; Zheng, X.; Zhao, C.; Jin, X.; Wang, W.; Lin, T. A review: Polyacrylonitrile as high-performance piezoelectric materials. Nano Energy 2023, 118, 108987. [Google Scholar] [CrossRef]
- Zhang, W.; You, L.; Meng, X.; Wang, B.; Lin, D. Recent Advances on Conducting Polymers Based Nanogenerators for Energy Harvesting. Micromachines 2021, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Shanbedi, M.; Ardebili, H.; Karim, A. Polymer-based triboelectric nanogenerators: Materials, characterization, and applications. Prog. Polym. Sci. 2023, 144, 101723. [Google Scholar] [CrossRef]
- Tao, X.; Chen, X.; Wang, Z.L. Design and synthesis of triboelectric polymers for high performance triboelectric nanogenerators. Energy Environ. Sci. 2023, 16, 3654–3678. [Google Scholar] [CrossRef]
- Zhu, M.; Yi, Z.; Yang, B.; Lee, C. Making use of nanoenergy from human—Nanogenerator and self-powered sensor enabled sustainable wireless IoT sensory systems. Nano Today 2021, 36, 101016. [Google Scholar] [CrossRef]
- Wang, Z.L. On the first principle theory of nanogenerators from Maxwell’s equations. Nano Energy 2019, 68, 104272. [Google Scholar] [CrossRef]
- Wang, Z.L. On Maxwell’s displacement current for energy and sensors: The origin of nanogenerators. Mater. Today 2017, 20, 74–82. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, B.; Li, Z.; Wang, Z.L. Recent Progress on Piezoelectric and Triboelectric Energy Harvesters in Biomedical Systems. Adv. Sci. 2017, 4, 1700029. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; In-na, P.; Sahu, M.; Rajaitha, P.M.; Kim, H.J. Piezoelectric energy harvesting systems for biomedical applications. Nano Energy 2022, 100, 107514. [Google Scholar] [CrossRef]
- Dobashi, Y.; Yao, D.; Petel, Y.; Nguyen, T.N.; Sarwar, M.S.; Thabet, Y.; Ng, C.L.W.; Glitz, E.S.; Nguyen, G.T.M.; Plesse, C.; et al. Piezoionic mechanoreceptors: Force-induced current generation in hydrogels. Science 2022, 376, 502–507. [Google Scholar] [CrossRef]
- Zou, H.; Guo, L.; Xue, H.; Zhang, Y.; Shen, X.; Liu, X.; Wang, P.; He, X.; Dai, G.; Jiang, P.; et al. Quantifying and understanding the triboelectric series of inorganic non-metallic materials. Nat. Commun. 2020, 11, 2093. [Google Scholar] [CrossRef]
- Sun, S.; Li, M.; Shi, X.; Chen, Z. Advances in Ionic Thermoelectrics: From Materials to Devices. Adv. Energy Mater. 2023, 13, 2203692. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Comparison of Energy Efficiency and Power Density in Pressure Retarded Osmosis and Reverse Electrodialysis. Environ. Sci. Technol. 2014, 48, 11002–11012. [Google Scholar] [CrossRef]
- Yan, H.; Liu, Z.; Qi, R. A review of humidity gradient-based power generator: Devices, materials and mechanisms. Nano Energy 2022, 101, 107591. [Google Scholar] [CrossRef]
- Sapkal, S.; Kandasubramanian, B.; Panda, H.S. A review of piezoelectric materials for nanogenerator applications. J. Mater. Sci. Mater. Electron. 2022, 33, 26633–26677. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Guo, L.; Wang, P.; He, X.; Dai, G.; Zheng, H.; Chen, C.; Wang, A.C.; Xu, C.; et al. Quantifying the triboelectric series. Nat. Commun. 2019, 10, 1427. [Google Scholar] [CrossRef]
- Zhang, R.; Olin, H. Material choices for triboelectric nanogenerators: A critical review. EcoMat 2020, 2, e12062. [Google Scholar] [CrossRef]
- Gao, J.; Miao, L.; Zhang, B.; Chen, Y. Advances in Flexible Thermoelectric Materials and Devices. J. Funct. Polym. 2017, 30, 142–167. [Google Scholar]
