The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Preparation
2.3. Morphological Evaluation of Scaffold Variants
2.4. Pycnometry and Thermal Gravimetric Analysis for Porosity Measurement
2.5. hMSC Isolation and Culture
2.6. Phenotypic Characterization of Undifferentiated hMSCs
2.7. Porcine Articular Chondrocyte Isolation and Culture
2.8. Cytotoxicity Testing
2.9. Cell Seeding
2.9.1. Unilayer Scaffolds Seeded with hMSCs
2.9.2. Flow Seeding Procedure to Coculture hMSCs and pACs on Bilayer Scaffolds
2.10. Immunofluorescence Labeling
2.11. Live–Dead Assay
2.12. Histological Staining Procedures
2.13. Trilineage Differentiation of hMSCs Cultured on Coverslips and in Pellet Culture
2.14. Alizarin Red Staining
2.15. Oil Red Staining
2.16. Total DNA and GAG Content Measurement
2.17. RNA Isolation
2.18. Gene Expression Analysis of Bilayer Scaffolds Seeded with pACs and hMSCs
2.19. Statistics
3. Results
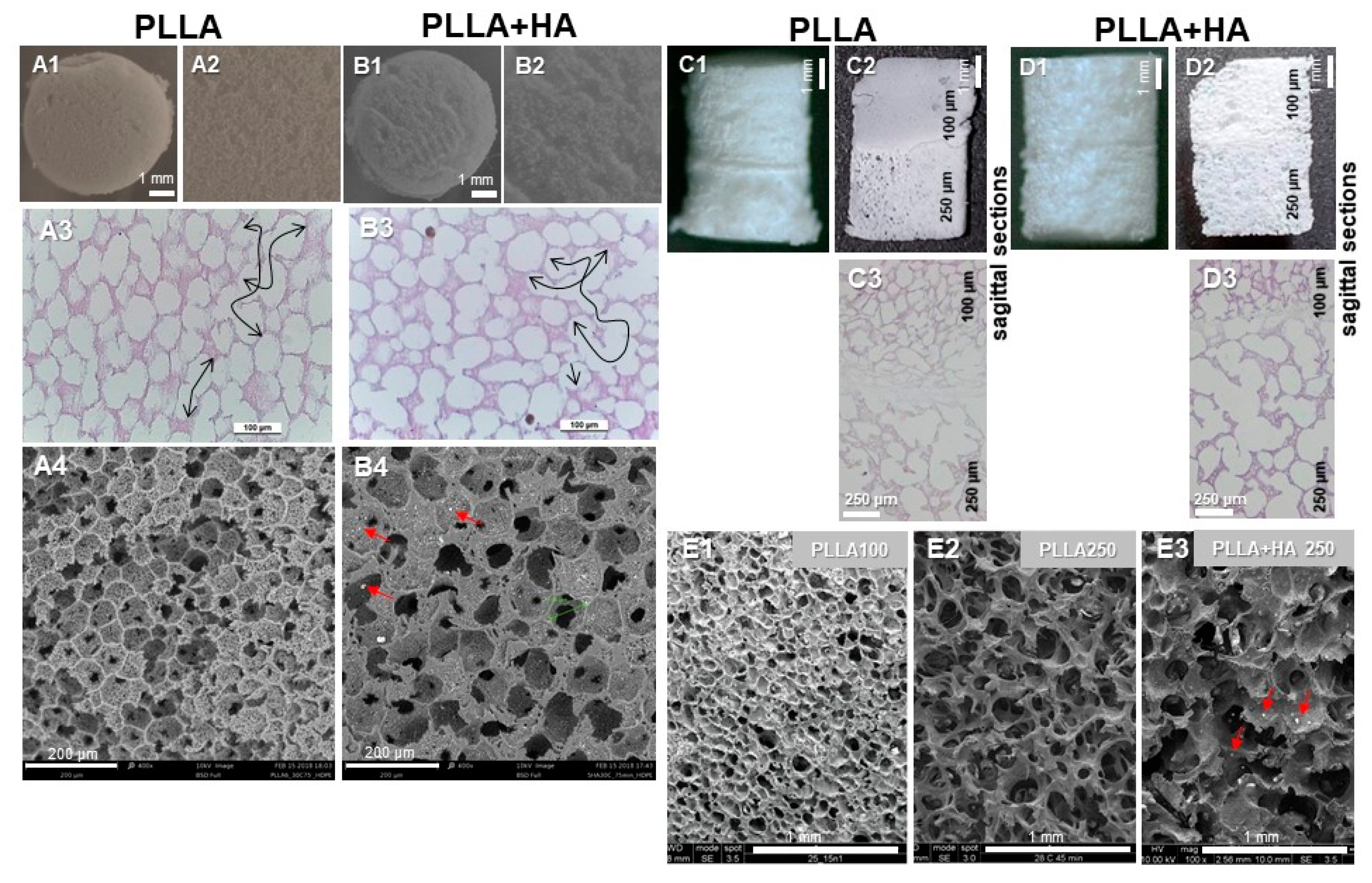
3.1. Structure of Unseeded Scaffold Variants as Determined by SEM, Pycnometry and Histology
3.2. Cytocompatibility of the Scaffold
3.3. hMSC Characterization
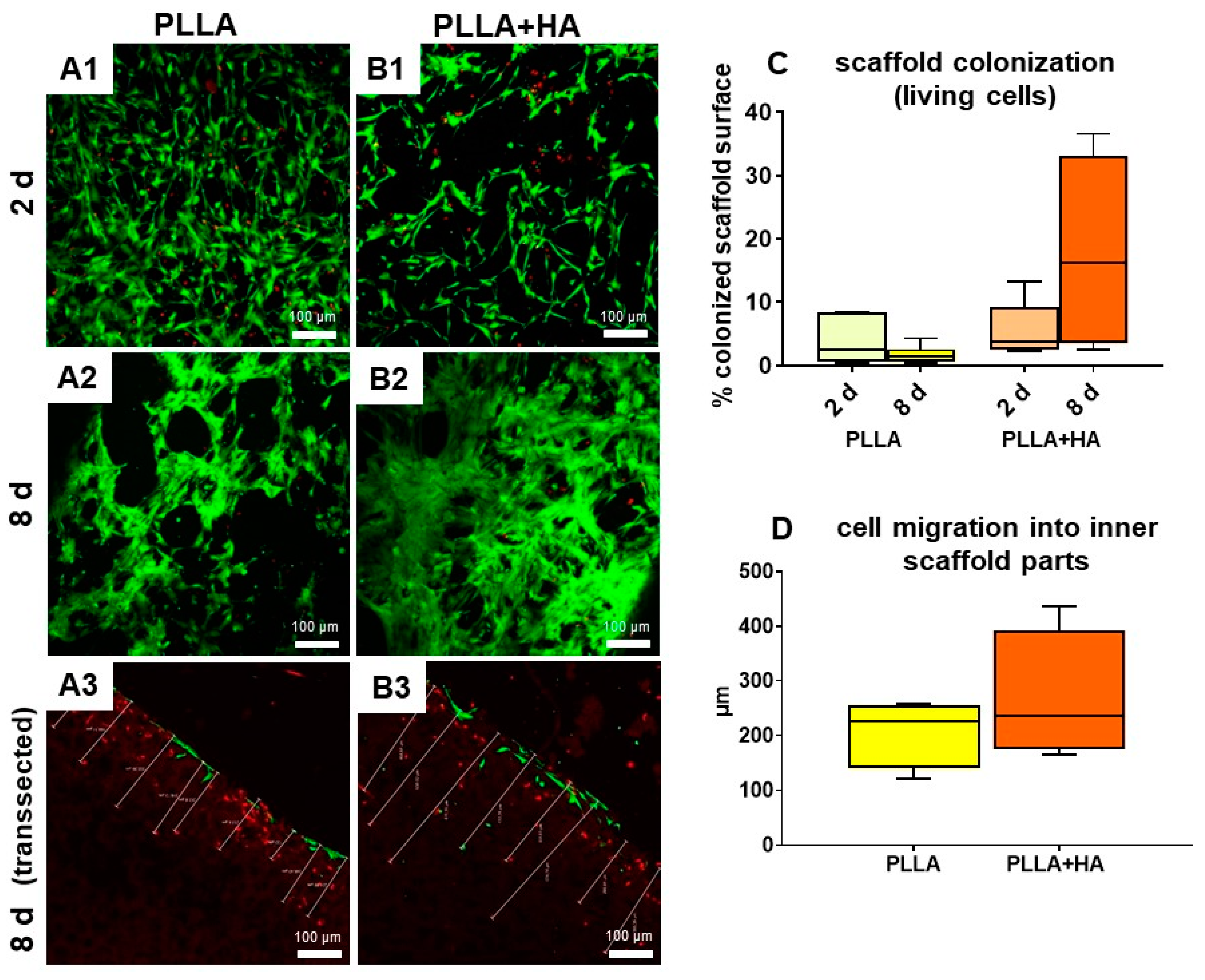
3.4. Cell Survival and Colonization of the Scaffolds Supplemented with HA or Not
3.5. Osteogenic Differentiation
3.6. Cell Survival in Bilayer Scaffolds as Shown by Life–Death Assay
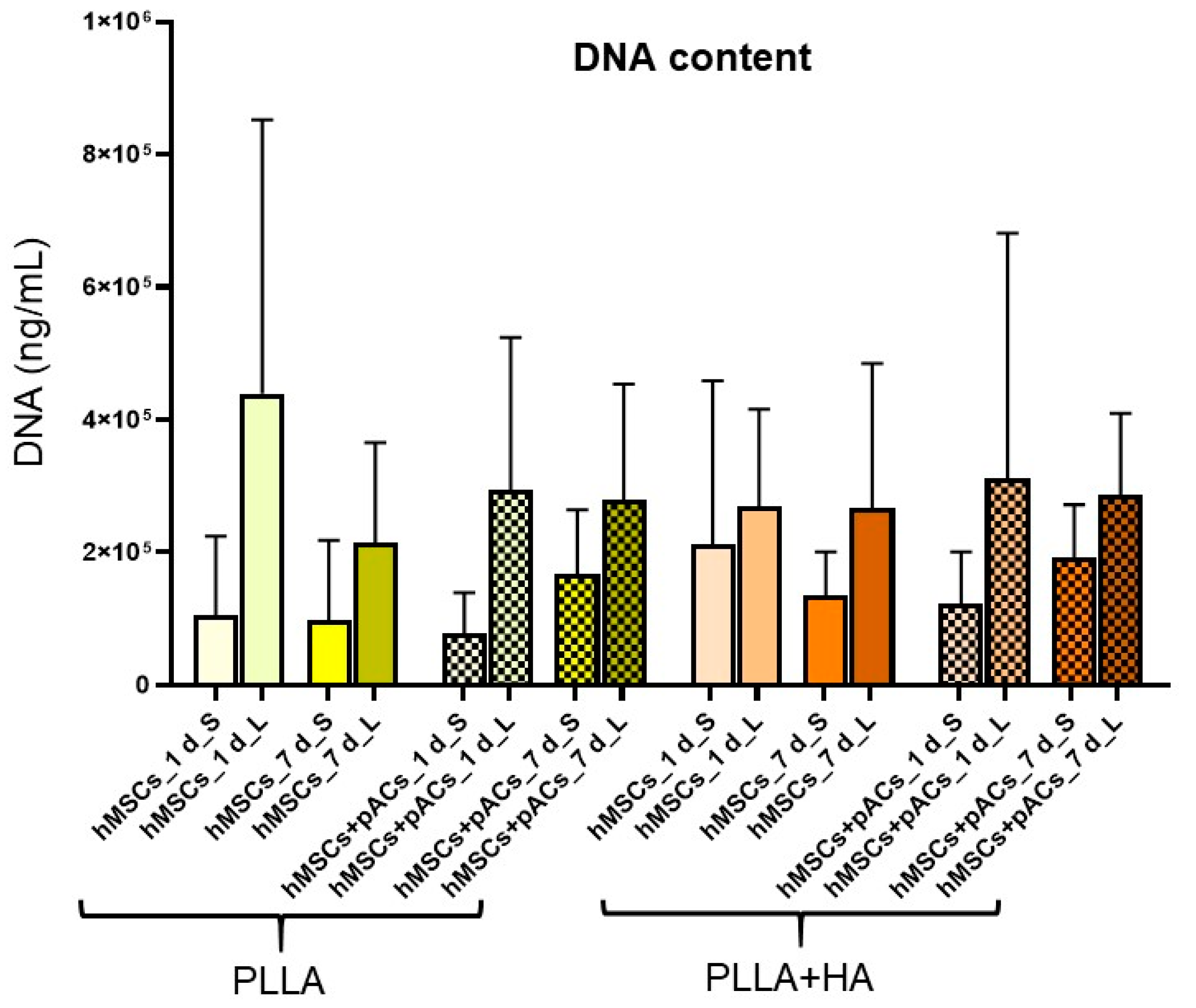
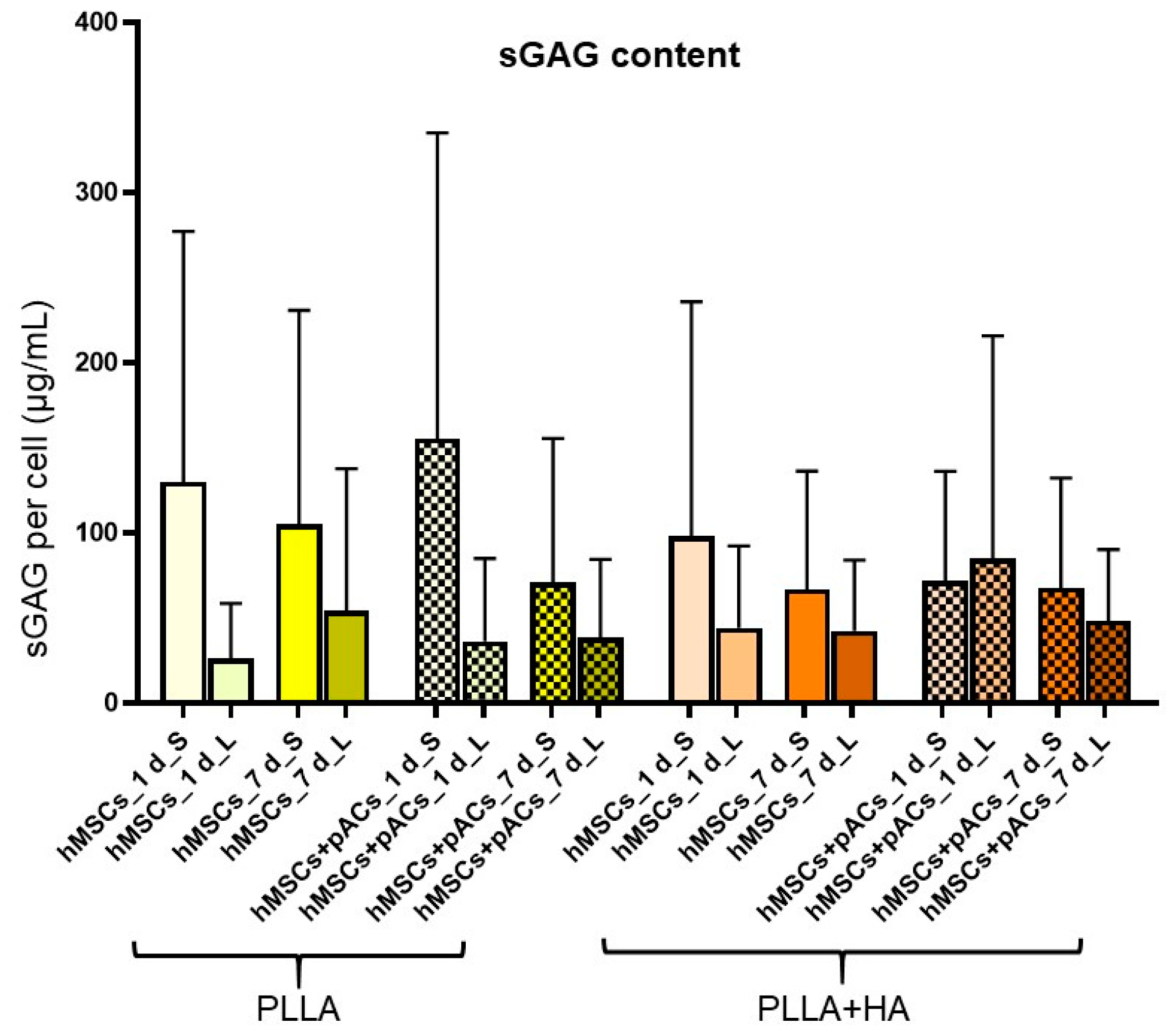
