Balanced Thermal Insulation, Flame-Retardant and Mechanical Properties of PU Foam Constructed via Cost-Effective EG/APP/SA Ternary Synergistic Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
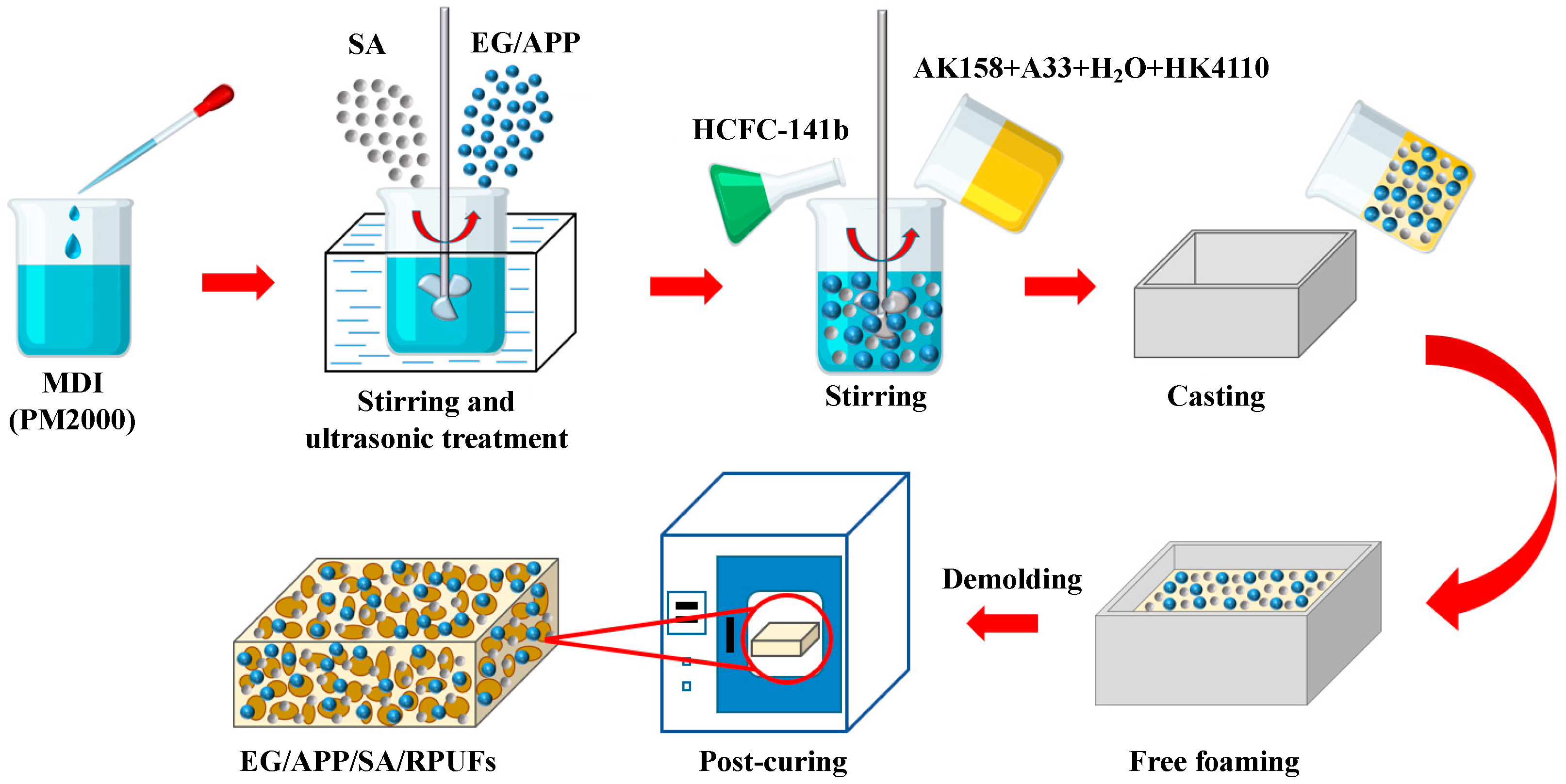
2.2. Preparation of EG/APP/SA/RPUFs
2.3. Characterization
2.3.1. Density and Microstructure
2.3.2. Chemical Structure
2.3.3. Mechanical and Thermal Conductivity Properties
2.3.4. Thermal Stability and Flame Retardancy
| Samples | SA | EG | APP | RPUF | Formulation Density b | Foam Density | Pore Fraction c | Compressive Strength | Specific Strength | Thermal Conductivity Coefficient | T5% | T50% | Tmax1 | Tmax2 | Residues after TGA | Residues after CCT | LOI | UL-94 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | wt.% | wt.% | wt.% | wt.% | kg/m3 | kg/m3 | vt.% | kPa | MPa/(g/cm3) | mW/mK | °C | °C | °C | °C | wt.% | wt.% | % | |
| SA | 100 | 13 | 100 | |||||||||||||||
| EG | 540 | 290 | 72.6 | |||||||||||||||
| APP | 1740 | / | 34.9 | |||||||||||||||
| RPUF | 0 | 0 | 0 | 100 | 1200 a | 45.6 ± 0.2 | 96.2 | 229 ± 12 | 5.02 | 24.4 ± 0.2 | 266.1 | 355.8 | 345.9 | 475.2 | 17.5 | 0.23 | 18.6 | NR d |
| SA/RPUF | 1 | 0 | 0 | 99 | 1081 | 49.8 ± 0.2 | 95.4 | 396 ± 8 | 7.95 | 19.8 ± 0.2 | 267.7 | 358.2 | 346.4 | 476.8 | 19.2 | 0.24 | 18.3 | NR |
| 8EG/SA/RPUF | 1 | 8 | 0 | 91 | 994 | 57.9 ± 0.6 | 94.2 | 268 ± 11 | 4.63 | 25.8 ± 0.1 | 266.5 | 357.0 | 343.8 | 458.8 | 22.3 | 7.24 | 24.8 | V-1 |
| 8APP/SA/RPUF | 1 | 0 | 8 | 91 | 1106 | 52.1 ± 0.8 | 95.3 | 348 ± 8 | 6.68 | 24.7 ± 0.2 | 249.8 | 332.4 | 314.3 | 478.7 | 23.4 | 4.35 | 24.4 | V-1 |
| 2EG/6APP/SA/RPUF | 1 | 2 | 6 | 91 | 1075 | 54.5 ± 0.7 | 94.9 | 431 ± 8 | 7.91 | 24.8 ± 0.1 | 257.4 | 330.8 | 298.9 | 474.2 | 25.1 | 4.92 | 25.8 | V-0 |
| 4EG/4APP/SA/RPUF | 1 | 4 | 4 | 91 | 1047 | 55.8 ± 0.4 | 94.7 | 394 ± 7 | 7.01 | 25.1 ± 0.2 | 258.5 | 336.7 | 309.0 | 473.1 | 26 | 6.06 | 25.6 | V-0 |
| 6EG/2APP/SA/RPUF | 1 | 6 | 2 | 91 | 1019 | 55.2 ± 0.6 | 94.6 | 342 ± 5 | 6.20 | 25.3 ± 0.2 | 283.8 | 370.6 | 321.6 | 476.3 | 30.9 | 6.85 | 26.1 | V-0 |
3. Results and Discussion
3.1. Chemical Structure of EG/APP/SA/RPUFs
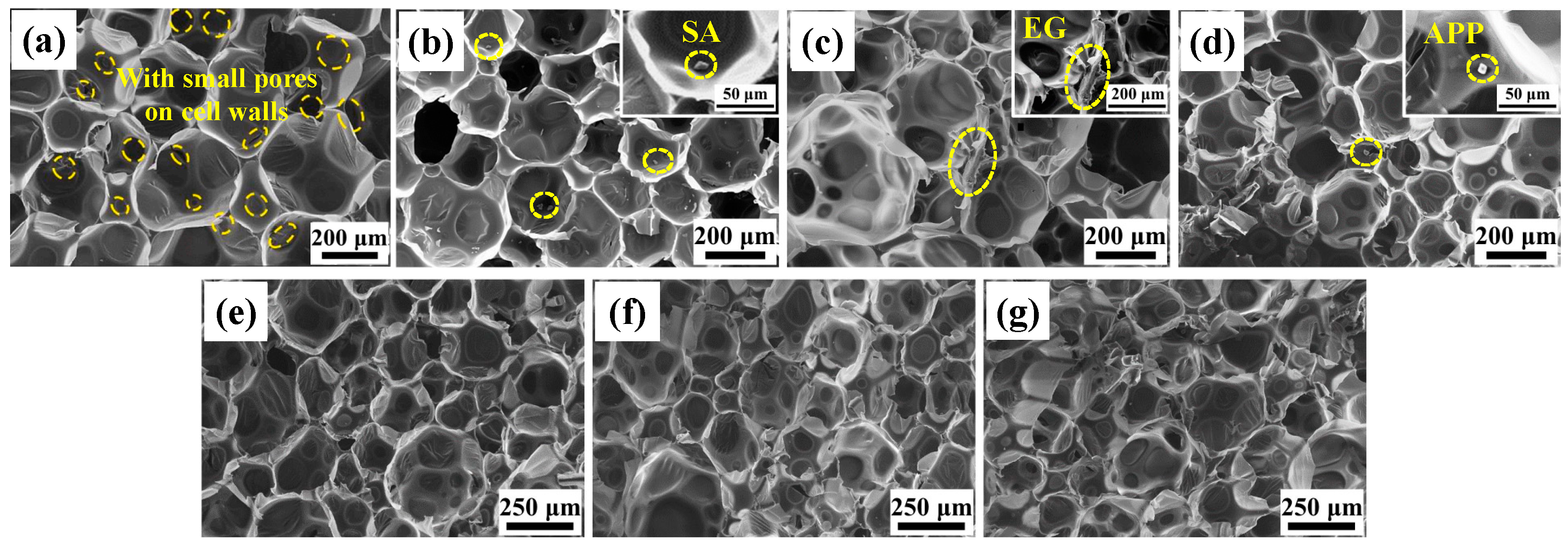
3.2. Microstructure of EG/APP/SA/PRUFs
3.2.1. Microstructure of SA, EG, and APP
3.2.2. Microstructure of EG/APP/SA/PRUFs
3.3. Compressive Properties of EG/APP/SA/RPUFs
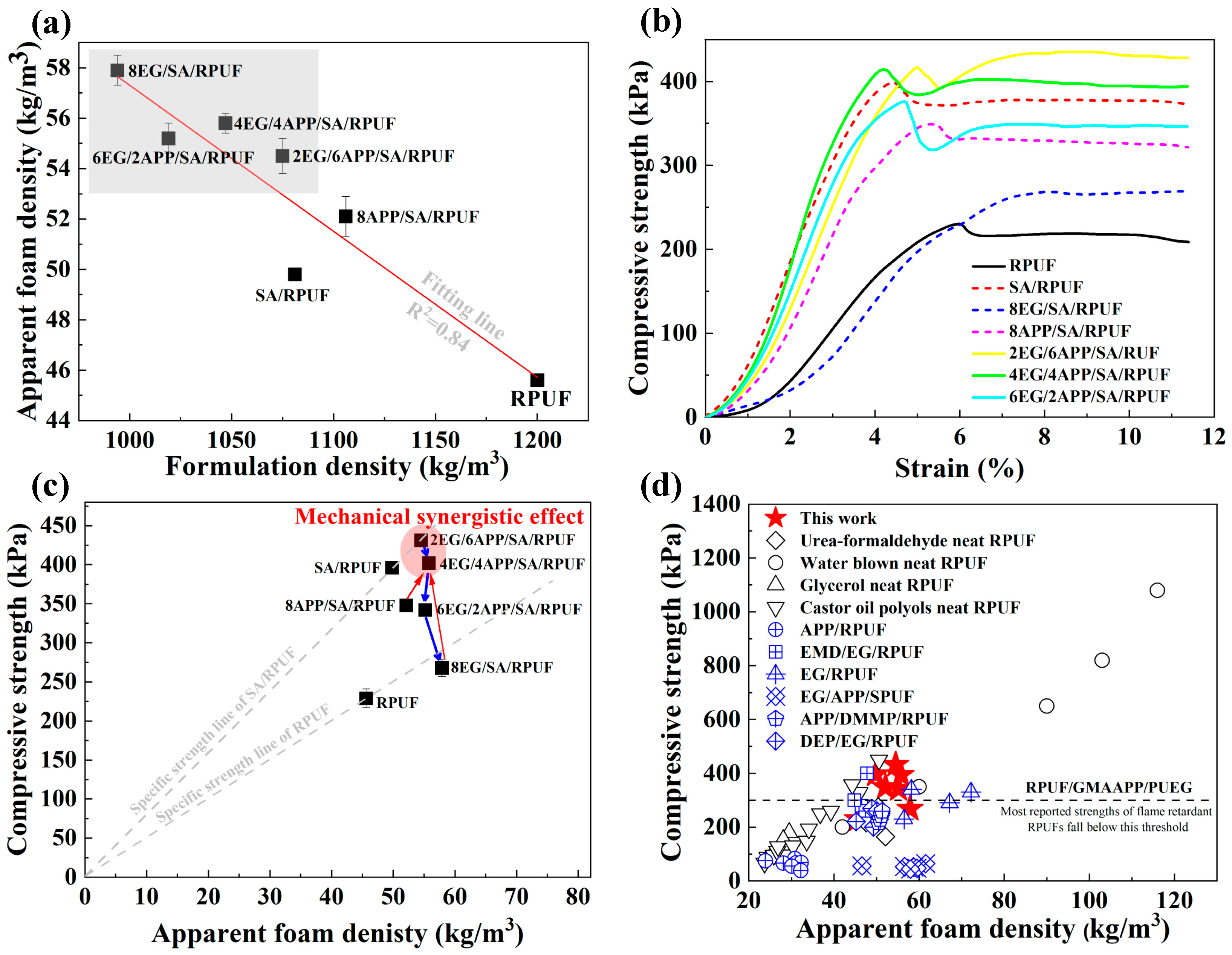
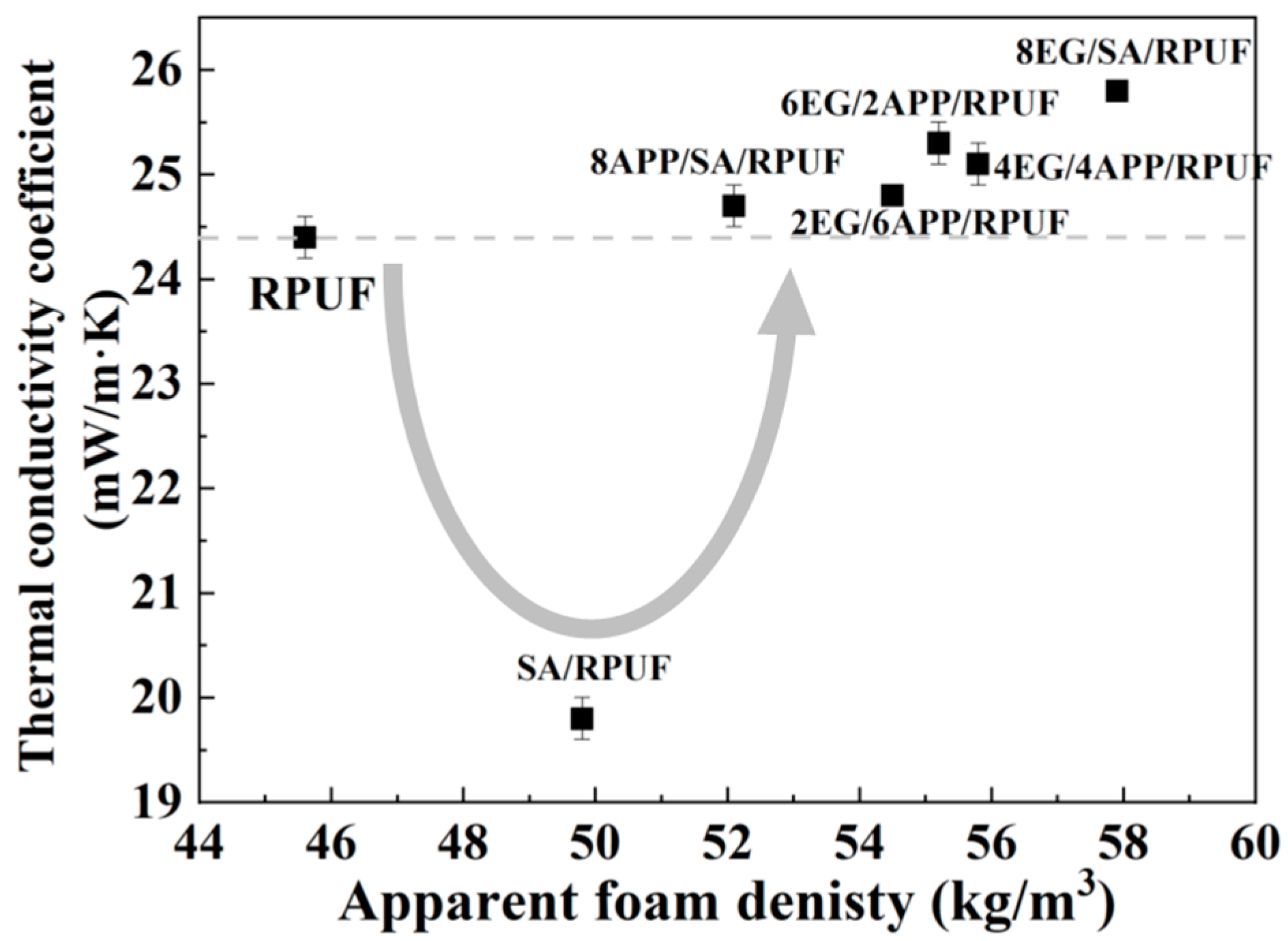
3.4. Thermal Conductivity Behaviors of EG/APP/SA/PRUFs
3.5. Thermal Stability of EG/APP/SA/PRUFs
3.6. Flame-Retardant Properties of EG/APP/SA/PRUFs
3.6.1. Flammability Behaviors
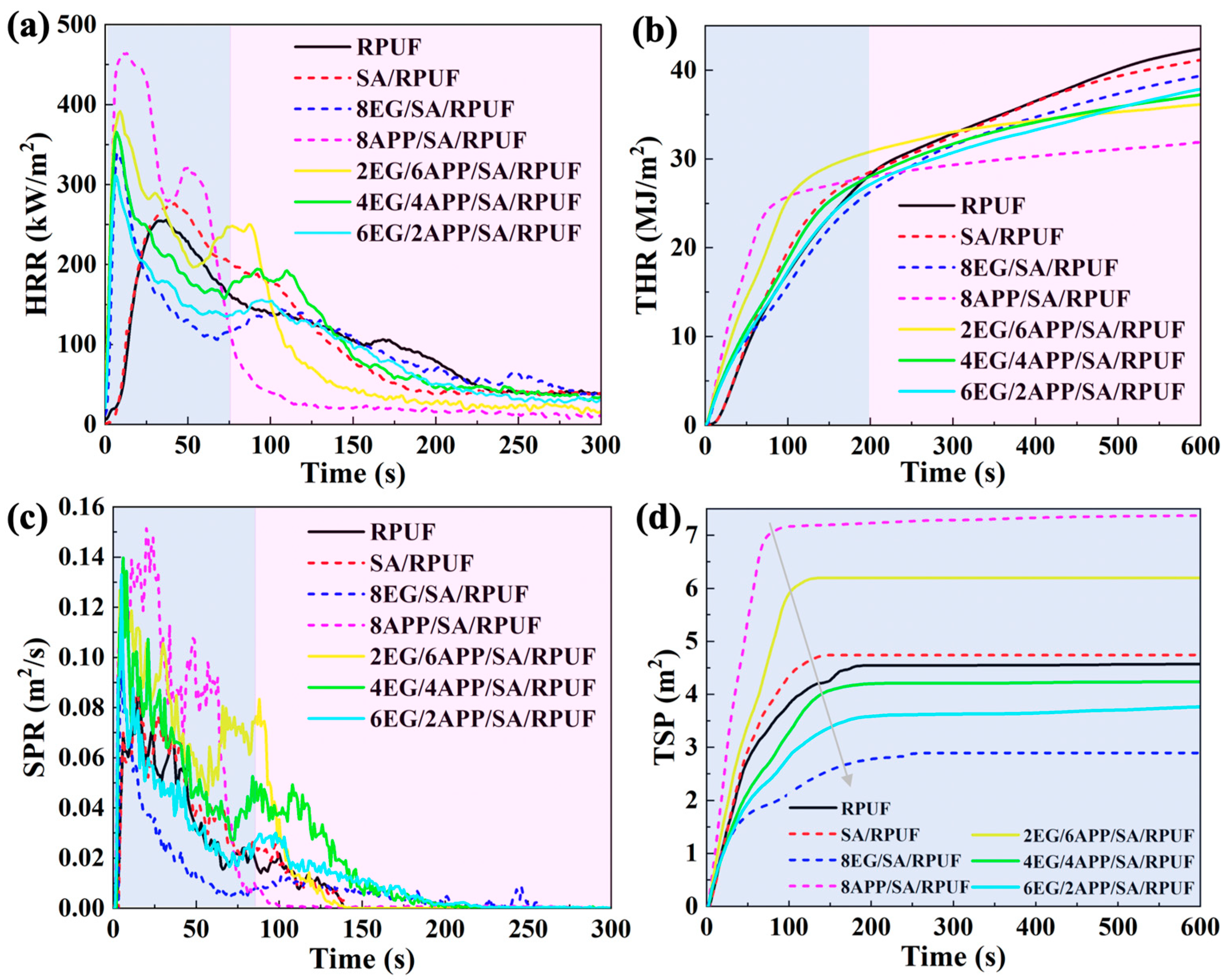
3.6.2. Fire Behaviors
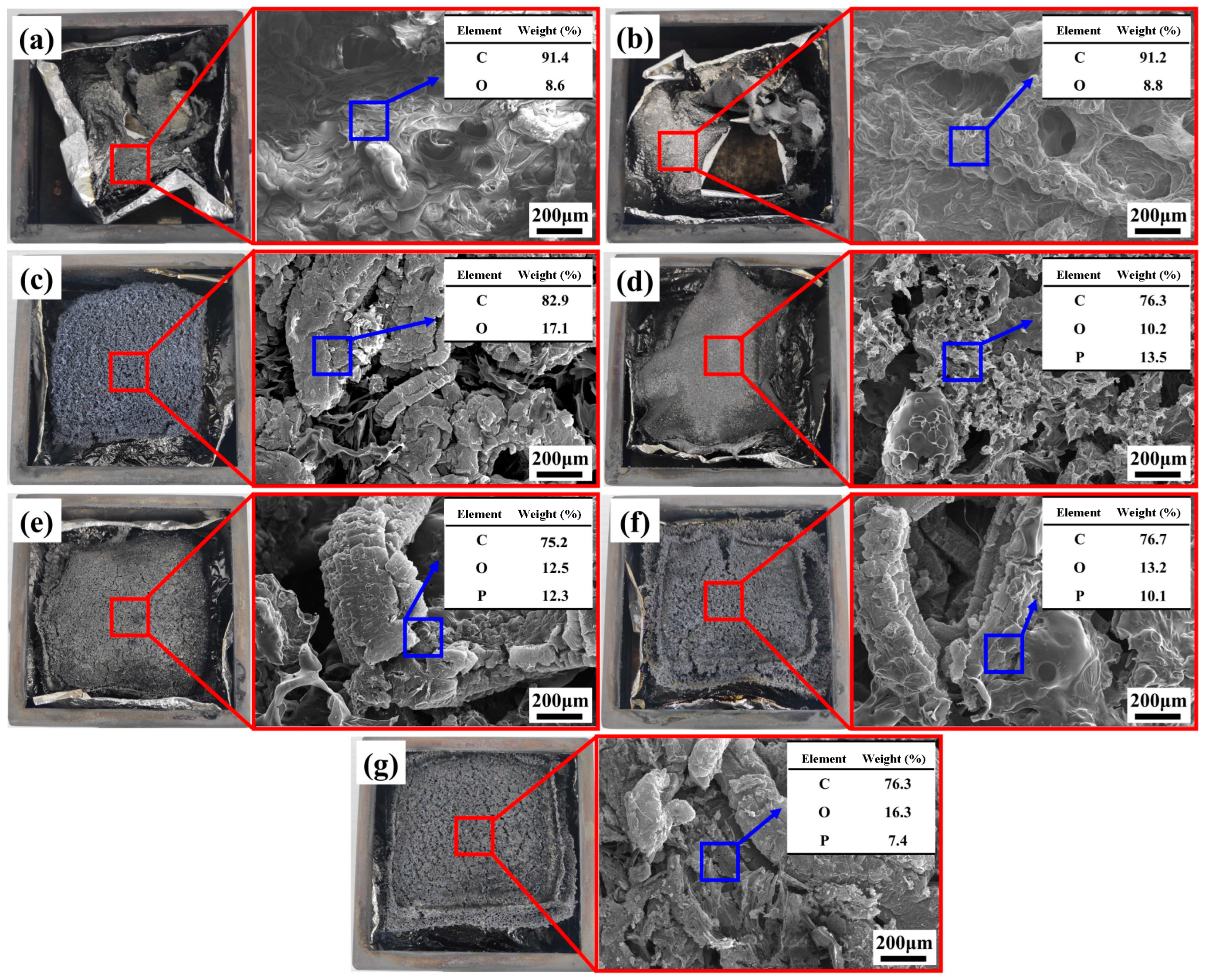
3.6.3. Residual Char Morphology and Elemental Composition
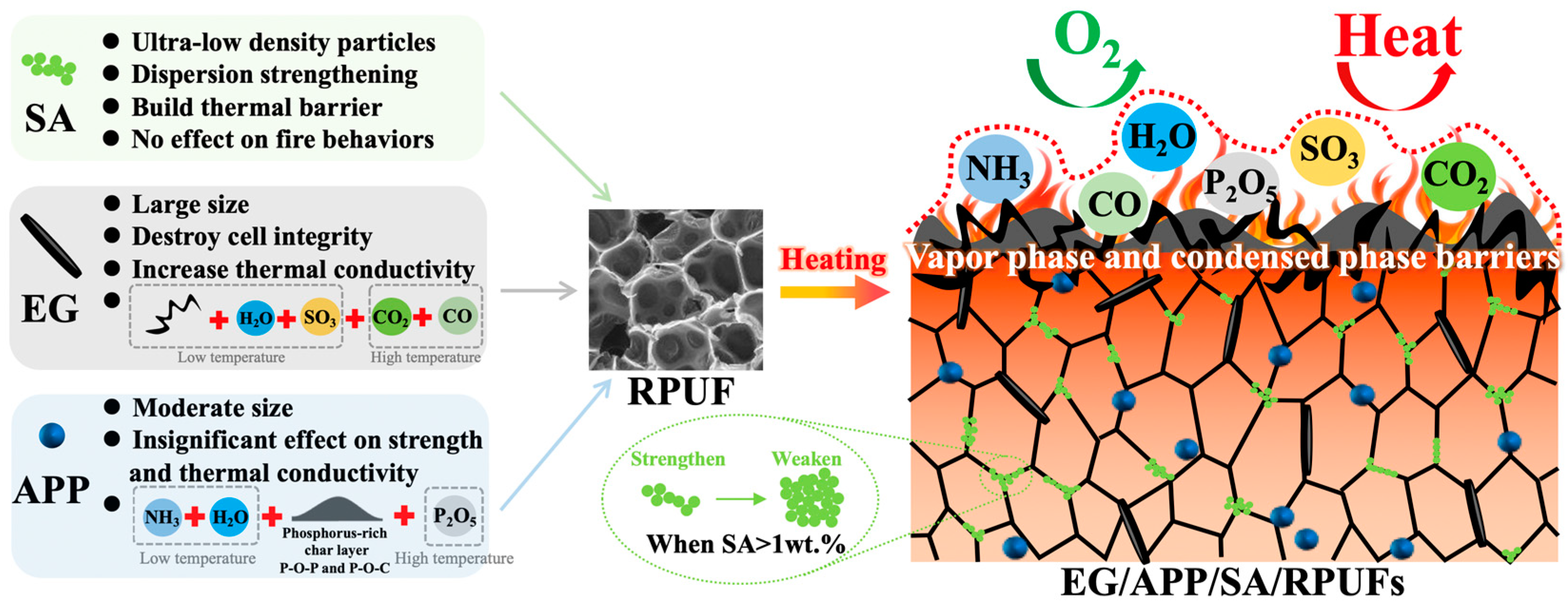
3.6.4. Flame Retardant Mechanism for EG/APP/SA/RPUFs
3.7. Ternary Synergistic Mechanism of EG/APP/SA in RPUF