- Chen, G.; Dresselhaus, M.S.; Dresselhaus, G.; Fleurial, J.-P.; Caillat, T. Recent developments in thermoelectric materials. Int. Mater. Rev. 2003, 48, 45–66. [Google Scholar] [CrossRef]
- Horike, S.; Wei, Q.; Kirihara, K.; Mukaida, M.; Sasaki, T.; Koshiba, Y.; Fukushima, T.; Ishida, K. Outstanding Electrode-Dependent Seebeck Coefficients in Ionic Hydrogels for Thermally Chargeable Supercapacitor near Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 43674–43683. [Google Scholar] [CrossRef]
- Wang, H.; Xie, W.; Yu, B.; Qi, B.; Liu, R.; Zhuang, X.; Liu, S.; Liu, P.; Duan, J.; Zhou, J. Simultaneous Solar Steam and Electricity Generation from Synergistic Salinity—Temperature Gradient. Adv. Energy Mater. 2021, 11, 2100481. [Google Scholar] [CrossRef]
- Majumdar, A. Microscale transport phenomena. Handb. Heat Transf. 1998, 8, 1–88. [Google Scholar]
- Jin, D.; Xi, R.; Xu, S.; Wang, P.; Wu, X. Numerical simulation of salinity gradient power generation using reverse electrodialysis. Desalination 2021, 512, 115132. [Google Scholar] [CrossRef]
- Komazaki, Y.; Kanazawa, K.; Nobeshima, T.; Hirama, H.; Watanabe, Y.; Suemori, K.; Uemura, S. Energy harvesting by ambient humidity variation with continuous milliampere current output and energy storage. Sustain. Energy Fuels 2021, 5, 3570–3577. [Google Scholar] [CrossRef]
- Komazaki, Y.; Kanazawa, K.; Nobeshima, T.; Hirama, H.; Watanabe, Y.; Suemori, K.; Uemura, S. Mathematical Modeling of a Hygroelectric Cell Based on Deliquescent Electrolyte Solution Partitioned by Cation-exchange Membrane. Chem. Lett. 2022, 51, 561–565. [Google Scholar] [CrossRef]
- Chae, S.; Kim, H.; Hong, G.J.; Jang, J.; Higa, M.; Pishnamazi, M.; Choi, J.-Y.; Chandula Walgama, R.; Bae, C.; Kim, I.S.; et al. Clean power generation from salinity gradient using reverse electrodialysis technologies: Recent advances, bottlenecks, and future direction. Chem. Eng. J. 2023, 452, 139482. [Google Scholar] [CrossRef]
- Zeng, Z.; Yeh, L.-H.; Zhang, M.; Qian, S. Ion transport and selectivity in biomimetic nanopores with pH-tunable zwitterionic polyelectrolyte brushes. Nanoscale 2015, 7, 17020–17029. [Google Scholar] [CrossRef]
- Graf, M.; Lihter, M.; Unuchek, D.; Sarathy, A.; Leburton, J.-P.; Kis, A.; Radenovic, A. Light-Enhanced Blue Energy Generation Using MoS2 Nanopores. Joule 2019, 3, 1549–1564. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Xue, G.; Ding, T.; Yang, P.; Chen, Q.; Shen, Y.; Li, S.; Feng, G.; Shen, A.; et al. Electricity generation from water droplets via capillary infiltrating. Nano Energy 2018, 48, 211–216. [Google Scholar] [CrossRef]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef]
- Xia, M.; Pan, N.; Zhang, C.; Zhang, C.; Fan, W.; Xia, Y.; Wang, Z.; Sui, K. Self-Powered Multifunction Ionic Skins Based on Gradient Polyelectrolyte Hydrogels. ACS Nano 2022, 16, 4714–4725. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Q.; Benetti, D.; Jin, L.; Ni, Y.; Rosei, F. Biomimetic polyelectrolyte-gradient hydrogel electricity generator: A green and portable energy source. J. Mater. Chem. A 2023, 11, 19506–19513. [Google Scholar] [CrossRef]
- Xu, T.; Ding, X.; Huang, Y.; Shao, C.; Song, L.; Gao, X.; Zhang, Z.; Qu, L. An efficient polymer moist-electric generator. Energy Environ. Sci. 2019, 12, 972–978. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Q. Novel Ferroelectric Polymers for High Energy Density and Low Loss Dielectrics. Macromolecules 2012, 45, 2937–2954. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, S.; Zhai, B.; Lu, X.; Sun, B.; Ding, T. Plasmon-Assisted Nanopoling of Poly(Vinyl Difluoride) Films. Adv. Opt. Mater. 2021, 9, 2100084. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Zhang, F.; Zhang, M.; Li, D.; Deng, G.; Guan, L.; Dong, M. Studies on the electrostatic effects of stretched PVDF films and nanofibers. Nanoscale Res. Lett. 2021, 16, 79. [Google Scholar] [CrossRef]