3.7. Cell Content and Glycosaminoglycan Deposition in Bilayer Scaffolds
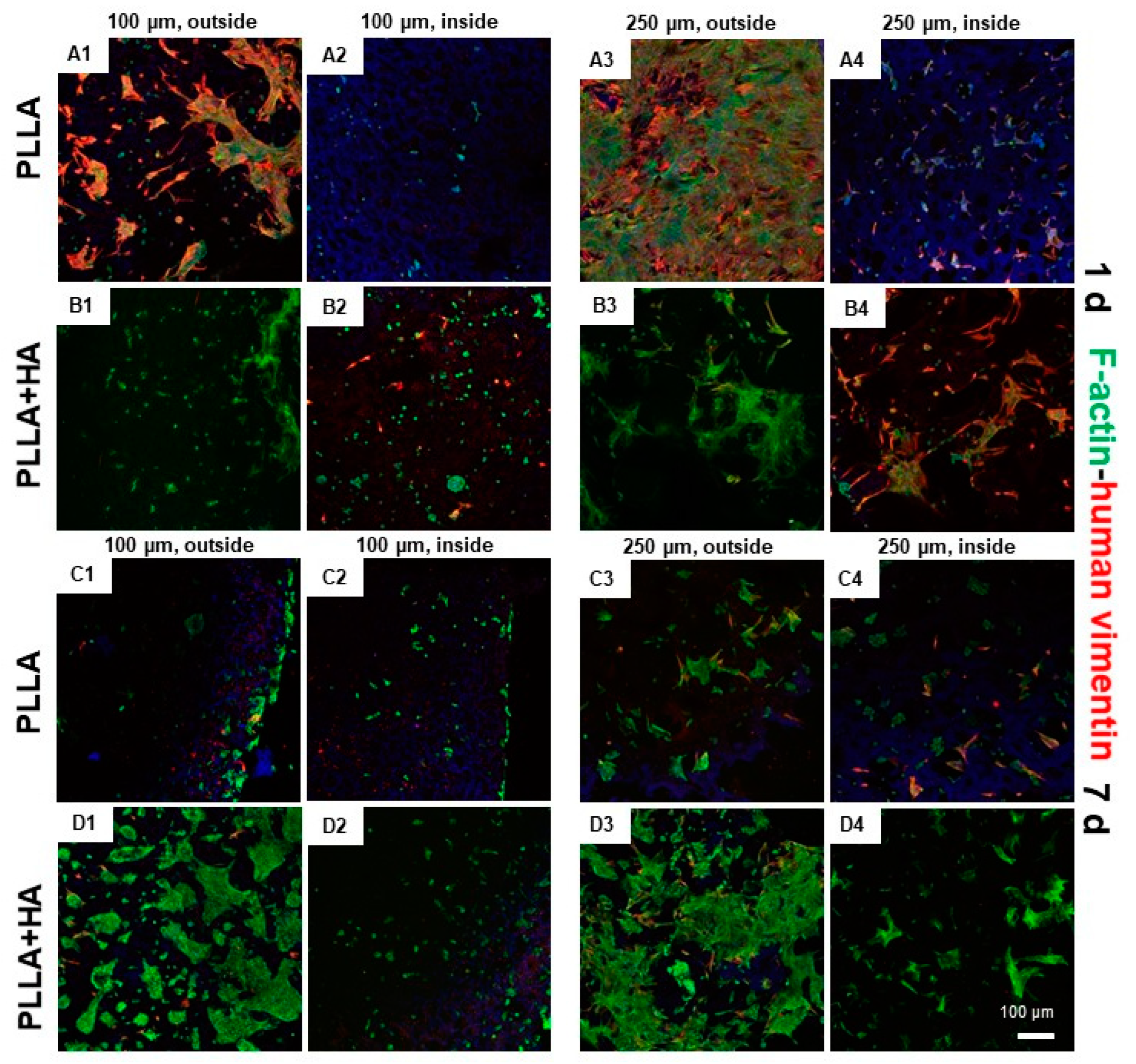
3.8. Cell Distribution and Cytoskeletal Architecture in hMSC/pAC Coculture on Bilayer Scaffolds as Shown by Immunolabeling
3.9. Gene Expression Profile in Bilayer Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimek, K.; Benko, A.; Vandrovcova, M.; Travnickova, M.; Douglas, T.E.; Tarczynska, M.; Broz, A.; Gaweda, K.; Ginalska, G.; Bacakova, L. Biomimetic biphasic curdlan-based scaffold for osteochondral tissue engineering applications—Characterization and preliminary evaluation of mesenchymal stem cell response in vitro. Biomater. Adv. 2022, 135, 212724. [Google Scholar] [CrossRef]
- Ghosh, S.; Viana, J.C.; Reis, R.L.; Mano, J.F. Development of porous lamellar poly(l-lactic acid) scaffolds by conventional injection molding process. Acta Biomater. 2008, 4, 887–896. [Google Scholar] [CrossRef]
- Ma, W.; Zhou, Z.; Ismail, N.; Tocci, E.; Figoli, A.; Khayet, M.; Matsuura, T.; Cui, Z.; Tavajohi, N. Membrane formation by thermally induced phase separation: Materials, involved parameters, modeling, current efforts and future directions. J. Membr. Sci. 2023, 669, 121303. [Google Scholar] [CrossRef]
- Pavia, F.C.; La Carrubba, V.; Piccarolo, S.; Brucato, V. Polymeric scaffolds prepared via thermally induced phase separation: Tuning of structure and morphology. J. Biomed. Mater. Res. Part A 2007, 86, 459–466. [Google Scholar]