- (a)
- APP demonstrates reduced high-temperature heat release characteristics along with a certain degree of mechanical enhancement. However, it exhibits elevated smoke generation and accelerates RPUF degradation at lower temperatures.
- (b)
- EG exhibits lower smoke emission, higher residual char yield, and relatively lower heat release. Nonetheless, it has a higher thermal conductivity and larger particle size, leading to more pronounced disruption in mechanical properties of RPUFs.
- (c)
- SA does not alter the fire-retardant, heat release, or smoke characteristics of RPUF, yet it plays a vital role in reducing thermal conductivity and enhancing mechanical performance. And it effectively compensates for the shortcomings of APP and EG.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; Liu, B.W.; Zhang, L.; Peng, Z.C.; Zhang, Y.Y.; Wang, H.; Zhao, H.B.; Wang, Y.Z. Fully biomass-based aerogels with ultrahigh mechanical modulus, enhanced flame retardancy, and great thermal insulation applications. Compos. Part. B-Eng. 2021, 225, 109309. [Google Scholar] [CrossRef]
- Berardi, U. A cross-country comparison of the building energy consumptions and their trends. Resour. Conserv. Recycl. 2017, 123, 230–241. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions—Properties, requirements and possibilities. Energ Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework. Nat. Chem. 2016, 8, 718–724. [Google Scholar] [CrossRef]
- Wi, S.; Yang, S.; Yun, B.Y.; Kang, Y.; Kim, S. Fire retardant performance, toxicity and combustion characteristics, and numerical evaluation of core materials for sandwich panels. Environ. Pollut. 2022, 312, 120067. [Google Scholar] [CrossRef]
- Al-Homoud, M.S. Performance characteristics and practical applications of common building thermal insulation materials. Build. Environ. 2005, 40, 353–366. [Google Scholar] [CrossRef]
- Kumar, D.; Alam, M.; Zou, P.X.; Sanjayan, J.G.; Memon, R.A. Comparative analysis of building insulation material properties and performance. Renew. Sustain. Energy Rev. 2020, 131, 110038. [Google Scholar] [CrossRef]
- Schiavoni, S.; Bianchi, F.; Asdrubali, F. Insulation materials for the building sector: A review and comparative analysis. Renew. Sustain. Energ Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Pan, H.; Yang, W.; Liew, K.M.; Song, L.; Hu, Y. Synthesis and characterization of MnO2 nanosheets based multilayer coating and applications as a flame retardant for flexible polyurethane foam. Compos. Sci. Technol. 2016, 123, 212–221. [Google Scholar] [CrossRef]
- Visakh, P.M.; Semkin, A.O.; Rezaev, I.A.; Fateev, A.V. Review on soft polyurethane flame retardant. Constr. Build. Mater. 2019, 227, 116673. [Google Scholar] [CrossRef]
