Thermal Resistance Enhancement and Wettability Amelioration of Poly(benzimidazole-aramid) Film by Silica Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
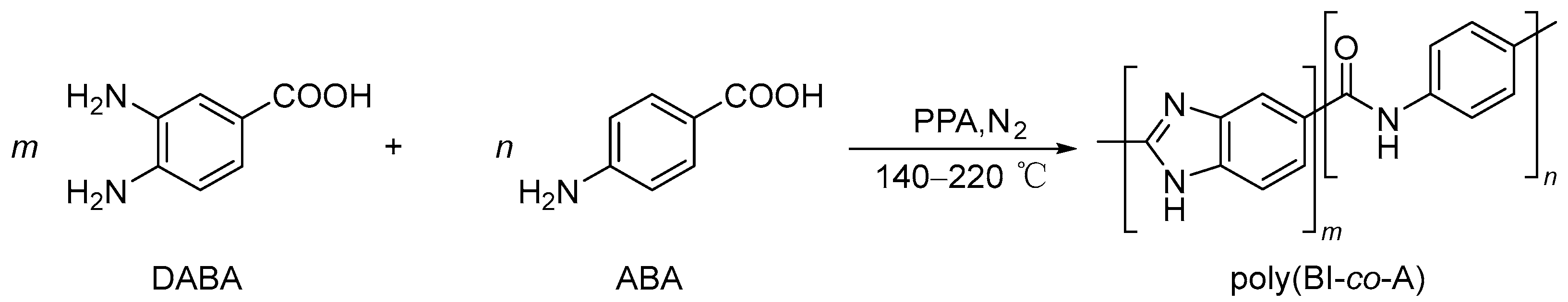
2.3. Synthesis of Poly(benzimidazole-co-aramid)
2.4. Fabrication of Pure Poly(BI-co-A) Film
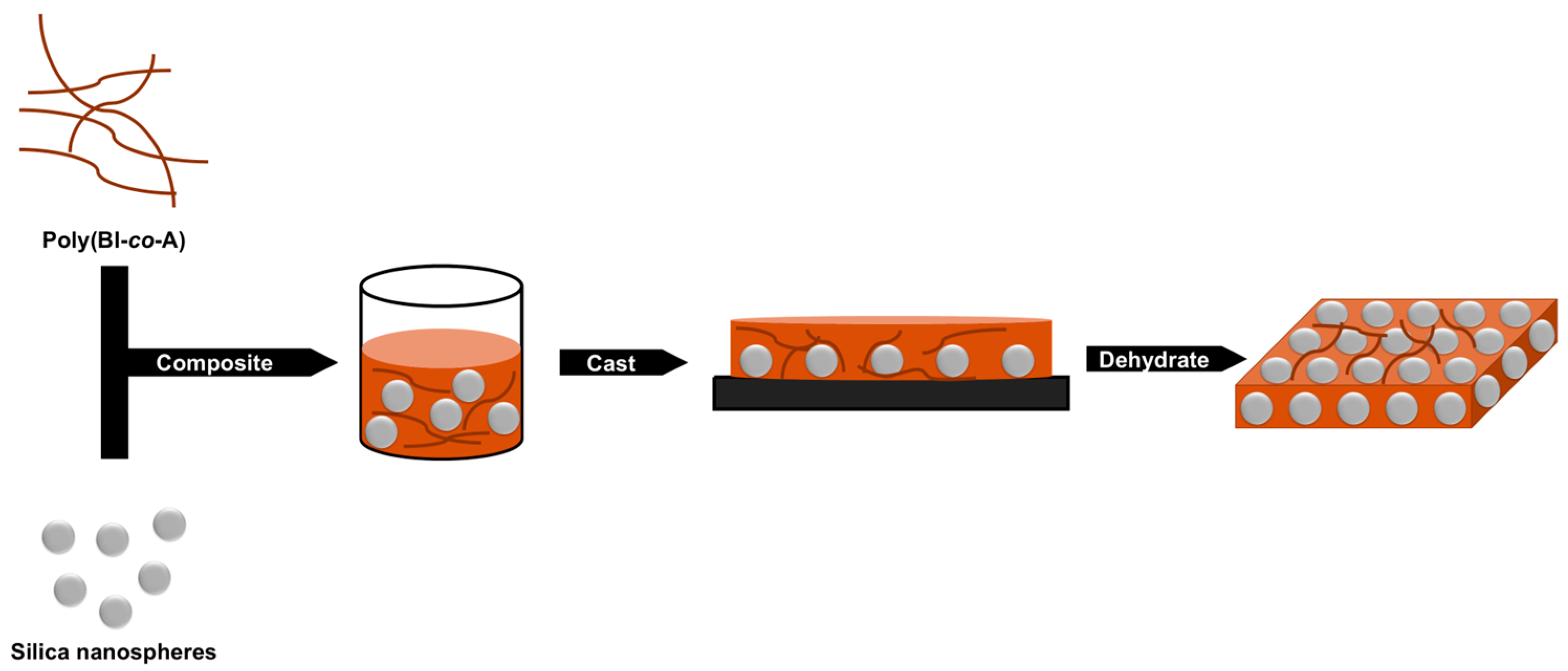
2.5. Fabrication of Silica Nanocomposite Poly(BI-co-A) Films
2.6. Dehydration Treatment
3. Results and Discussion
3.1. FT-IR Spectra
3.2. SEM Morphologies
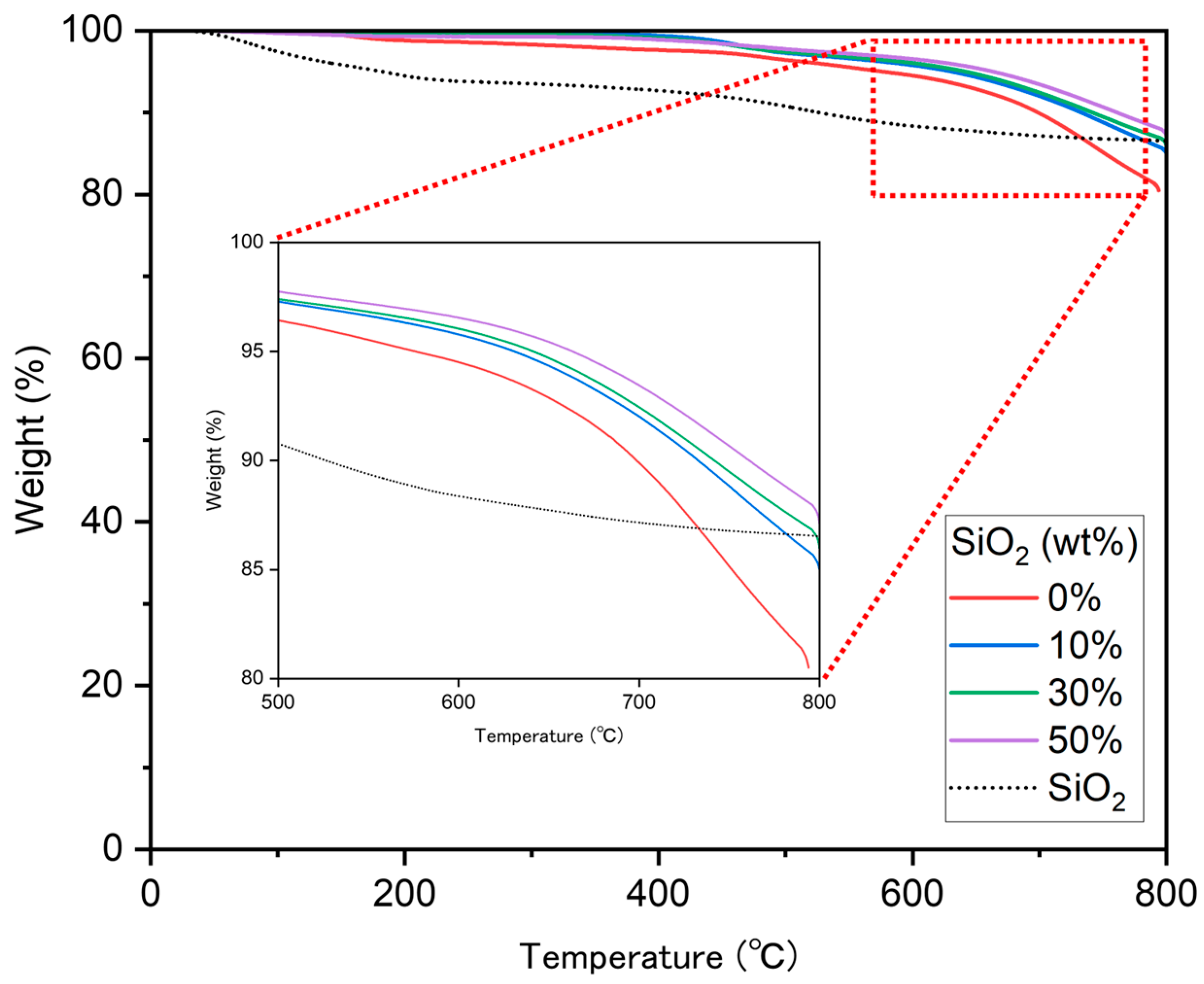
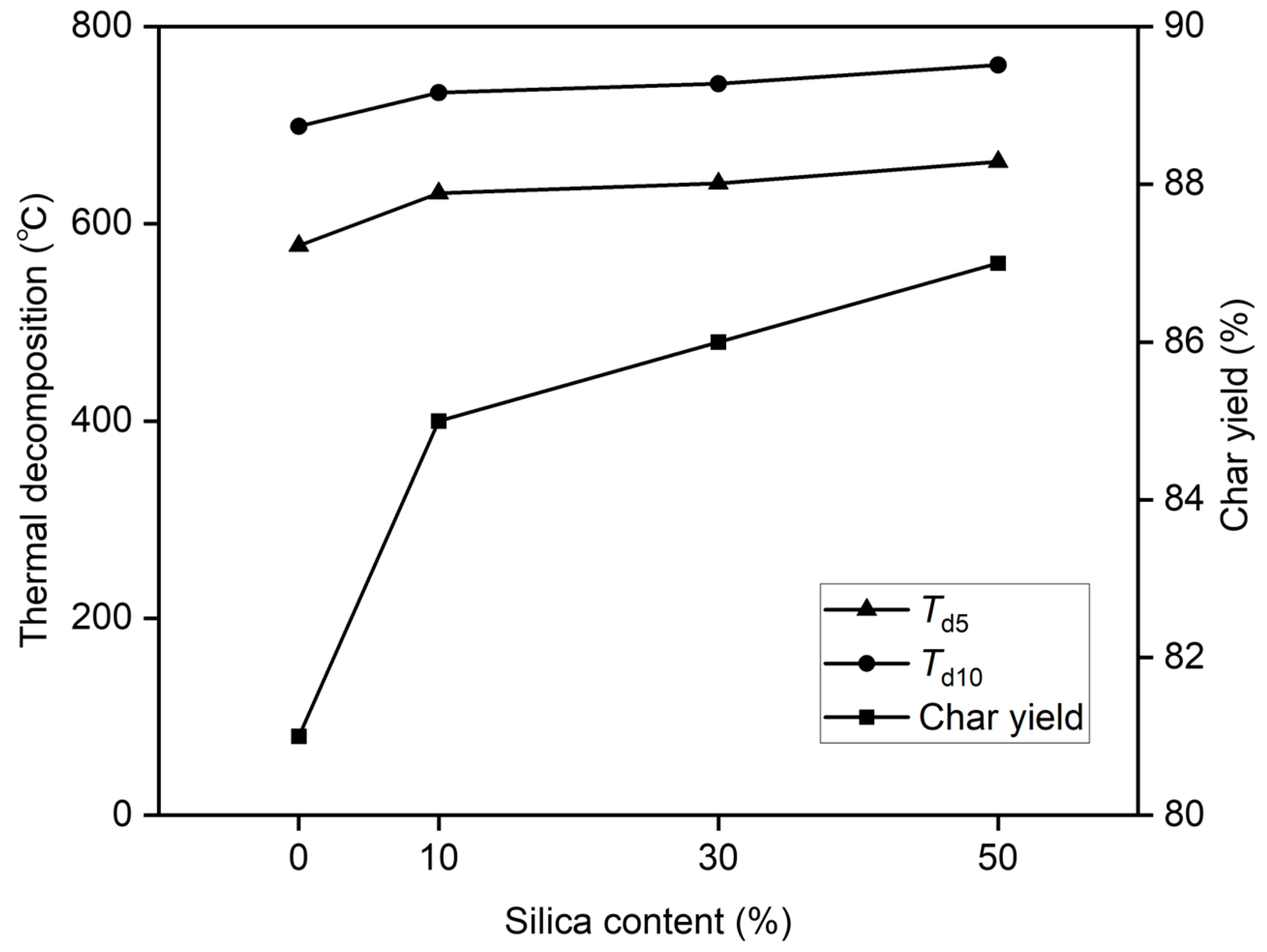
3.3. TGA
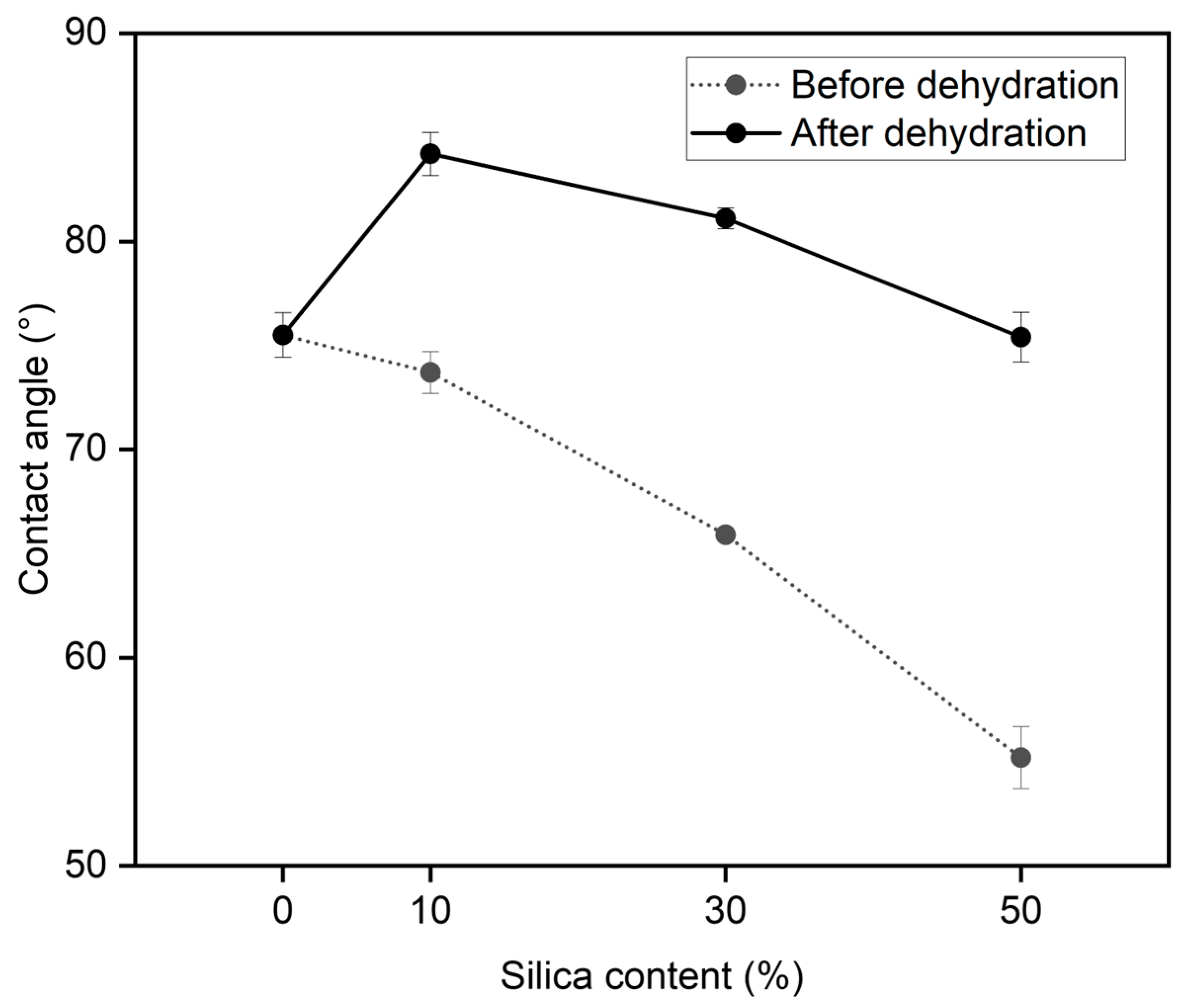
3.4. Contact Angle Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Zhao, H.-Y.; Shi, R.; Lin, W.-F.; Jia, X.-M.; Qian, H.-J.; Lu, Z.-Y.; Zhang, X.-X.; Li, Y.-K.; Sun, Z.-Y. An Unexpected N-Dependence in the Viscosity Reduction in All-Polymer Nanocomposite. Nat. Commun. 2019, 10, 5552. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Polymers and Nanotechnology for Industry 4.0. Polymers 2023, 15, 3556. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Robeson, L.M. Polymer Nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Sun, L.; Liang, T.; Zhang, C.; Chen, J. The rheological performance of shear-thickening fluids based on carbon fiber and silica nanocomposite. Phys. Fluids 2023, 35, 032002. [Google Scholar] [CrossRef]