- Duan, W.; Chen, C.; Haque, M.; Hayes, D.; Lopez, M.J. Polymer-mineral scaffold augments in vivo equine multipotent stromal cell osteogenesis. Stem Cell Res. Ther. 2018, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dai, W.; Gupta, A.; Zhang, B.; Wu, Z.; Zhang, Y.; Pan, L.; Wang, L. Frontiers of Hydroxyapatite Composites in Bionic Bone Tissue Engineering. Materials 2022, 15, 8475. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Vitrano, I.; Costa, V.; Raimondi, L.; Carina, V.; Bellavia, D.; Conoscenti, G.; Di Falco, R.; Pavia, F.C.; La Carrubba, V.; et al. Improvement of osteogenic differentiation of human mesenchymal stem cells on composite poly l-lactic acid/nano-hydroxyapatite scaffolds for bone defect repair. J. Biosci. Bioeng. 2019, 129, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, K.; Lu, W.; Sun, Q.; Peng, L.; Fen, W.; Li, H.; Ou, Y.; Liu, H.; Wang, D.; et al. In vitro study of nano-HA/PLLA composite scaffold for rabbit BMSC differentiation under TGF-β1 induction. Vitr. Cell. Dev. Biol. Anim. 2013, 50, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Pavia, F.C.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C 2017, 80, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Popkov, A.V.; Stogov, M.V.; Gorbach, E.N.; Kononovich, N.A.; Tushina, N.V.; Tverdokhlebov, S.I.; Dubinenko, G.E.; Akimchenko, I.O.; Bolbasov, E.N.; Popkov, D.A. Hydrolysis