- Wi, S.; Yang, S.; Kim, Y.U.; Kang, Y.; Kim, S. Toxicity characteristics and fire retardant performance of commercially manufactured organic insulation materials for building applications. Constr. Build. Mater. 2022, 341, 127898. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S.A. Synthesis and properties of rigid polyurethane foams synthesized from modified urea-formaldehyde resin. Constr. Build. Mater. 2019, 202, 718–726. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Kirpluks, M.; Cabulis, U.; Zeltins, V.; Stiebra, L.; Avots, A. Rigid polyurethane foam thermal insulation protected with mineral intumescent mat. AUTEX Res. J. 2014, 14, 259–269. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The pyrolysis behaviors of phosphorus-containing organosilicon compound modified APP with different polyether segments and their flame retardant mechanism in polyurethane foam. Compos. Part. B-Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Han, S.; Zhu, X.; Chen, F.; Chen, S.; Liu, H. Flame-retardant system for rigid polyurethane foams based on diethyl bis (2-hydroxyethyl) aminomethylphosphonate and in-situ exfoliated clay. Polym. Degrad. Stab. 2020, 177, 109178. [Google Scholar] [CrossRef]
- Wi, S.; Berardi, U.; Di Loreto, S.; Kim, S. Microstructure and thermal characterization of aerogel–graphite polyurethane spray-foam composite for high efficiency thermal energy utilization. J. Hazard. Mater. 2020, 397, 122656. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, Q.; Xie, M.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M.; Zheng, G. Core-shell expandable graphite@ aluminum hydroxide as a flame-retardant for rigid polyurethane foams. Polym. Degrad. Stab. 2017, 146, 267–276. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Melamine, silica, and ionic liquid as a novel flame retardant for rigid polyurethane foams with enhanced flame retardancy and mechanical properties. Polym. Test. 2020, 87, 106511. [Google Scholar] [CrossRef]
- Guo, K.Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.D.; Zhao, L.; Gao, J.F.; Song, P.; Tang, L.C. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response. Compos. Part. B-Eng. 2020, 193, 108017. [Google Scholar] [CrossRef]
- Yang, H.; Yu, B.; Song, P.; Maluk, C.; Wang, H. Surface-coating engineering for flame retardant flexible polyurethane foams: A critical review. Compos. Part. B-Eng. 2019, 176, 107185. [Google Scholar] [CrossRef]
- Vo, D.K.; Do, T.D.; Nguyen, B.T.; Tran, C.K.; Nguyen, T.A.; Nguyen, D.M.; Pham, L.H.; Nguyen, T.D.; Nguyen, T.-D.; Hoang, D. Effect of metal oxide nanoparticles and aluminum hydroxide on the physicochemical properties and flame-retardant behavior of rigid polyurethane foam. Constr. Build. Mater. 2022, 356, 129268. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, Z.; Liu, M.; Jiang, H.; Fan, C.; Wang, B.; Tang, G.; Wang, H. Flame retardant rigid polyurethane foam composites based on microencapsulated ammonium polyphosphate and microencapsulated expanded graphite. J. Macromol. Sci. A 2021, 58, 659–668. [Google Scholar] [CrossRef]
