Synthesis of Network Biobased Aliphatic Polyesters Exhibiting Better Tensile Properties than the Linear Polymers by ADMET Polymerization in the Presence of Glycerol Tris(undec-10-enoate)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
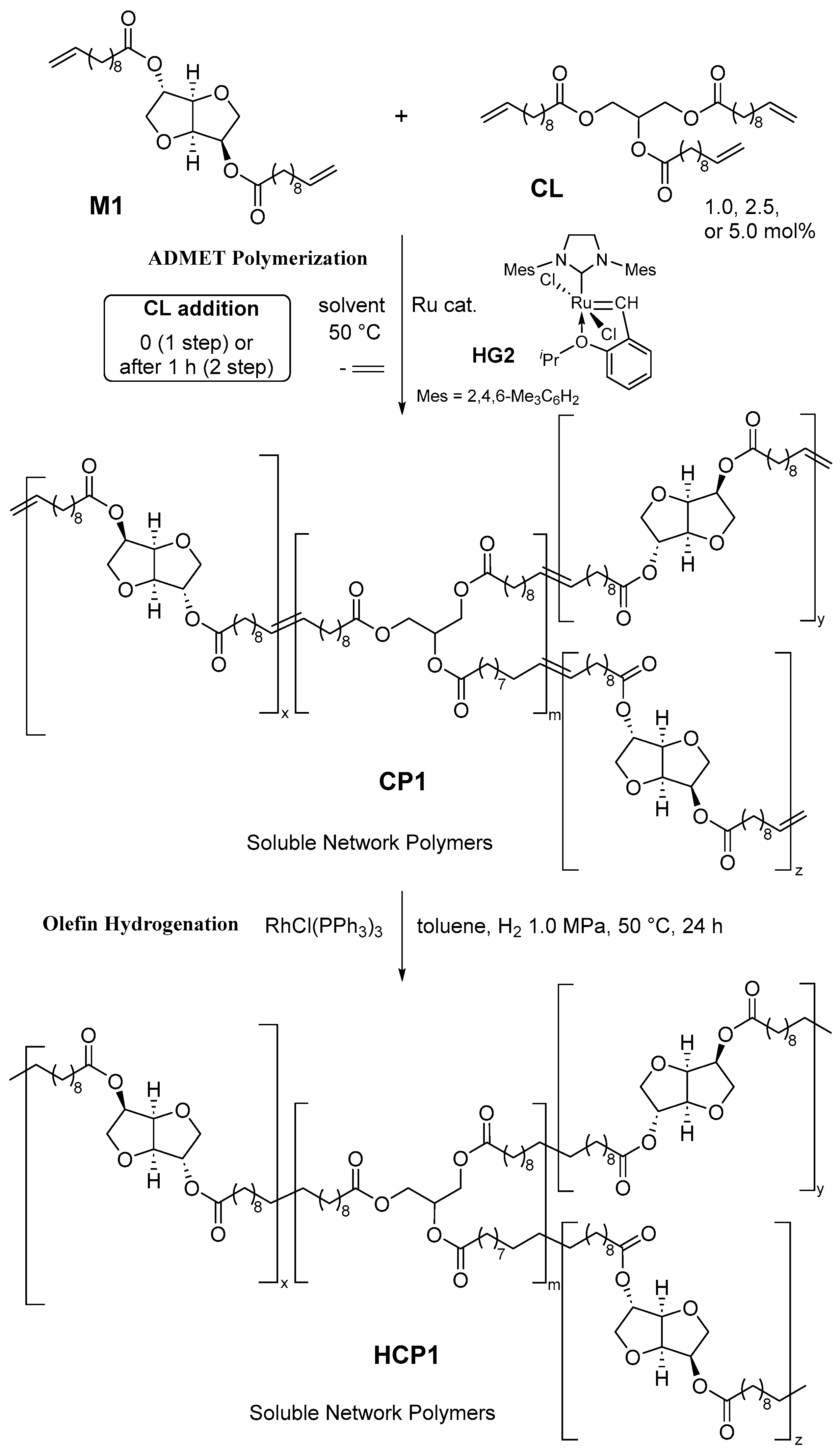
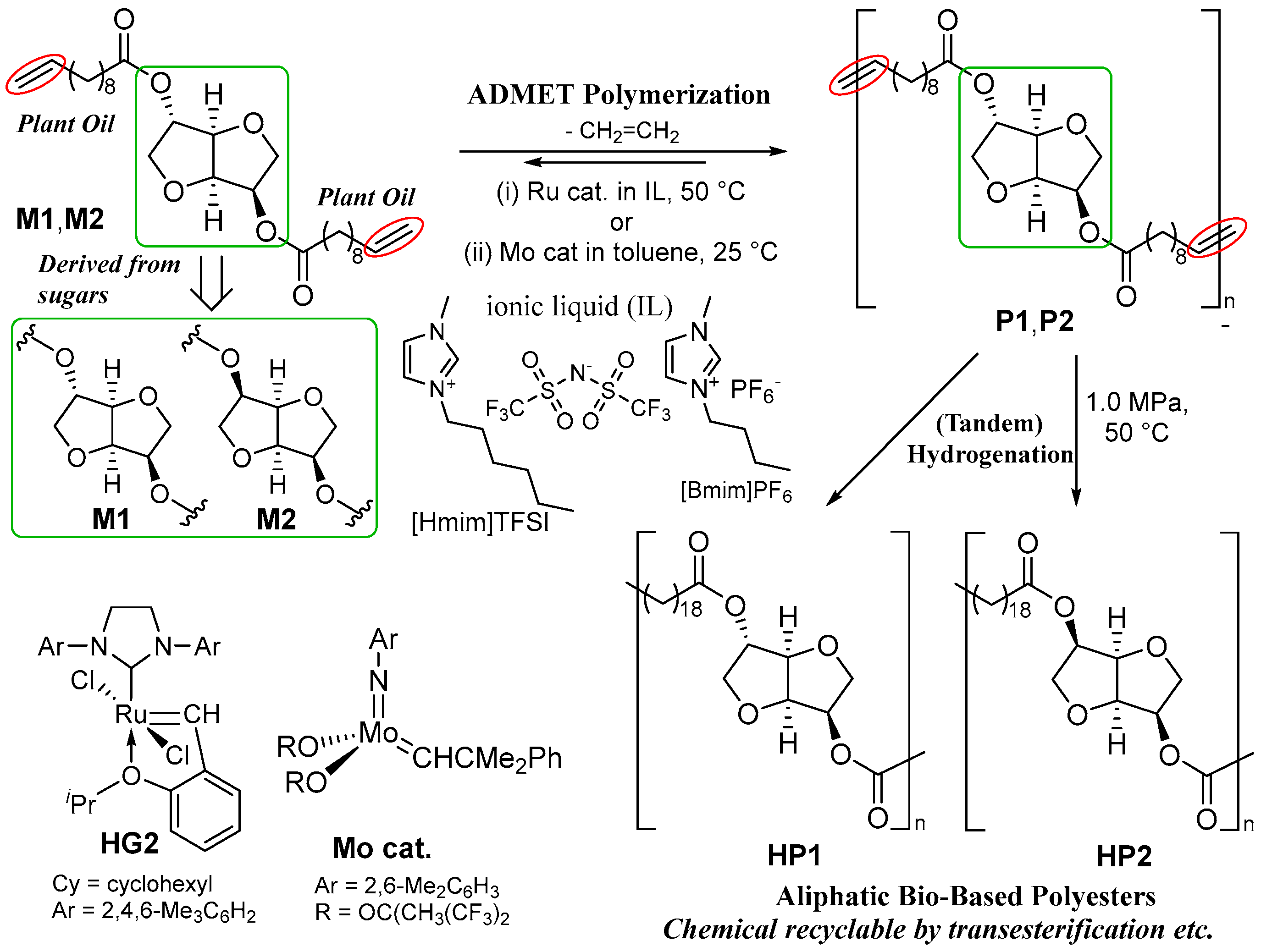
3.1. Synthesis of Network Polymers (CP1 and HCP1) by ADMET Polymerization and Subsequent Hydrogenation
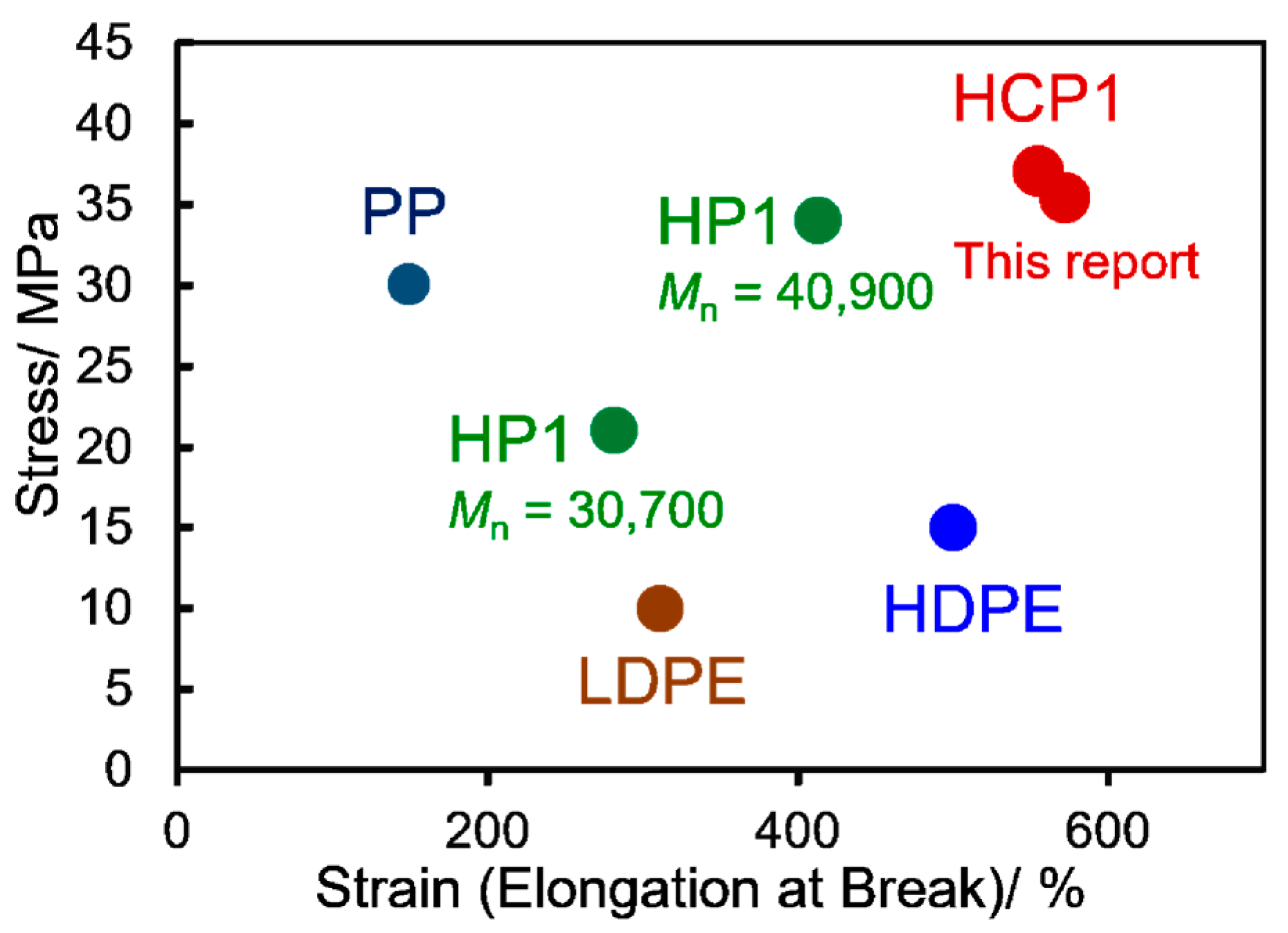
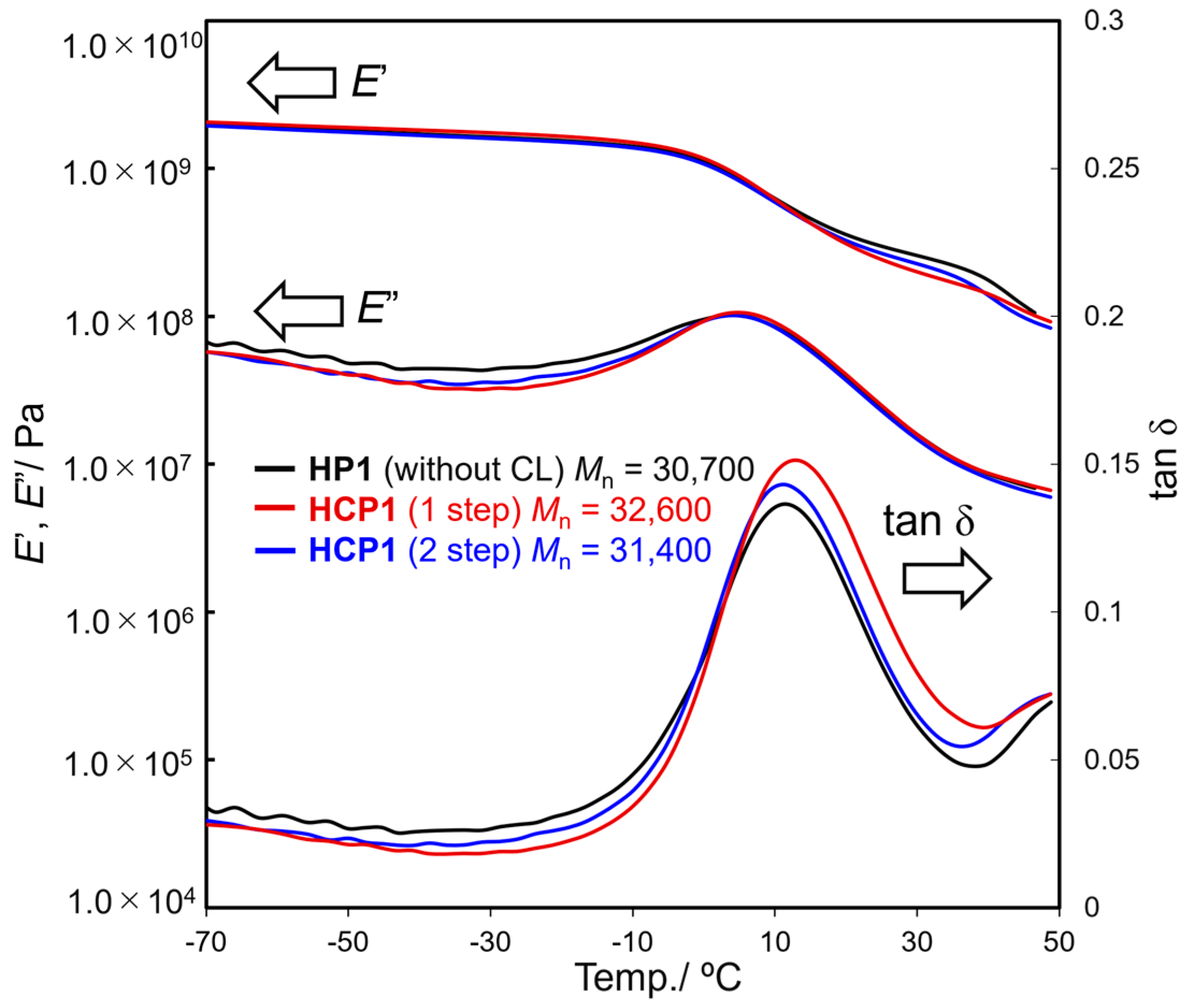
3.2. Tensile Properties in the Polymer Films (CP1s, HCP1s)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stempfle, F.; Ortmann, P.; Mecking, S. Long-chain aliphatic polymers to bridge the gap between semicrystalline polyolefins and traditional polycondensates. Chem. Rev. 2016, 116, 4597–4641. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic polyester block polymers: Renewable, degradable, and sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. Monomers and polymers from chemically modified plant oils and their fatty acids. In Polymers from Plant Oils, 2nd ed.; Gandini, A., Lacerda, T.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 33–82. [Google Scholar]
- Nomura, K.; Awang, N.W.B. Synthesis of bio-based aliphatic polyesters from plant oils by efficient molecular catalysis: A selected survey from recent reports. ACS Sustain. Chem. Eng. 2021, 9, 5486–5505. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.R.; Metzger, J.O. Fatty acids and their derivatives as renewable platform molecules for the chemical industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165. [Google Scholar] [CrossRef]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mat. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Häußler, M.; Eck, M.; Rothauer, D.; Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 2021, 590, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.C.; Dove, A.P. 100th Anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 2020, 9, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Aoki, T.; Ohki, Y.; Kikkawa, S.; Yamazoe, S. Transesterification of methyl-10-undecenoate and poly(ethylene adipate) catalyzed by (cyclopentadienyl)titanium trichlorides as model chemical conversions of plant oils and acid-, base-free chemical recycling of aliphatic polyesters. ACS Sustain. Chem. Eng. 2022, 10, 12504–12509. [Google Scholar] [CrossRef]
- Ohki, Y.; Ogiwara, Y.; Nomura, K. Depolymerization of polyesters by transesterification with ethanol using (cyclopentadienyl)titanium trichlorides. Catalysts 2023, 13, 421. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Nomura, K. Synthesis of high molecular weight biobased aliphatic polyesters by acyclic diene metathesis polymerization in ionic liquids. ACS Omega 2023, 8, 7222–7233. [Google Scholar] [CrossRef]
- Roesle, P.; Stempfle, F.; Hess, S.K.; Zimmerer, J.; Río Bartulos, C.; Lepetit, B.; Eckert, A.; Kroth, P.G.; Mecking, S. Synthetic polyester from Algae oil. Angew. Chem. Int. Ed. 2014, 53, 6800–6804. [Google Scholar] [CrossRef]
- Quinzler, D.; Mecking, S. Linear semicrystalline polyesters from fatty acids by complete feedstock molecule utilization. Angew. Chem. Int. Ed. 2010, 49, 4306–4308. [Google Scholar] [CrossRef] [PubMed]
- Stempfle, F.; Quinzler, D.; Heckler, I.; Mecking, S. Long-chain linear C19 and C23 monomers and polycondensates from unsaturated fatty acid esters. Macromolecules 2011, 44, 4159–4166. [Google Scholar] [CrossRef]
- Roumanet, P.-J.; Laflèche, F.; Jarroux, N.; Raoul, Y.; Claude, S.; Guégan, P. Novel aliphatic polyesters from an oleic acid based monomer. Synthesis, epoxidation, cross-linking and biodegradation. Eur. Polym. J. 2013, 49, 813–822. [Google Scholar] [CrossRef]