- Yi, J.; Ye, Z.; Zhang, S.; Song, Y.; Cao, Z.; Liu, B.; Li, C.; Liu, S.; Nie, S.; Xiong, C. Corona: An effective polarization strategy of polymer composites with high-k filler for piezoelectric nanogenerators. Appl. Energy 2024, 353, 122005. [Google Scholar] [CrossRef]
- Liu, Y.; Aziguli, H.; Zhang, B.; Xu, W.; Lu, W.; Bernholc, J.; Wang, Q. Ferroelectric polymers exhibiting behaviour reminiscent of a morphotropic phase boundary. Nature 2018, 562, 96–100. [Google Scholar] [CrossRef]
- Kawai, H. The Piezoelectricity of Poly (vinylidene Fluoride). Jpn. J. Appl. Phys. 1969, 8, 975–976. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. PEDOT:PSS films with significantly enhanced conductivities induced by preferential solvation with cosolvents and their application in polymer photovoltaic cells. J. Mater. Chem. 2011, 21, 4927. [Google Scholar] [CrossRef]
- Zhu, Q.; Yildirim, E.; Wang, X.; Soo, X.Y.D.; Zheng, Y.; Tan, T.L.; Wu, G.; Yang, S.W.; Xu, J. Improved Alignment of PEDOT:PSS Induced by in-situ Crystallization of “Green” Dimethylsulfone Molecules to Enhance the Polymer Thermoelectric Performance. Front. Chem. 2019, 7, 783. [Google Scholar] [CrossRef]
- Yemata, T.A.; Kyaw, A.K.K.; Zheng, Y.; Wang, X.; Zhu, Q.; Chin, W.S.; Xu, J. Enhanced Thermoelectric Performance of PEDOT:PSS with Long-term Humidity Stability via Sequential Treatment with Trifluoroacetic Acid. Polym. Int. 2020, 69, 84–92. [Google Scholar] [CrossRef]
- Yu, Z.D.; Lu, Y.; Wang, Z.Y.; Un, H.I.; Zelewski, S.J.; Cui, Y.; You, H.Y.; Liu, Y.; Xie, K.F.; Yao, Z.F.; et al. High n-type and p-type conductivities and power factors achieved in a single conjugated polymer. Sci. Adv. 2023, 9, eadf3495. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, M.; Dan, Z.; Lan, S.; Ren, W.; Zhou, L.; Wang, Y.; Liang, Y.; Zheng, Y.; Pan, J.; et al. Large-area atomic-smooth polyvinylidene fluoride Langmuir-Blodgett film exhibiting significantly improved ferroelectric and piezoelectric responses. Sci. Bull. 2021, 66, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Yuan, M.; Ma, R.; Ye, Q.; Bai, X.; Li, H.; Yan, F.; Liu, C.; Ren, Y.; Wang, Z. Melt-stretched poly(vinylidene fluoride)/zinc oxide nanocomposite films with enhanced piezoelectricity by stress concentrations in piezoelectric domains for wearable electronics. Chem. Eng. J. 2023, 455, 140771. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Jin, Q.; Hu, Z.; Zhou, Z.; Zhu, M. Self-poled PVDF/recycled cellulose composite fibers utilizing cellulose nanocrystals to induce PVDF β-phase formation through wet-spinning as a flexible fabric piezoelectric sensor. Chem. Eng. J. 2024, 479, 147742. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Liu, J.; Liu, X.; Zhang, C. Electrospinning of Highly Bi-Oriented Flexible Piezoelectric Nanofibers for Anisotropic-Responsive Intelligent Sensing. Small Methods 2023, 7, 2300701. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Shi, K.; Wang, Y.; Liu, Y.; Liu, F.; Jiang, P.; Sheng, G.; Wang, S.; Xu, P.; Xu, X.; et al. Modulus-Modulated All-Organic Core–Shell Nanofiber with Remarkable Piezoelectricity for Energy Harvesting and Condition Monitoring. Nano Lett. 2023, 23, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, X.; Song, Z.; Liu, J.; Liu, X.; Zhang, C. Simulation Guided Coaxial Electrospinning of Polyvinylidene Fluoride Hollow Fibers with Tailored Piezoelectric Performance. Small 2023, 19, 2303285. [Google Scholar] [CrossRef]