- Marconnet, A.M.; Yamamoto, N.; Panzer, M.A.; Wardle, B.L.; Goodson, K.E. Thermal Conduction in Aligned Carbon Nanotube–Polymer Nanocomposites with High Packing Density. ACS Nano 2011, 5, 4818–4825. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Cheng, Y.; Zhang, Y. A Molecular Dynamics Study on Thermal and Mechanical Properties of Graphene–Paraffin Nanocomposites. RSC Adv. 2015, 5, 82638–82644. [Google Scholar] [CrossRef]
- Czel, G.; Sycheva, A.; Janovszky, D. Effect of Different Fillers on Thermal Conductivity, Tribological Properties of Polyamide 6. Sci. Rep. 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, B.; Wang, J.; Li, S.; Zhen, X.; Zhi, J.; Zou, J.; Li, B.; Shen, Z.; Zhang, X.; et al. High-Temperature Capacitive Energy Storage in Polymer Nanocomposites through Nanoconfinement. Nat. Commun. 2024, 15, 6655. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, Y.; Zhou, D.; Li, D.; Pang, L.; Liu, W.; Su, J.; Shi, Z.; Sun, S. High-Temperature Flexible Nanocomposites with Ultra-High Energy Storage Density by Nanostructured MgO Fillers. Adv. Funct. Mater. 2022, 32, 202204155. [Google Scholar] [CrossRef]
- Faiza; Khattak, A.; Alahmadi, A.A.; Ishida, H.; Ullah, N. Improved PVC/ZnO Nanocomposite Insulation for High Voltage and High Temperature Applications. Sci. Rep. 2023, 13, 7235. [Google Scholar] [CrossRef] [PubMed]
- Haruna, M.A.; Wen, D. Stabilization of Polymer Nanocomposites in High-Temperature and High-Salinity Brines. ACS Omega 2019, 4, 11631–11641. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Baleizão, C.; Farinha, J. Functional Films from Silica/Polymer Nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, J.; Hall, A.J.; Haupt, K.; Tse Sum Bui, B. Water-compatible Silica Sol–Gel Molecularly Imprinted Polymer as a Potential Delivery System for the Controlled Release of Salicylic Acid. J. Mol. Recognit. 2014, 27, 559–565. [Google Scholar] [CrossRef]
- Kontou, E.; Christopoulos, A.; Koralli, P.; Mouzakis, D.E. The Effect of Silica Particle Size on the Mechanical Enhancement of Polymer Nanocomposites. Nanomaterials 2023, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Duan, R. Leak-Proof Reversible Thermochromic Microcapsule Phase Change Materials with High Latent Thermal Storage for Thermal Management. ACS Appl. Energy Mater. 2024, 7, 5944–5956. [Google Scholar] [CrossRef]
- Fan, W.; Du, T.; Droce, A.; Jensen, L.R.; Youngman, R.E.; Ren, X.; Gurevich, L.; Bauchy, M.; Kristensen, P.; Xing, B.; et al. Resolving the Conflict between Strength and Toughness in Bioactive Silica-Polymer Hybrid Materials. ACS Nano 2022, 16, 9748–9761. [Google Scholar] [CrossRef]
- Sorkin, V.; Pei, Q.X.; Liu, P.; Thitsartarn, W.; He, C.B.; Zhang, Y.W. Atomistic-Scale Analysis of the Deformation and Failure of Polypropylene Composites Reinforced by Functionalized Silica Nanoparticles. Sci. Rep. 2021, 11, 23108. [Google Scholar] [CrossRef]