- Roumanet, P.-J.; Jarroux, N.; Goujard, L.; Le Petit, J.; Raoul, Y.; Bennevault, V.; Guégan, P. Synthesis of linear polyesters from monomers based on 1,18-(Z)-octadec-9-enedioic acid and their biodegradability. ACS Sustain. Chem. Eng. 2020, 8, 16853–16860. [Google Scholar] [CrossRef]
- Rybak, A.; Meier, M.A.R. Acyclic diene metathesis with a monomer from renewable resources: Control of molecular weight and one-step preparation of block copolymers. ChemSusChem 2008, 1, 542–547. [Google Scholar] [CrossRef]
- Fokou, P.A.; Meier, M.A.R. Use of a renewable and degradable monomer to study the temperature-dependent olefin isomerization during ADMET polymerizations. J. Am. Chem. Soc. 2009, 131, 1664–1665. [Google Scholar] [CrossRef] [PubMed]
- Trzaskowski, J.; Quinzler, D.; Bährle, C.; Mecking, S. Aliphatic long-chain C20 polyesters from olefin metathesis. Macromol. Rapid Commun. 2011, 32, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Silvestre, A.J.D.; Meier, M.A.R. Plant oil-based long-chain C26 monomers and their polymers. Macromol. Chem. Phys. 2012, 213, 2220–2227. [Google Scholar] [CrossRef]
- Stempfle, F.; Ortmann, P.; Mecking, S. Which polyesters can mimic polyethylene? Macromol. Rapid Commun. 2013, 34, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, P.; Mecking, S. Long-spaced aliphatic polyesters. Macromolecules 2013, 46, 7213–7218. [Google Scholar] [CrossRef]
- Lebarbé, T.; Neqal, M.; Grau, E.; Alfos, C.; Cramail, H. Branched polyethylene mimicry by metathesis copolymerization of fatty acid-based α,ω-dienes. Green Chem. 2014, 16, 1755–1758. [Google Scholar] [CrossRef]