- Soulestin, T.; Ladmiral, V.; Dos Santos, F.D.; Améduri, B. Vinylidene fluoride- and trifluoroethylene-containing fluorinated electroactive copolymers. How does chemistry impact properties? Prog. Polym. Sci. 2017, 72, 16–60. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Qian, X.; Zhu, W.; Li, B.; Zhang, B.; Lu, W.; Li, R.; Zhang, S.; Zhu, L.; et al. Relaxor ferroelectric polymer exhibits ultrahigh electromechanical coupling at low electric field. Science 2022, 375, 1418–1422. [Google Scholar] [CrossRef]
- Wang, Z.X.; Liao, W.Q. Giant electromechanical effects in polymers. Science 2022, 375, 1353–1354. [Google Scholar] [CrossRef]
- Park, K.I.; Lee, M.; Liu, Y.; Moon, S.; Hwang, G.T.; Zhu, G.; Kim, J.E.; Kim, S.O.; Kim, D.K.; Wang, Z.L.; et al. Flexible Nanocomposite Generator Made of BaTiO3 Nanoparticles and Graphitic Carbons. Adv. Mater. 2012, 24, 2999–3004. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, S.H.; Seo, H.J.; Jeong, S.M.; Lim, S.K. Effects of biomimetic cross-sectional morphology on the piezoelectric properties of BaTiO3 nanorods-contained PVDF fibers. Nano Energy 2022, 97, 107216. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, S.; Zhang, M.; Xiong, J.; Gu, H.; Wang, Z.; Hu, Y.; Zhang, X.; Du, Y.; Ren, L. Giant Piezoelectric Output and Stability Enhancement in Piezopolymer Composites with Liquid Metal Nanofillers. Adv. Sci. 2023, 10, e2304096. [Google Scholar] [CrossRef]
- Li, H.; Lim, S. High-performance piezoelectric nanogenerators featuring embedded organic nanodroplets for self-powered sensors. J. Mater. Chem. A 2022, 10, 14894–14905. [Google Scholar] [CrossRef]
- Li, H.; Lee, H.B.; Kang, J.W.; Lim, S. Three-dimensional polymer-nanoparticle-liquid ternary composite for ultrahigh augmentation of piezoelectric nanogenerators. Nano Energy 2023, 113, 108576. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Niu, S.; Lin, L.; Wang, Z.L. Freestanding Triboelectric-Layer-Based Nanogenerators for Harvesting Energy from a Moving Object or Human Motion in Contact and Non-contact Modes. Adv. Mater. 2014, 26, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Liu, L.; Zhou, L.; Liu, D.; Li, S.; Chen, S.; Dai, Y.; Wang, J.; Wang, Z.L. Improved Output Performance of Triboelectric Nanogenerator by Fast Accumulation Process of Surface Charges. Adv. Energy Mater. 2021, 11, 2100050. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, G.; Yuan, M.; Zhong, S.; Wang, Y.; Liu, X.; Cao, D.; Peng, W.; Liu, J.; Wang, G.; et al. Core–Shell Engineering of Conductive Fillers toward Enhanced Dielectric Properties: A Universal Polarization Mechanism in Polymer Conductor Composites. Adv. Mater. 2023, 35, 2207829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, L.; Zhang, C.; Feng, Y.; Wang, J.; Shen, Z.; Chen, Q.; Lei, Q.; Chi, Q. Polymer dielectric films exhibiting superior high-temperature capacitive performance by utilizing an inorganic insulation interlayer. Mater. Horiz. 2022, 9, 1273–1282. [Google Scholar] [CrossRef]
- An, L.; Boggs, S.A.; Calame, J.P. Energy Storage in Polymer Films with High Dielectric Constant Fillers. In Proceedings of the 2008 IEEE International Power Modulators and High-Voltage Conference 2008, Las Vegas, NV, USA, 27–31 May 2008; pp. 552–555. [Google Scholar]