- Yang, C.; Shao, S. Rigid polyurethane foams containing modified ammonium polyphosphate having outstanding charring ability and increased flame retardancy. Front. Mater. 2021, 8, 712809. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Synergistic effects of expandable graphite and ammonium pentaborate octahydrate on the flame-retardant, thermal insulation, and mechanical properties of rigid polyurethane foam. Polym. Compos. 2020, 41, 1749–1762. [Google Scholar] [CrossRef]
- Yan, J.; Xu, P.; Zhang, P.; Fan, H. Surface-modified ammonium polyphosphate for flame-retardant and reinforced polyurethane composites. Colloid Surf. A 2021, 626, 127092. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Qian, L.; Xu, B.; Xi, W. Addition flame-retardant effect of nonreactive phosphonate and expandable graphite in rigid polyurethane foams. J. Appl. Polym. 2018, 135, 45960. [Google Scholar] [CrossRef]
- Li, J.; Mo, X.; Li, Y.; Zou, H.; Liang, M.; Chen, Y. Influence of expandable graphite particle size on the synergy flame retardant property between expandable graphite and ammonium polyphosphate in semi-rigid polyurethane foam. Polym. Bull. 2018, 75, 5287–5304. [Google Scholar] [CrossRef]
- Xu, W.Z.; Liu, L.; Wang, S.Q.; Hu, Y. Synergistic effect of expandable graphite and aluminum hypophosphite on flame-retardant properties of rigid polyurethane foam. J. Appl. Polym. 2015, 132, 42842. [Google Scholar] [CrossRef]
- Luo, F.B.; Wu, K.; Lu, M.G.; Nie, S.B.; Li, X.Y.; Guan, X.X. Thermal degradation and flame retardancy of microencapsulated ammonium polyphosphate in rigid polyurethane foam. J. Therm. Anal. Calorim. 2015, 120, 1327–1335. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Meng, X.Y.; Ye, L.; Zhang, X.G.; Tang, P.M.; Tang, J.H.; Ji, X.; Li, Z.M. Effects of expandable graphite and ammonium polyphosphate on the flame-retardant and mechanical properties of rigid polyurethane foams. J. Appl. Polym. 2009, 114, 853–863. [Google Scholar] [CrossRef]
- Bartczak, P.; Siwińska-Ciesielczyk, K.; Haak, N.; Parus, A.; Piasecki, A.; Jesionowski, T.; Borysiak, S. Closed-cell polyurethane spray foam obtained with novel TiO2–ZnO hybrid fillers—Mechanical, insulating properties and microbial purity. J. Build. Eng. 2023, 65, 105760. [Google Scholar] [CrossRef]
- Ou, M.; Lian, R.; Li, R.; Cui, J.; Guan, H.; Zhu, J.; Liu, L.; Jiao, C.; Chen, X. Facile strategy of preparing excellently thermal-insulating and fire-safe building materials based on an aromatic Schiff base dicarboxylic acid without additional flame retardants. J. Build. Eng. 2023, 78, 107572. [Google Scholar] [CrossRef]