- Shearouse, W.C.; Lillie, L.M.; Reineke, T.M.; Tolman, W.B. Sustainable Polyesters Derived from Glucose and Castor Oil: Building Block Structure Impacts Properties. ACS Macro Lett. 2015, 4, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Grau, E.; Carlotti, S.; Greliera, S.; Cramail, H. ADMET polymerization of bio-based biphenyl compounds. Polym. Chem. 2015, 6, 7693–7700. [Google Scholar] [CrossRef]
- Hibert, G.; Grau, E.; Pintori, D.; Lecommandoux, S.; Cramail, H. ADMET polymerization of α,ω-unsaturated glycolipids: Synthesis and physico-chemical properties of the resulting polymers. Polym. Chem. 2017, 8, 3731–3739. [Google Scholar] [CrossRef]
- Lillie, L.M.W.; Tolman, B.T.; Reineke, M. Structure/property relationships in copolymers comprising renewable isosorbide, glucarodilactone, and 2,5-bis(hydroxymethyl)-furan subunits. Polym. Chem. 2017, 8, 3746–3754. [Google Scholar] [CrossRef]
- Le, D.; Samart, C.; Kongparakul, S.; Nomura, K. Synthesis of new polyesters by acyclic diene metathesis polymerization of bio-based α,ω-dienes prepared from eugenol and castor oil (undecenoate). RSC Adv. 2019, 9, 10245–10252. [Google Scholar] [CrossRef]
- Nomura, K.; Chaijaroen, P.; Abdellatif, M.M. Synthesis of biobased long-chain polyesters by acyclic diene metathesis polymerization and tandem hydrogenation and depolymerization with ethylene. ACS Omega 2020, 5, 18301–18312. [Google Scholar] [CrossRef]
- Piccini, M.; Leak, D.J.; Chuck, C.J.; Buchard, A. Polymers from sugars and unsaturated fatty acids: ADMET polymerisation of monomers derived from d-xylose, d-mannose and castor oil. Polym. Chem. 2020, 11, 2681–2691. [Google Scholar] [CrossRef]
- Piccini, M.; Lightfoot, J.; Castro, D.B.; Buchard, A. Xylose-Based Polyethers and Polyesters via ADMET Polymerization toward Polyethylene-like Materials. ACS Appl. Polym. Mater. 2021, 3, 5870–5881. [Google Scholar] [CrossRef]
- Kojima, M.; Abdellatif, M.M.; Nomura, K. Synthesis of semi-crystalline long chain aliphatic polyesters by ADMET copolymerization of dianhydro-D-glucityl bis(undec-10-enoate) with 1,9-decadiene and tandem hydrogenation. Catalysts 2021, 11, 1098. [Google Scholar] [CrossRef]