- Huang, X.; Sun, B.; Zhu, Y.; Li, S.; Jiang, P. High-k Polymer Nanocomposites with 1D Filler for Dielectric and Energy Storage Applications. Prog. Mater. Sci. 2019, 100, 187–225. [Google Scholar] [CrossRef]
- Fan, Y.; Li, S.; Tao, X.; Wang, Y.; Liu, Z.; Chen, H.; Wu, Z.; Zhang, J.; Ren, F.; Chen, X.; et al. Negative triboelectric polymers with ultrahigh charge density induced by ion implantation. Nano Energy 2021, 90, 106574. [Google Scholar] [CrossRef]
- Xu, S.; Shi, X.-L.; Dargusch, M.; Di, C.; Zou, J.; Chen, Z.G. Conducting polymer-based flexible thermoelectric materials and devices: From mechanisms to applications. Prog. Mater. Sci. 2021, 121, 100840. [Google Scholar] [CrossRef]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT:PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Bounioux, C.; Díaz-Chao, P.; Campoy-Quiles, M.; Campoy-Quiles, M.; Martín-González, M.S.; Goñi, A.R.; Yerushalmi-Rozen, R.; Müller, C. Thermoelectric composites of poly(3-hexylthiophene) and carbon nanotubes with a large power factor. Energy Environ. Sci. 2013, 6, 918. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Z.; Wang, S.; Gao, C.; Wang, L. High-Performance thermoelectric generator based on n-Type flexible composite and its application in Self-Powered temperature sensor. Chem. Eng. J. 2024, 479, 147569. [Google Scholar] [CrossRef]
- Prunet, G.; Pawula, F.; Fleury, G.; Cloutet, E.; Robinson, A.J.; Hadziioannou, G.; Pakdel, A. A review on conductive polymers and their hybrids for flexible and wearable thermoelectric applications. Mater. Today Phys. 2021, 18, 100402. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Sarwar, M.S.U.; Dobashi, Y.; Glitz, E.F.S.; Farajollahi, M.; Mirabbasi, S.; Nafici, S.; Spinks, G.M.; Madden, J.D.W. Transparent and conformal “piezoionic” touch sensor. Electroact. Polym. Actuators Devices (EAPAD) 2015, 9430, 943026. [Google Scholar]
- Woehling, V.; Nguyen, G.T.M.; Plesse, C.; Petel, Y.; Dobashi, Y.; Madden, J.D.W.; Michal, C.A.; Vidal, F. Study of the piezoionic effect and influence of electrolyte in conducting polymer based soft strain sensors. Multifunct. Mater. 2019, 2, 45002. [Google Scholar] [CrossRef]
- Vinikoor, T.; Dzidotor, G.K.; Le, T.T.; Liu, Y.; Kan, H.M.; Barui, S.; Chorsi, M.T.; Curry, E.J.; Reinhardt, E.; Wang, H.; et al. Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 6257. [Google Scholar] [CrossRef]
- Xu, C.; Sun, Y.; Zhang, J.; Xu, W.; Tian, H. Adaptable and Wearable Thermocell Based on Stretchable Hydrogel for Body Heat Harvesting. Adv. Energy Mater. 2022, 12, 2201542. [Google Scholar] [CrossRef]
- Sun, Z.; Feng, L.; Xiong, C.; He, X.; Wang, L.; Qin, X.; Yu, J. Electrospun nanofiber fabric: An efficient, breathable and wearable moist-electric generator. J. Mater. Chem. A 2021, 9, 7085–7093. [Google Scholar] [CrossRef]
- Yang, M.; Hu, Y.; Zheng, S.; Liu, Z.; Li, W.; Yan, F. Integrated Moist-Thermoelectric Generator for Efficient Waste Steam Energy Utilization. Adv. Sci. 2023, 10, e2206071. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, W.; Guo, J.; Song, J.; Deng, X. Is Heat Really Beneficial to Water Evaporation-Driven Electricity? J. Phys. Chem. Lett. 2021, 12, 12370–12375. [Google Scholar] [CrossRef]