- Buratti, C.; Merli, F.; Moretti, E. Aerogel-based materials for building applications: Influence of granule size on thermal and acoustic performance. Energ. Build. 2017, 152, 472–482. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as promising thermal insulating materials: An overview. J. Mater. 2014, 2014, 127049. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A review of state-of-the-art aerogel applications in buildings. Int. J. Low-Carbon Technol. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Yang, W.J.; Wei, C.X.; Yuen, A.C.Y.; Lin, B.; Yeoh, G.H.; Lu, H.D.; Yang, W. Fire-retarded nanocomposite aerogels for multifunctional applications: A review. Compos. Part. B-Eng. 2022, 237, 109866. [Google Scholar] [CrossRef]
- Soltani, R.; Dinari, M.; Mohammadnezhad, G. Ultrasonic-assisted synthesis of novel nanocomposite of poly (vinyl alcohol) and amino-modified MCM-41: A green adsorbent for Cd (II) removal. Ultrason. Sonochem. 2018, 40, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Verdolotti, L.; Lavorgna, M.; Lamanna, R.; Di Maio, E.; Iannace, S. Polyurethane-silica hybrid foam by sol–gel approach: Chemical and functional properties. Polymer 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non-Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Bonab, S.A.; Moghaddas, J.; Rezaei, M. In-situ synthesis of silica aerogel/polyurethane inorganic-organic hybrid nanocomposite foams: Characterization, cell microstructure and mechanical properties. Polymer 2019, 172, 27–40. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Vaitkus, S. Composites of rigid polyurethane foams and silica powder filler enhanced with ionic liquid. Polym. Test. 2019, 75, 12–25. [Google Scholar] [CrossRef]
- Cimavilla-Román, P.; Pérez-Tamarit, S.; Santiago-Calvo, M.; Rodríguez-Pérez, M.Á. Influence of silica aerogel particles on the foaming process and cellular structure of rigid polyurethane foams. Eur. Polym. J. 2020, 135, 109884. [Google Scholar] [CrossRef]
- Hou, L.; Li, H.; Liu, Y.; Niu, K.; Shi, Z.; Liang, L.; Yao, Z.; Liu, C.; Tian, D. Synergistic effect of silica aerogels and hollow glass microspheres on microstructure and thermal properties of rigid polyurethane foam. J. Non-Cryst. Solids 2022, 592, 121753. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.; Naik, Y.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Xu, X.; Bai, Z.; Guo, X.; Chen, Y.; Chen, X.; Lu, Z.; Wu, H. Effect of blowing agent content on the structure and flame-retardant properties of rigid polyurethane foam/expanded graphite composites. Polym. Adv. Technol. 2022, 34, 195–204. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Zhu, M. Flame retardant, mechanical and thermal insulating properties of rigid polyurethane foam modified by nano zirconium amino-tris-(methylenephosphonate) and expandable graphite. Polym. Degrad. Stab. 2019, 170, 108997. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y. Synthesis and characterization of polyols and polyurethane roams from PET waste and crude glycerol. J. Polym. Environ. 2014, 22, 318–328. [Google Scholar] [CrossRef]