- Kojima, M.; Wang, X.; Go, L.O.P.; Makino, R.; Matsumoto, Y.; Shimoyama, D.; Abdellatif, M.M.; Kadota, J.; Higashi, S.; Hirano, H.; et al. Synthesis of high molecular weight biobased aliphatic polyesters exhibiting tensile properties beyond polyethylene. ACS Macro Lett. 2023, 12, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.D.; Wagener, K.B. ADMET Polymerization. In Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2015; Volume 3, pp. 315–355. [Google Scholar]
- da Silva, L.C.; Rojas, G.; Schulz, M.D.; Wagener, K.B. Acyclic diene metathesis polymerization: History, methods and applications. Prog. Polym. Sci. 2017, 69, 79–107. [Google Scholar] [CrossRef]
- Chen, Y.; Abdellatif, M.M.; Nomura, K. Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET). Tetrahedron 2018, 74, 619–692. [Google Scholar] [CrossRef]
- Pribyl, J.; Wagener, K.B.; Rojas, G. ADMET polymers: Synthesis, structure elucidation, and function. Mater. Chem. Front. 2021, 5, 14–43. [Google Scholar] [CrossRef]
- Go, L.O.P.; Nomura, K. Functional Bio-based Polymer Network Synthesis via ADMET Polymerization. In Proceedings of the 11th The International Symposium on Feedstock Recycling of Polymeric Materials, ISFR, Pattaya, Thailand, 29 November–2 December 2022. [Google Scholar]
- Abdellatif, M.M.; Nomura, K. Synthesis of polyesters containing long aliphatic methylene units by ADMET polymerization and synthesis of the ABA-triblock copolymers by one-pot end modification and the subsequent living ring-opening polymerization. ACS Omega 2024. accepted. [Google Scholar]
- Wang, X.; Chin, A.L.; Zhou, J.; Wang, H.; Tong, R. Resilient poly(α-hydroxy acids) with improved strength and ductility via scalable stereosequence-controlled polymerization. J. Am. Chem. Soc. 2021, 143, 16813–16823. [Google Scholar] [CrossRef]