- Watanabe, R.; Kunioka, M.; Sato, H.; Mizukado, J.; Hagihara, H. Management of Both Toughness and Stiffness of Polypropylene Nanocomposites Using Poly(5-hexen-1-ol- co -propylene) and Silica Nanospheres. Polym. Adv. Technol. 2018, 29, 417–423. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Zhu, L.; Ye, H.; Liu, H. Polymer–Silica Hybrid Self-Healing Nano/Microcapsules with Enhanced Thermal and Mechanical Stability. RSC Adv. 2019, 9, 1782–1791. [Google Scholar] [CrossRef]
- Zhao, J.; Du, F.; Cui, W.; Zhu, P.; Zhou, X.; Xie, X. Effect of Silica Coating Thickness on the Thermal Conductivity of Polyurethane/SiO2 Coated Multiwalled Carbon Nanotube Composites. Compos. Part A Appl. Sci. Manuf. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Guo, H.; Ge, J.; Wu, H.; Zhang, T.; Zhao, A. Enhancing Temperature Resistance of Polymer Gels by Hybrid with Silica Nanoparticles. Pet. Sci. Technol. 2022, 40, 3037–3059. [Google Scholar] [CrossRef]
- Eslami, R.; Bagheri, R.; Hashemzadeh, Y.; Salehi, M. Optical and Mechanical Properties of Transparent Acrylic Based Polyurethane Nano Silica Composite Coatings. Prog. Org. Coat. 2014, 77, 1184–1190. [Google Scholar] [CrossRef]
- Ernawati, L.; Ogi, T.; Balgis, R.; Okuyama, K.; Stucki, M.; Hess, S.C.; Stark, W.J. Hollow Silica as an Optically Transparent and Thermally Insulating Polymer Additive. Langmuir 2016, 32, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Suthabanditpong, W.; Takai, C.; Fuji, M.; Buntem, R.; Shirai, T. Improved Optical Properties of Silica/UV-Cured Polymer Composite Films Made of Hollow Silica Nanoparticles with a Hierarchical Structure for Light Diffuser Film Applications. Phys. Chem. Chem. Phys. 2016, 18, 16293–16301. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Brajović, L.; Orlović, A.; Dramlić, D.; Radmilović, V.; Uskoković, P.S.; Aleksić, R. Transparent PMMA/Silica Nanocomposites Containing Silica Nanoparticles Coating under Supercritical Conditions. Prog. Org. Coat. 2013, 76, 626–631. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hong, Y.; Cheng, L.; Li, Z. Bonding Strength and Water Resistance of Starch-Based Wood Adhesive Improved by Silica Nanoparticles. Carbohydr. Polym. 2011, 86, 72–76. [Google Scholar] [CrossRef]
- Pingan, H.; Mengjun, J.; Yanyan, Z.; Ling, H. A Silica/PVA Adhesive Hybrid Material with High Transparency, Thermostability and Mechanical Strength. RSC Adv. 2017, 7, 2450–2459. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, J.; Wu, X. Study on the Silica-Polymer Hybrid Coated SrAl2O4:Eu2+, Dy3+ Phosphor as a Photoluminescence Pigment in a Waterborne UV Acrylic Coating. J. Mater. Res. Technol. 2021, 13, 1230–1242. [Google Scholar] [CrossRef]
- Jean-Fulcrand, A.; Masen, M.A.; Bremner, T.; Wong, J.S.S. High Temperature Tribological Properties of Polybenzimidazole (PBI). Polymer 2017, 128, 159–168. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, J.; Byun, J.J.; Perez–Page, M.; Ji, Z.; Zhao, Z.; Holmes, S.M. Insights into the Performance and Degradation of Polybenzimidazole/Muscovite Composite Membranes in High–Temperature Proton Exchange Membrane Fuel Cells. J. Memb. Sci. 2022, 641, 119868. [Google Scholar] [CrossRef]