of Bone-Replacing Materials Based on Polylactic Acid and Containing Hydroxyapatite in an In Vitro Experiment. Bull. Exp. Biol. Med. 2022, 174, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Pavia, F.C.; Conoscenti, G.; Greco, S.; La Carrubba, V.; Ghersi, G.; Brucato, V. Preparation, characterization and in vitro test of composites poly-lactic acid/hydroxyapatite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 945–953. [Google Scholar] [CrossRef]
- Marycz, K.; Smieszek, A.; Targonska, S.; Walsh, S.A.; Szustakiewicz, K.; Wiglusz, R.J. Three dimensional (3D) printed polylactic acid with nano-hydroxyapatite doped with europium(III) ions (nHAp/PLLA@Eu3+) composite for osteochondral defect regeneration and theranostics. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110634. [Google Scholar] [CrossRef]
- Fu, L.; Zhao, W.; Zhang, L.; Gao, C.; Zhang, X.; Yang, X.; Cai, Q. Mimicking osteochondral interface using pre-differentiated BMSCs/fibrous mesh complexes to promote tissue regeneration. J. Biomater. Sci. Polym. Ed. 2022, 33, 2081–2103. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, D.; Peng, L.; Chen, Y.F.; Cui, J.; Xiong, J.; Lu, W.; Duan, L.; Chen, K.; Zeng, Y.; et al. Repair of rabbit cartilage defect based on the fusion of rabbit bone marrow stromal cells and Nano-HA/PLLA composite material. Artif. Cells Nanomed. Biotechnol. 