- Nomura, K.; Hatanaka, Y.; Okumura, H.; Fujiki, M.; Hasegawa, K. Polymerization of 1,5-hexadiene by the nonbridged half-titanocene complex-MAO catalyst system: Remarkable difference in the selectivity of repeated 1,2-insertion. Macromolecules 2004, 37, 1693–1695. [Google Scholar] [CrossRef]


| Run | Solvent | Polymerization | CP1 | ||||
|---|---|---|---|---|---|---|---|
| (Times of Solvent Replacement) 2 | CL | Time (2) 4 | Mn 5 × 10−3 | Mw/Mn 5 | Yield 6 | ||
| /mol% | Time (1) 3/h | /h | /% | ||||
| 1 | chloroform (1) | -- | -- | 24 | 30.3 | 2.11 | 96 |
| 2 | chloroform (1) | 0.5 | 0 | 24 | 36.5 | 2.20 | 92 |
| 3 | chloroform (1) | 1.0 | 0 | 24 | 36.6 | 3.00 | 97 |
| 4 | chloroform (1) | 1.0 | 0 | 24 | 34.8 | 3.11 | 92 |
| 5 | chloroform (1) | 1.0 | 0 | 24 | 32.2 | 2.64 | 91 |
| 6 | toluene (1) | 1.0 | 0 | 24 | 19.3 | 2.04 | 89 |
| 7 | toluene (2) | 1.0 | 0 | 24 | 26.0 | 2.82 | 94 |
| 8 | tetrachloroethane (1) | 1.0 | 0 | 24 | 20.1 | 1.99 | 98 |
| 9 | chloroform (1) | 2.5 | 0 | 24 | 29.6 | 4.11 | 98 |
| 10 | chloroform (1) | 2.5 | 0 | 24 | 29.6 | 4.14 | 91 |
| 11 | toluene (1) | 2.5 | 0 | 24 | 23.0 | 3.66 | 87 |
| 12 | tetrachloroethane (1) | 2.5 | 0 | 24 | 23.1 | 2.59 | 96 |
| 13 | toluene (1) | 5.0 | 0 | 24 | 20.0 | 4.63 * | 89 |
| 14 | chloroform (1) | 5.0 | 0 | 6 | 19.7 | 8.63 * | 77 |
| 15 | chloroform (0) | 5.0 | 0 | 6 | 6.70 | 1.67 | 75 |
| 16 | chloroform (0) | 5.0 | 0 | 12 | 7.50 | 1.83 | 89 |
| 17 | chloroform (0) | 5.0 | 0 | 24 | 20.0 | 4.64 * | 84 |
| 18 | chloroform (1) | 1.0 | 1 | 24 | 26.8 | 2.53 | 94 |
| 19 | toluene (1) | 1.0 | 1 | 24 | 21.0 | 2.18 | 88 |
| 20 | tetrachloroethane (1) | 1.0 | 1 | 24 | 17.6 | 1.95 | 97 |
| 21 | chloroform (5) | 1.0 | 1 | 24 | 36.6 | 2.79 | 91 |
| 22 | chloroform (5) | 1.0 | 1 | 24 | 35.8 | 3.00 | 90 |