- Chae, I.; Jeong, C.K.; Ounaies, Z.; Kim, S.H. A Review on Electromechanical Coupling Properties of Biomaterials. ACS Appl. Bio Mater. 2018, 1, 936–953. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Liu, K.; Nie, S.; Xu, T.; Zhang, X.; Si, C. Fabrication and applications of cellulose-based nanogenerators. Adv. Compos. Hybrid Mater. 2021, 4, 865–884. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Z.; Hu, G.; Xiong, C.; Isogai, A.; Yang, Q. Recent Advances of Cellulose-based Piezoelectric and Triboelectric Nanogenerators for Energy Harvesting: A Review. J. Mater. Chem. A 2021, 9, 1910–1937. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Ribera, J.; Wu, C.; Tu, K.; Binelli, M.; Panzarasa, G.; Schwarze, F.W.M.R.; Wang, Z.L.; Burgert, I. Sustainable and Biodegradable Wood Sponge Piezoelectric Nanogenerator for Sensing and Energy Harvesting Applications. ACS Nano 2020, 14, 14665–14674. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, Y.; Liu, T.; Mo, J.; Zhang, W.; Zhang, S.; Luo, B.; Wang, J.; Qin, Y.; Wang, S.; et al. Air-permeable cellulosic triboelectric materials for self-powered healthcare products. Nano Energy 2022, 102, 107739. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, Y.; Hu, L.; Guan, P.; Su, D.; Zhang, S.; Liu, C.; Feng, Z.; Hu, G.; Chen, F.; et al. Lab free protein-based moisture electric generators with a high electric output. Energy Environ. Sci. 2023, 16, 2338–2345. [Google Scholar] [CrossRef]
- Lin, Z.; Meng, Z.; Miao, H.; Wu, R.; Qiu, W.; Lin, N.; Liu, X.Y. Biomimetic Salinity Power Generation Based on Silk Fibroin Ion-Exchange Membranes. ACS Nano 2021, 15, 5649–5660. [Google Scholar] [CrossRef]
- Liu, X.; Gao, H.; Ward, J.E.; Liu, X.; Yin, B.; Fu, T.; Chen, J.; Lovley, D.R.; Yao, J. Power generation from ambient humidity using protein nanowires. Nature 2020, 578, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, L.; Zhu, C.; Qian, Y.; Wen, L.; Jiang, L. Improved osmotic energy conversion in heterogeneous membrane boosted by three-dimensional hydrogel interface. Nat. Commun. 2020, 11, 875. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 16631–16634. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, L.; Bai, S.; Yao, Q.; Wang, Q. Thermoelectric properties of p-type (Bi2Te3)x(Sb2Te3)1−x crystals prepared via zone melting. J. Cryst. Growth 2005, 277, 258–263. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Surjadi, J.U.; Li, X.; Fan, R.; Theja, V.C.S.; Li, W.J.; Lu, Y.; Roy, V.A.L. Three dimensional architected thermoelectric devices with high toughness and power conversion efficiency. Nat. Commun. 2023, 14, 2069. [Google Scholar] [CrossRef]
- Chen, G. Thermal conductivity and ballistic-phonon transport in the cross-plane direction of superlattices. Phys. Rev. B 1998, 57, 14958–14973. [Google Scholar] [CrossRef]
- Majumdar, A. Thermoelectricity in Semiconductor Nanostructures. Science 2004, 303, 777–778. [Google Scholar] [CrossRef]
- Zebarjadi, M.; Esfarjani, K.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Perspectives on thermoelectrics: From fundamentals to device applications. Energy Environ. Sci. 2012, 5, 5147–5162. [Google Scholar] [CrossRef]
- Balandin, A.; Wang, K.L. Effect of phonon confinement on the thermoelectric figure of merit of quantum wells. J. Appl. Phys. 1998, 84, 6149–6153. [Google Scholar] [CrossRef]
- Lu, C.; Yu, X.; Chen, Y.; Chen, X.; Zhang, X. Giant piezoionic effect of ultrathin MXene nanosheets toward highly-sensitive sleep apnea diagnosis. Chem. Eng. J. 2023, 463, 142523. [Google Scholar] [CrossRef]
- Li, F.; Cai, X.; Liu, G.; Xu, H.; Chen, W. Piezoionic SnSe Nanosheets-Double Network Hydrogel for Self-Powered Strain Sensing and Energy Harvesting. Adv. Funct. Mater. 2023, 33, 2300701. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, S.; Wang, Y.; Liu, A.; Wu, H.; Huang, L.; Chen, L.; Ni, Y.; Liu, K. Vascular bundle-inspired MXene ion-conducting microchannels enabled tough ionic hydrogels with high-sensitivity sensing and high-efficiency mechanical-electric conversion. Nano Energy 2023, 113, 108540. [Google Scholar] [CrossRef]