2016, 45, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, T.; Zhao, W.; Song, G.; Li, C.; Cui, G. The effect of fiber size and pore size on cell proliferation and infiltration in PLLA scaffolds on bone tissue engineering. J. Biomater. Appl. 2016, 30, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Sicchieri, L.G.; Crippa, G.E.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Pore size regulates cell and tissue interactions with PLGA-CaP scaffolds used for bone engineering. J. Tissue Eng. Regen. Med. 2011, 6, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, K.; Włodarczyk, M.; Gazińska, M.; Rudnicka, K.; Płociński, P.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Biernat, M.; Sieja, K.; Grzymajło, M.; et al. The Effect of Pore Size Distribution and l-Lysine Modified Apatite Whiskers (HAP) on Osteoblasts Response in PLLA/HAP Foam Scaffolds Obtained in the Thermally Induced Phase Separation Process. Int. J. Mol. Sci. 2021, 22, 3607. [Google Scholar] [CrossRef]
- Erickson, A.E.; Sun, J.; Levengood, S.K.L.; Swanson, S.; Chang, F.-C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21, 34. [Google Scholar] [CrossRef]
- DIN EN ISO 10993-5:2009-10; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Kim, Y.-J.; Sah, R.L.Y.; Doong, J.-Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C T difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef]