- Yusuf, A.; Mamza, P.; Ahmed, A.; Agunwa, U. Physico-mechanical properties of rigid polyurethane foams synthesized from modified castor oil polyols. Int. J. Sci. Res. Publ. 2016, 6, 548–556. [Google Scholar]
- Yao, W.; Zhang, D.; Zhang, Y.; Fu, T.; Guan, D.; Dou, Y. Synergistic flame retardant effects of expandable graphite and ammonium polyphosphate in water-blow polyurethane foam. Adv. Mater. Sci. Eng. 2019, 2019, 6921474. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Preparation of flame-retardant rigid polyurethane foams by combining modified melamine-formaldehyde resin and phosphorus flame retardants. ACS Omega 2020, 5, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, B.; Wang, X.; Liu, Y. A phosphorous-based bi-functional flame retardant for rigid polyurethane foam. Polym. Degrad. Stab. 2021, 186, 109516. [Google Scholar] [CrossRef]
- Yurtseven, R. Effects of ammonium polyphosphate/melamine additions on mechanical, thermal and burning properties of rigid polyurethane foams. Acta Phys. Pol. A 2019, 135, 775–777. [Google Scholar] [CrossRef]
- Zhou, S.; Chiang, S.; Xu, J.; Du, H.; Li, B.; Xu, C.; Kang, F. Modeling the in-plane thermal conductivity of a graphite/polymer composite sheet with a very high content of natural flake graphite. Carbon 2012, 50, 5052–5061. [Google Scholar] [CrossRef]
- Acuña, P.; Lin, X.; Calvo, M.S.; Shao, Z.; Pérez, N.; Villafañe, F.; Rodríguez-Pérez, M.Á.; Wang, D.-Y. Synergistic effect of expandable graphite and phenylphosphonic-aniline salt on flame retardancy of rigid polyurethane foam. Polym. Degrad. Stab. 2020, 179, 109274. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Han, L.; Yuan, W.; Cheng, J.; Zhang, A.; Zhao, H.; Wang, Y. Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef]
- Cao, Z.J.; Liao, W.; Wang, S.X.; Zhao, H.B.; Wang, Y.Z. Polyurethane foams with functionalized graphene towards high fire-resistance, low smoke release, superior thermal insulation. Chem. Egn. J. 2019, 361, 1245–1254. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhao, H.B.; Rao, W.H.; Huang, S.C.; Wang, T.; Liao, W.; Wang, Y.Z. Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer 2018, 153, 616–625. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z. Synergistic effect of nano magnesium amino-tris-(methylenephosphonate) and expandable graphite on improving flame retardant, mechanical and thermal insulating properties of rigid polyurethane foam. Mater. Chem. Phys. 2018, 219, 318–327. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Cheng, J.; Ma, Q.; Zhang, F.; Wang, Y.; Liu, M.; Wang, D.; Qu, W. Mechanical property improvement and fire hazard reduction of ammonium polyphosphate microencapsulated in rigid polyurethane foam. J. Appl. Polym. 2020, 137, 48307. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, G.; Xu, W. Roles of organically-modified montmorillonite and phosphorous flame retardant during the combustion of rigid polyurethane foam. Polym. Degrad. Stab. 2014, 101, 32–39. [Google Scholar] [CrossRef]