- Zhang, H.; He, N.; Wang, B.; Ding, B.; Jiang, B.; Tang, D.; Li, L. High-Performance, Highly Stretchable, Flexible Moist-Electric Generators via Molecular Engineering of Hydrogels. Adv. Mater. 2023, 35, 2300398. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Lv, Y.; Cui, Z.; Wang, L. Nanomaterials-Based Nanochannel Membrane for Osmotic Energy Harvesting. Adv. Funct. Mater. 2023, 34, 2308176. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, M.; Wang, L.; Zhang, X.; Liu, L. Anti-bacterial, anti-freezing starch/ionic liquid/PVA ion-conductive hydrogel with high performance for multi-stimulation sensitive responsive sensors. Chem. Eng. J. 2023, 477, 147065. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, J.; Liu, Y.; Bai, P.; Zhou, Y.S.; Jing, Q.; Pan, C.; Wang, Z.L. Linear-Grating Triboelectric Generator Based on Sliding Electrification. Nano Lett. 2013, 13, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.B.; Bai, G.; Wong, M.C.; Yang, Z.; Xu, W.; Hao, J. Magnetic-Assisted Noncontact Triboelectric Nanogenerator Converting Mechanical Energy into Electricity and Light Emissions. Adv. Mater. 2016, 28, 2744–2751. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, B.; Suo, Z.; Hayward, R.C. Ionoelastomer junctions between polymer networks of fixed anions and cations. Science 2020, 367, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids—An Overview. Aust. J. Chem. 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Zaidi, S.S.H.; Li, X. Li–O2/Air Batteries Using Ionic Liquids—A Comprehensive Review. Adv. Energy Mater. 2023, 13, 2300985. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Gu, K.; Yao, J.; Saho, Z.; Chen, X. Poly(vinyl alcohol) Hydrogels with Integrated Toughness, Conductivity, and Freezing Tolerance Based on Ionic Liquid/Water Binary Solvent Systems. ACS Appl. Mater. Interfaces 2021, 13, 29008–29020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Cho, C.; Oh, J.H. Highly flexible triboelectric nanogenerators with electrospun PVDF-TrFE nanofibers on MWCNTs/PDMS/AgNWs composite electrodes. Compos. Part B Eng. 2023, 255, 110622. [Google Scholar] [CrossRef]
- Sohn, A.; Lee, J.H.; Yoon, H.J.; Lee, H.H.; Kim, S.W. Self-boosted power generation of triboelectric nanogenerator with glass transition by friction heat. Nano Energy 2020, 74, 104840. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, H.; Yang, C.; Yao, H.; Li, C.; Qu, L. All-region-applicable, continuous power supply of graphene oxide composite. Energy Environ. Sci. 2019, 12, 1848–1856. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; He, T.; Huang, Y.; Cheng, H.; Li, C.; Xie, D.; Yang, P.; Zhang, Y.; Qu, L. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1000 V output. Nat. Nanotechnol. 2021, 16, 811–819. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Han, Y.; Wang, J.; Wang, Y.; Li, D.; Lv, J.; Wu, W.; Wang, Z.; Wei, X.; et al. Long Cycle Life Rechargeable Moisture-Enabled Electricity Cell. Adv. Energy Mater. 2023, 2303815. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, L.; Wang, Y.C.; Li, J.; Nie, J.; Wang, Z.L. A Flexible Multifunctional Triboelectric Nanogenerator Based on MXene/PVA Hydrogel. Adv. Funct. Mater. 2021, 31, 2104928. [Google Scholar] [CrossRef]