- Paglia, L.; Genova, V.; Bracciale, M.P.; Bartuli, C.; Marra, F.; Natali, M.; Pulci, G. Thermochemical Characterization of Polybenzimidazole with and without Nano-ZrO2 for Ablative Materials Application. J. Therm. Anal. Calorim. 2020, 142, 2149–2161. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, M.; Zhao, Y.; Qiu, Y. Theoretical Studies on the Chemical Degradation and Proton Dissociation Property of PBI Used in High-Temperature Polymer Electrolyte Membrane Fuel Cells. J. Phys. Chem. B 2024, 128, 6167–6177. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Takada, K.; Mori, Y.; Ali, M.A.; Kaneko, T. Syntheses of Organic Solvent-Soluble Polybenzimidazole Derivatives with a Guanidinoid Structure. Macromolecules 2024, 57, 3328–3337. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Sasaki, K.; Uematsu, K.; Tsuge, Y.; Teramura, H.; Okai, N.; Nakamura-Tsuruta, S.; Katsuyama, Y.; Sugai, Y.; Ohnishi, Y.; et al. 3-Amino-4-Hydroxybenzoic Acid Production from Sweet Sorghum Juice by Recombinant Corynebacterium Glutamicum. Bioresour. Technol. 2015, 198, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watanabe, A.; Suda, M.; Kogure, T.; Hiraga, K.; Inui, M. Production of Para-Aminobenzoate by Genetically Engineered Corynebacterium Glutamicum and Non-Biological Formation of an N-Glucosyl Byproduct. Metab. Eng. 2016, 38, 322–330. [Google Scholar] [CrossRef]