- Khaleel, M.; Soykan, U.; Çetin, S. Influences of turkey feather fiber loading on significant characteristics of rigid polyurethane foam: Thermal degradation, heat insulation, acoustic performance, air permeability and cellular structure. Constr. Build. Mater. 2021, 308, 125014. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G. Influence of thermal behavior of phosphorus compounds on their flame retardant effect in PU rigid foam. Fire Mater. 2016, 40, 826–835. [Google Scholar] [CrossRef]
- Sun, G.; Duan, T.; Liu, C.; Zhang, L.; Chen, R.; Wang, J.; Han, S. Fabrication of flame-retardant and smoke-suppressant isocyanate-based polyimide foam modified by silica aerogel thermal insulation and flame protection layers. Polym. Test. 2020, 91, 106738. [Google Scholar] [CrossRef]
- Pang, X.Y.; Xin, Y.P.; Shi, X.Z.; Xu, J.Z. Effect of different size-modified expandable graphite and ammonium polyphosphate on the flame retardancy, thermal stability, physical, and mechanical properties of rigid polyurethane foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- Lian, R.; Ou, M.; Guan, H.; Cui, J.; Zhao, Z.; Liu, L.; Chen, X.; Jiao, C. Cu (II) and Co (II) complexes decorated ammonium polyphosphate as co-curing agents on improving fire safety and mechanical properties of epoxy-based building coatings. Constr. Build. Mater. 2023, 389, 131786. [Google Scholar] [CrossRef]
- Lian, R.; Ou, M.; Guan, H.; Cui, J.; Piao, J.; Feng, T.; Ren, J.; Wang, Y.; Wang, Y.; Liu, L. Facile fabrication of multifunctional energy-saving building materials with excellent thermal insulation, robust mechanical property and ultrahigh flame retardancy. Energy 2023, 277, 127773. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Chen, L.; Shi, H.; Hao, J. Ammonium polyphosphate modified with β-cyclodextrin crosslinking rigid polyurethane foam: Enhancing thermal stability and suppressing flame spread. Polym. Degrad. Stab. 2019, 161, 166–174. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Gama, N.; Costa, L.C.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Insights into the physical properties of biobased polyurethane/expanded graphite composite foams. Compos. Sci. Technol. 2017, 138, 24–31. [Google Scholar] [CrossRef]
- Harikrishnan, G.; Singh, S.N.; Kiesel, E.; Macosko, C.W. Nanodispersions of carbon nanofiber for polyurethane foaming. Polymer 2010, 51, 3349–3353. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chen, W.J.; Kuan, C.F.; Kuan, H.C.; Yang, J.M.; Chiang, C.L. Preparation, characterization of microencapsulated ammonium polyphosphate and its flame retardancy in polyurethane composites. Mater. Chem. Phys. 2016, 173, 205–212. [Google Scholar] [CrossRef]
- Wang, B.B.; Qian, X.D.; Shi, Y.Q.; Yu, B.; Hong, N.N.; Song, L.; Hu, Y. Cyclodextrin microencapsulated ammonium polyphosphate: Preparation and its performance on the thermal, flame retardancy and mechanical properties of ethylene vinyl acetate copolymer. Compos. Part. B-Eng. 2015, 69, 22–30. [Google Scholar] [CrossRef]