- Conoscenti, G.; Pavia, F.C.; Ongaro, A.; Brucato, V.; Goegele, C.; Schwarz, S.; Boccaccini, A.R.; Stoelzel, K.; La Carrubba, V.; Schulze-Tanzil, G. Human nasoseptal chondrocytes maintain their differentiated phenotype on PLLA scaffolds produced by thermally induced phase separation and supplemented with bioactive glass 1393. Connect. Tissue Res. 2019, 60, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, L.; Song, X.; Yang, H.; Ren, X.; Gong, X.; Wang, F.; Yang, L. Effects of vimentin disruption on the mechanoresponses of articular chondrocyte. Biochem. Biophys. Res. Commun. 2016, 469, 132–137. [Google Scholar] [CrossRef]
- Jamal, S.A.; Ye, Q. Hydroxyapatite-Based Colloidal Gels Facilitate the Proliferation and Migration of Chondrocytes and the Adhesion of Umbilical Cord Mesenchymal Stem Cells. Int. Sch. Res. Not. 2014, 2014, 935689. [Google Scholar] [CrossRef] [PubMed]





| Target | Primary Antibody | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| CD29 (β1-integrin) | mouse anti-human, Merck KGaA, Darmstadt, Germany | 1:50 | donkey anti-mouse cy-3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| CD34 | mouse anti-human, BD Pharmingen, San Diego, CA, USA, APC labeled | 1:50 | donkey anti-mouse cy-3, Invitrogen | 1:200 |
| CD44 | mouse anti-human, Cell Signalling Technology, Danvers, MA, USA | 1:70 | donkey anti-mouse A488, Invitrogen | 1:200 |