| 23 | chloroform (5) | 2.5 | 1 | 24 | 31.1 | 4.13 | 90 |
| 24 | chloroform (5) | 2.5 | 1 | 24 | 32.0 | 4.48 | 91 |
| 25 | chloroform (1) | 2.5 | 1 | 24 | 25.0 | 5.94 * | 99 |
| 26 | toluene (1) | 2.5 | 1 | 24 | 19.5 | 2.61 | 90 |
| 27 | chloroform (1) | 5.0 | 1 | 24 | 16.4 | 5.45 * | 89 |
| 28 | chloroform (5) | 1.0 | 3 | 24 | 31.2 | 2.54 | 89 |
| 29 | chloroform (1) | 1.0 | 3 | 24 | 26.7 | 3.04 | 94 |
| 30 | toluene (1) | 1.0 | 3 | 24 | 19.4 | 2.00 | 88 |
| 31 | chloroform (1) | 2.5 | 3 | 24 | 25.7 | 3.90 | 97 |
| 32 | toluene (1) | 2.5 | 3 | 21 | 15.1 | 2.60 | 92 |
| 33 | toluene (2) | 2.5 | 3 | 21 | 20.6 | 2.46 | 89 |
| Run | Before Hydrogenation | After Hydrogenation | ||||
|---|---|---|---|---|---|---|
| Sample No. 2 | Mn3 × 10−4 | Mw/Mn3 | Mn3 × 10−4 | Mw/Mn3 | Yield 4/% | |
| 34 | run 1 | 3.03 | 2.11 | 3.07 | 2.22 | 99 |
| 35 | run 4 | 3.48 | 3.11 | 3.48 | 3.10 | 98 |
| 36 | run 5 | 3.22 | 2.64 | 3.26 | 2.47 | >99 |
| 37 | run 10 | 2.96 | 4.11 | 2.83 | 4.05 | 98 |
| 38 | run 21 | 3.66 | 2.79 | 3.73 | 2.92 | 96 |
| 39 | run 22 | 3.58 | 3.00 | 3.53 | 2.98 | 97 |
| 40 | run 23 | 3.11 | 4.13 | 3.05 | 3.91 | >99 |
| 41 | run 24 | 3.20 | 4.48 | 3.22 | 4.39 | 95 |
| 42 | run 28 | 3.12 | 2.54 | 3.14 | 2.46 | >99 |
| Sample | Run No. 2 | CL /mol% | Method 3 | Mn4 | Mw/ Mn4 | Tensile Strength/MPa | Elongation at Break/% |
|---|---|---|---|---|---|---|---|
| HP1 | ref 5 | -- | -- | 40.9 | 2.41 | 33.7 (±2.2) | 413 (±13) |
| HP1 | 34 | -- | -- | 30.7 | 2.22 | 20.8 (±1.3) | 282 (±14) |
| HCP1 | 36 | 1.0 | 1 step | 32.6 | 2.47 | 34.7 (±0.6) | 537 (±7) |
| HCP1 | 42 | 1.0 | 2 step | 31.4 | 2.46 | 35.4 (±0.6) | 572 (±1) |
| HCP1 | 38 | 1.0 | 2 step | 37.3 | 2.92 | 36.9 (±3.8) | 555 (±21) |
| HCP1 | 40 | 2.5 | 2 step | 30.5 | 3.91 | 31.9 (±1.6) | 457 (±65) |
| P1 | 1 | -- | -- | 30.3 | 2.11 | 15.4 (±1.2) | 444 (±28) |