- Xing, F.; Ou, Z.; Gao, X.; Chen, B.; Wang, Z.L. Harvesting Electrical Energy from High Temperature Environment by Aerogel Nano-Covered Triboelectric Yarns. Adv. Funct. Mater. 2022, 32, 2205275. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Kong, X.Y.; Xin, W.; Zhou, T.; Qian, Y.; Yang, L.; Pang, J.; Jiang, L.; Wen, L. Robust sulfonated poly (ether ether ketone) nanochannels for high-performance osmotic energy conversion. Natl. Sci. Rev. 2020, 7, 1349–1359. [Google Scholar] [CrossRef]
- Bian, G.; Pan, N.; Luan, Z.; Sui, X.; Fan, W.; Xia, Y.; Sui, K.; Jiang, L. Anti-Swelling Gradient Polyelectrolyte Hydrogel Membranes as High-Performance Osmotic Energy Generators. Angew. Chem. Int. Ed. 2021, 60, 20294–20300. [Google Scholar] [CrossRef]
- Yang, S.; Tao, X.; Chen, W.; Mao, J.; Luo, H.; Lin, S.; Zhang, L.; Hao, J. Ionic Hydrogel for Efficient and Scalable Moisture-Electric Generation. Adv. Mater. 2022, 34, 2200693. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Yu, Z.G.; Qu, H.; Sun, W.; Yang, J.; Suresh, L.; Zhang, X.; Koh, J.J.; Tan, S.C. An Asymmetric Hygroscopic Structure for Moisture-Driven Hygro-Ionic Electricity Generation and Storage. Adv. Mater. 2022, 34, 2201228. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Hu, Y.; Guang, T.; Zhu, K.; Wang, H.; Cheng, H.; Liu, F.; Qu, L. Vapor and heat dual-drive sustainable power for portable electronics in ambient environments. Energy Environ. Sci. 2022, 15, 3086–3096. [Google Scholar] [CrossRef]



| Type | Polymer Medium for Power Generation | Materials of Electrodes | Voc/V | Isc/μA | Power Density (mW/cm2) | Refs |
|---|---|---|---|---|---|---|
| PENG | GaIn NDs/PVDF-TrFE | Al | 212 | 0.04 | 23.4 | [90] |
| BTO/PVDF/PFOES | Ni | 102 | 10 | 0.07 | [92] | |
| Trbo ENG | PVDF-TrFE | AgNMW/MWCNTs/PDMS; Al | 508 | 16.5 | 0.528 | [142] |
| Ecoflex, Kapton | MXene, PVA | 180 | 1.2 | 0.033 | [147] | |
| PTFE, PI | Carbon | 80 | 1.7 | 0.0068 | [148] | |
| Thermo ENG | PVA/PEDOT/PAMPS | Au | 25.0 mV/K | 9.93 mW/(m K2) | [23] | |
| Emim-Cl/PVA | Al | 10.1 mV/K | [54] | |||
| SWCNT/PEI-PFA /NaOH | Cu | 0.02656 mV/K | 0.11577 mW/(m K2) | [103] | ||
| OPNG | SPEEK | Ag/AgCl | 0.349 | 0.63 | 0.58 | [149] |
| Polyelectrolyte hydrogel | Ag/AgCl | 0.043 | 7.5 | 0.787 | [150] | |
| MENG | TiO2-Co hydrogel | Carbon | 0.95 | 100 | 0.00179 | [33] |
| PVA-PA | 0.8 | 60 | 0.035 | [151] | ||
| CB-LiCl/PVA | Au | 0.6 | 6 | 0.07 mW/cm3 | [152] | |
| Thermo ENG and MENG | P(AMPS-SSS0.5) | C | 1.81 and 126.2 mV/K | <0.5 | 0.00475 mW/cm3 and 15.6 mW/(m K2) | [111] |
| PSSA-PEDOT: PSS-K4Fe(CN)6/K3Fe(CN)6 | C | 0.94 and 30.0 mV/K | 800 | 7.2 and 0.05 mW/(m K2) | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Yan, H.; Qi, R. A Review of Polymer-Based Environment-Induced Nanogenerators: Power Generation Performance and Polymer Material Manipulations. Polymers 2024, 16, 555. https://doi.org/10.3390/polym16040555
Xie S, Yan H, Qi R. A Review of Polymer-Based Environment-Induced Nanogenerators: Power Generation Performance and Polymer Material Manipulations. Polymers. 2024; 16(4):555. https://doi.org/10.3390/polym16040555
Chicago/Turabian StyleXie, Shuanghong, Huping Yan, and Ronghui Qi. 2024. "A Review of Polymer-Based Environment-Induced Nanogenerators: Power Generation Performance and Polymer Material Manipulations" Polymers 16, no. 4: 555. https://doi.org/10.3390/polym16040555
APA StyleXie, S., Yan, H., & Qi, R. (2024). A Review of Polymer-Based Environment-Induced Nanogenerators: Power Generation Performance and Polymer Material Manipulations. Polymers, 16(4), 555. https://doi.org/10.3390/polym16040555