| CD45 | mouse anti-human, DAKO, Cytomation, Hamburg, Germany | 1:70 | donkey anti-mouse cy-3, Invitrogen | 1:200 |
| CD90 | mouse anti-human, BD Pharmingen, FITC labeled | 1:70 | donkey anti-mouse A488, Invitrogen | 1:200 |
| CD105 | mouse anti-human, Miltenyi, Bergisch Gladbach, Germany, PE-labeled | 1:50 | donkey anti-mouse cy-3, Invitrogen | 1:200 |
| collagen type 1 | goat anti-human, Abcam, Cambridge, UK | 1:50 | donkey-anti-goat cy-3, Invitrogen, Carlsbad, CA, USA | 1:200 |
| collagen type 10 | mouse-anti human, Abcam, Cambridge, UK | 1:50 | donkey anti-mouse cy-3, Invitrogen | 1:200 |
| 4′,6′-diamidino-2-phenylindol (DAPI) | Roche, Mannheim, Germany | 1:100 | - | 1:200 |
| phalloidin-A488 | Santa Cruz Biotechnologies Inc., Dallas, TX, USA | 1:100 | - | 1:200 |
| vimentin | mouse anti-human, Dako Cytomation, Hamburg, Germany | 1:50 | donkey anti-mouse cy-3, Invitrogen | 1:200 |
| anti-human-specific vimentin | rabbit anti-human, Zytomed systems, Berlin, Germany | 1:50 | donkey anti-rabbit A555, Invitrogen | 1:200 |
| Gene Symbol | Species | Gene Name | Efficacy | Amplicon Length (Base Pairs) | Assay ID |
|---|---|---|---|---|---|
| ACAN | Sus scrofa | aggrecan | 1.69 | 60 | Ss03374823_m1 |
| ACTB | Homo sapiens | β-actin | 1.89 | 171 | Hs99999903_m1 |
| ACTB | Sus scrofa | β-actin | 1.71 | 77 | Ss03376081_u1 |
| Col2A1 | Homo sapiens | collagen type 2 | 2.06 (1.9 *) | 124 | Hs00264051_m1 |