| P1 | ref 5 | -- | -- | 39.6 | 1.89 | 17.3 (±2.2) | 506 (±44) |
| CP1 | 4 | 1.0 | 1 step | 34.8 | 3.11 | 18.6 (±0.5) | 807 (±5) |
| CP1 | 21 | 1.0 | 2 step | 36.6 | 2.79 | 24.6 (±1.1) | 798 (±47) |
| CP1 | 22 | 1.0 | 2 step | 35.8 | 3.00 | 21.3 (±2.0) | 816 (±48) |
| CP1 | 23 | 2.5 | 2 step | 31.1 | 4.13 | 19.7 (±1.5) | 704 (±59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, L.O.P.; Abdellatif, M.M.; Makino, R.; Shimoyama, D.; Higashi, S.; Hirano, H.; Nomura, K. Synthesis of Network Biobased Aliphatic Polyesters Exhibiting Better Tensile Properties than the Linear Polymers by ADMET Polymerization in the Presence of Glycerol Tris(undec-10-enoate). Polymers 2024, 16, 468. https://doi.org/10.3390/polym16040468
Go LOP, Abdellatif MM, Makino R, Shimoyama D, Higashi S, Hirano H, Nomura K. Synthesis of Network Biobased Aliphatic Polyesters Exhibiting Better Tensile Properties than the Linear Polymers by ADMET Polymerization in the Presence of Glycerol Tris(undec-10-enoate). Polymers. 2024; 16(4):468. https://doi.org/10.3390/polym16040468
Chicago/Turabian StyleGo, Lance O’Hari P., Mohamed Mehawed Abdellatif, Ryoji Makino, Daisuke Shimoyama, Seiji Higashi, Hiroshi Hirano, and Kotohiro Nomura. 2024. "Synthesis of Network Biobased Aliphatic Polyesters Exhibiting Better Tensile Properties than the Linear Polymers by ADMET Polymerization in the Presence of Glycerol Tris(undec-10-enoate)" Polymers 16, no. 4: 468. https://doi.org/10.3390/polym16040468
APA StyleGo, L. O. P., Abdellatif, M. M., Makino, R., Shimoyama, D., Higashi, S., Hirano, H., & Nomura, K. (2024). Synthesis of Network Biobased Aliphatic Polyesters Exhibiting Better Tensile Properties than the Linear Polymers by ADMET Polymerization in the Presence of Glycerol Tris(undec-10-enoate). Polymers, 16(4), 468. https://doi.org/10.3390/polym16040468