- Wan, Q.; Ramsey, C.; Baran, G. Thermal Pretreatment of Silica Composite Filler Materials. J. Therm. Anal. Calorim. 2010, 99, 237–243. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, S.K. Atomic Structure and Dehydration Mechanism of Amorphous Silica: Insights from 29Si and 1H Solid-State MAS NMR Study of SiO2 Nanoparticles. Geochim. Cosmochim. Acta 2013, 120, 39–64. [Google Scholar] [CrossRef]
- Nag, A.; Ali, M.A.; Kawaguchi, H.; Saito, S.; Kawasaki, Y.; Miyazaki, S.; Kawamoto, H.; Adi, D.T.N.; Yoshihara, K.; Masuo, S.; et al. Ultrahigh Thermoresistant Lightweight Bioplastics Developed from Fermentation Products of Cellulosic Feedstock. Adv. Sustain. Syst. 2021, 5, 2000193. [Google Scholar] [CrossRef]
- Liu, H.; Kaya, H.; Lin, Y.; Ogrinc, A.; Kim, S.H. Vibrational Spectroscopy Analysis of Silica and Silicate Glass Networks. J. Am. Ceram. Soc. 2022, 105, 2355–2384. [Google Scholar] [CrossRef]
- Uchino, T.; Sakka, T.; Lwasaki, M. Interpretation of Hydrated States of Sodium Silicate Glasses by Infrared and Raman Analysis. J. Am. Cerarn. Soc. 1991, 74, 306–319. [Google Scholar] [CrossRef]


| Element | Line Type | Weight % | Atomic % |
|---|---|---|---|
| C | K series | 44.24 | 68.20 |
| N | K series | 6.71 | 8.87 |
| O | K series | 11.28 | 13.05 |
| Si | K series | 11.21 | 7.39 |
| Au | M series | 26.56 | 2.50 |
| Total | 100.00 | 100.00 |
| Films | Td5 (°C) | Td10 (°C) | Char Yield (%) |
|---|---|---|---|
| poly(BI-co-A)-0% | 578 | 699 | 81 |