| RUNX2 | Homo sapiens | Runt-related protein | 1.94 | 116 | Hs00231692_m1 |
| COL10A1 | Homo sapiens | collagen type 10 alpha 1 | 2.15 | 76 | Hs00166657_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, W.; Civilleri, A.; Gögele, C.; Carbone, C.; Vitrano, I.; Carfi Pavia, F.; Brucato, V.; La Carrubba, V.; Werner, C.; Schäfer-Eckart, K.; et al. The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite. Polymers 2024, 16, 331. https://doi.org/10.3390/polym16030331
Ferraro W, Civilleri A, Gögele C, Carbone C, Vitrano I, Carfi Pavia F, Brucato V, La Carrubba V, Werner C, Schäfer-Eckart K, et al. The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite. Polymers. 2024; 16(3):331. https://doi.org/10.3390/polym16030331
Chicago/Turabian StyleFerraro, Wally, Aurelio Civilleri, Clemens Gögele, Camilla Carbone, Ilenia Vitrano, Francesco Carfi Pavia, Valerio Brucato, Vincenzo La Carrubba, Christian Werner, Kerstin Schäfer-Eckart, and et al. 2024. "The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite" Polymers 16, no. 3: 331. https://doi.org/10.3390/polym16030331
APA StyleFerraro, W., Civilleri, A., Gögele, C., Carbone, C., Vitrano, I., Carfi Pavia, F., Brucato, V., La Carrubba, V., Werner, C., Schäfer-Eckart, K., & Schulze-Tanzil, G. (2024). The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite. Polymers, 16(3), 331. https://doi.org/10.3390/polym16030331