- Huang, S.C.; Deng, C.; Wang, S.X.; Wei, W.C.; Chen, H.; Wang, Y.Z. Electrostatic action induced interfacial accumulation of layered double hydroxides towards highly efficient flame retardance and mechanical enhancement of thermoplastic polyurethane/ammonium polyphosphate. Polym. Degrad. Stab. 2019, 165, 126–136. [Google Scholar] [CrossRef]


| Materials | 4110 (g) | MDI (g) | AK158 (g) | A33 (g) | H2O (g) | 141b (g) |
|---|---|---|---|---|---|---|
| RPUF | 100 | 130 | 6 | 0.8 | 2 | 10 |
| Samples Units | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | TSP (m2) | MRC (wt.%) |
|---|---|---|---|---|---|
| RPUF | 2 | 256 | 42.9 | 4.58 | 0.23 |
| SA/RPUF | 2 | 276 | 42.3 | 4.74 | 0.24 |
| 8EG/SA/RPUF | 7 | 340 | 39.9 | 2.89 | 7.24 |
| 8APP/SA/RPUF | 5 | 464 | 32.1 | 7.37 | 4.35 |
| 2EG/6APP/SA/RPUF | 6 | 392 | 36.4 | 6.20 | 4.92 |
| 4EG/4APP/SA/RPUF | 7 | 365 | 37.6 | 4.23 | 6.06 |
| 6EG/2APP/SA/RPUF | 7 | 311 | 39.0 | 3.78 | 6.85 |
| Materials | Desired RPUF Performance | ||||||
|---|---|---|---|---|---|---|---|
| Low Thermal Conductivity | High Strength | High Flame Retardancy | |||||
| Low Heat Release | Low Smoke Production | High Residual Mass | High LOI | ||||
| <300 °C | >300 °C | ||||||
| APP | / | / | - - | + + | - - | + | + |
| EG | - - | - - | + | / | + + | + + | + |
| SA | + + | + + | / | / | / | / | / |
| EG/APP/SA | + | + | / | + | + | + + | + + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hou, L.; Liu, Y.; Yao, Z.; Liang, L.; Tian, D.; Liu, C.; Xue, J.; Zhan, L.; Liu, Y.; et al. Balanced Thermal Insulation, Flame-Retardant and Mechanical Properties of PU Foam Constructed via Cost-Effective EG/APP/SA Ternary Synergistic Modification. Polymers 2024, 16, 330. https://doi.org/10.3390/polym16030330
Li H, Hou L, Liu Y, Yao Z, Liang L, Tian D, Liu C, Xue J, Zhan L, Liu Y, et al. Balanced Thermal Insulation, Flame-Retardant and Mechanical Properties of PU Foam Constructed via Cost-Effective EG/APP/SA Ternary Synergistic Modification. Polymers. 2024; 16(3):330. https://doi.org/10.3390/polym16030330
Chicago/Turabian StyleLi, Hongfu, Longtao Hou, Yunpeng Liu, Zhiyu Yao, Lixing Liang, Dangxin Tian, Chunhui Liu, Junqiang Xue, Linshan Zhan, Yongqi Liu, and et al. 2024. "Balanced Thermal Insulation, Flame-Retardant and Mechanical Properties of PU Foam Constructed via Cost-Effective EG/APP/SA Ternary Synergistic Modification" Polymers 16, no. 3: 330. https://doi.org/10.3390/polym16030330
APA StyleLi, H., Hou, L., Liu, Y., Yao, Z., Liang, L., Tian, D., Liu, C., Xue, J., Zhan, L., Liu, Y., Zhen, Z., & Niu, K. (2024). Balanced Thermal Insulation, Flame-Retardant and Mechanical Properties of PU Foam Constructed via Cost-Effective EG/APP/SA Ternary Synergistic Modification. Polymers, 16(3), 330. https://doi.org/10.3390/polym16030330