| poly(BI-co-A)-10% | 631 | 733 | 85 |
| poly(BI-co-A)-30% | 641 | 742 | 86 |
| poly(BI-co-A)-50% | 663 | 761 | 87 |
| Films | Contact Angle (°) | |
|---|---|---|
| poly(BI-co-A)-0% | 75.5 ± 1.1 | |
| Before dehydration | After dehydration | |
| poly(BI-co-A)-10% | 73.7 ± 1.0 | 84.2 ± 1.0 |
| poly(BI-co-A)-30% | 65.9 ± 0.3 | 81.1 ± 0.5 |
| poly(BI-co-A)-50% | 55.2 ± 1.5 | 75.4 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhong, X.; Takada, K.; Yamaguchi, M.; Kaneko, T. Thermal Resistance Enhancement and Wettability Amelioration of Poly(benzimidazole-aramid) Film by Silica Nanocomposites. Polymers 2024, 16, 3563. https://doi.org/10.3390/polym16243563
Zhou J, Zhong X, Takada K, Yamaguchi M, Kaneko T. Thermal Resistance Enhancement and Wettability Amelioration of Poly(benzimidazole-aramid) Film by Silica Nanocomposites. Polymers. 2024; 16(24):3563. https://doi.org/10.3390/polym16243563
Chicago/Turabian StyleZhou, Jiabei, Xianzhu Zhong, Kenji Takada, Masayuki Yamaguchi, and Tatsuo Kaneko. 2024. "Thermal Resistance Enhancement and Wettability Amelioration of Poly(benzimidazole-aramid) Film by Silica Nanocomposites" Polymers 16, no. 24: 3563. https://doi.org/10.3390/polym16243563
APA StyleZhou, J., Zhong, X., Takada, K., Yamaguchi, M., & Kaneko, T. (2024). Thermal Resistance Enhancement and Wettability Amelioration of Poly(benzimidazole-aramid) Film by Silica Nanocomposites. Polymers, 16(24), 3563. https://doi.org/10.3390/polym16243563








