Exploring the Origins of Low-Temperature Thermochromism in Polydiacetylenes
Abstract
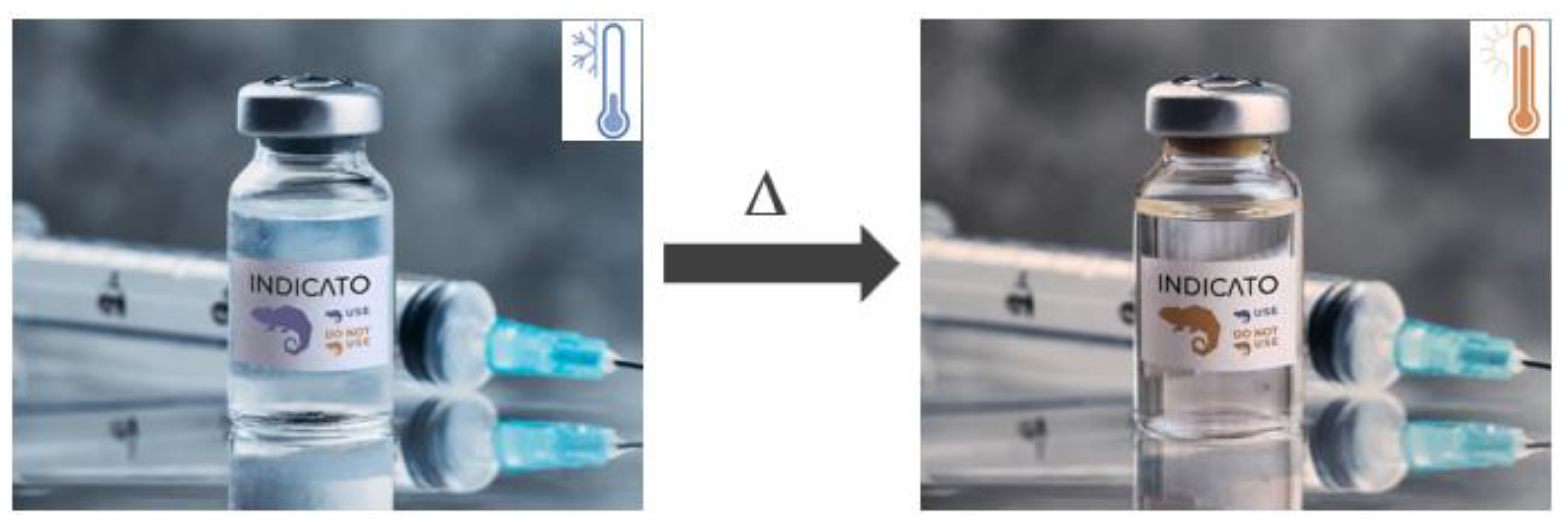
1. Introduction
2. Selection Criteria for Polydiacetylenes
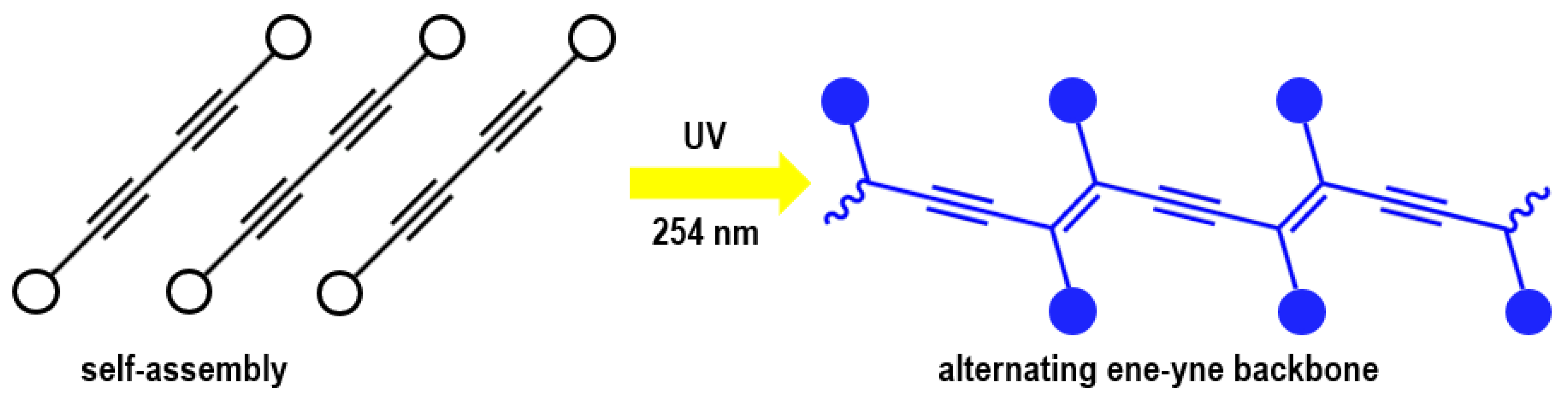
3. PDAs
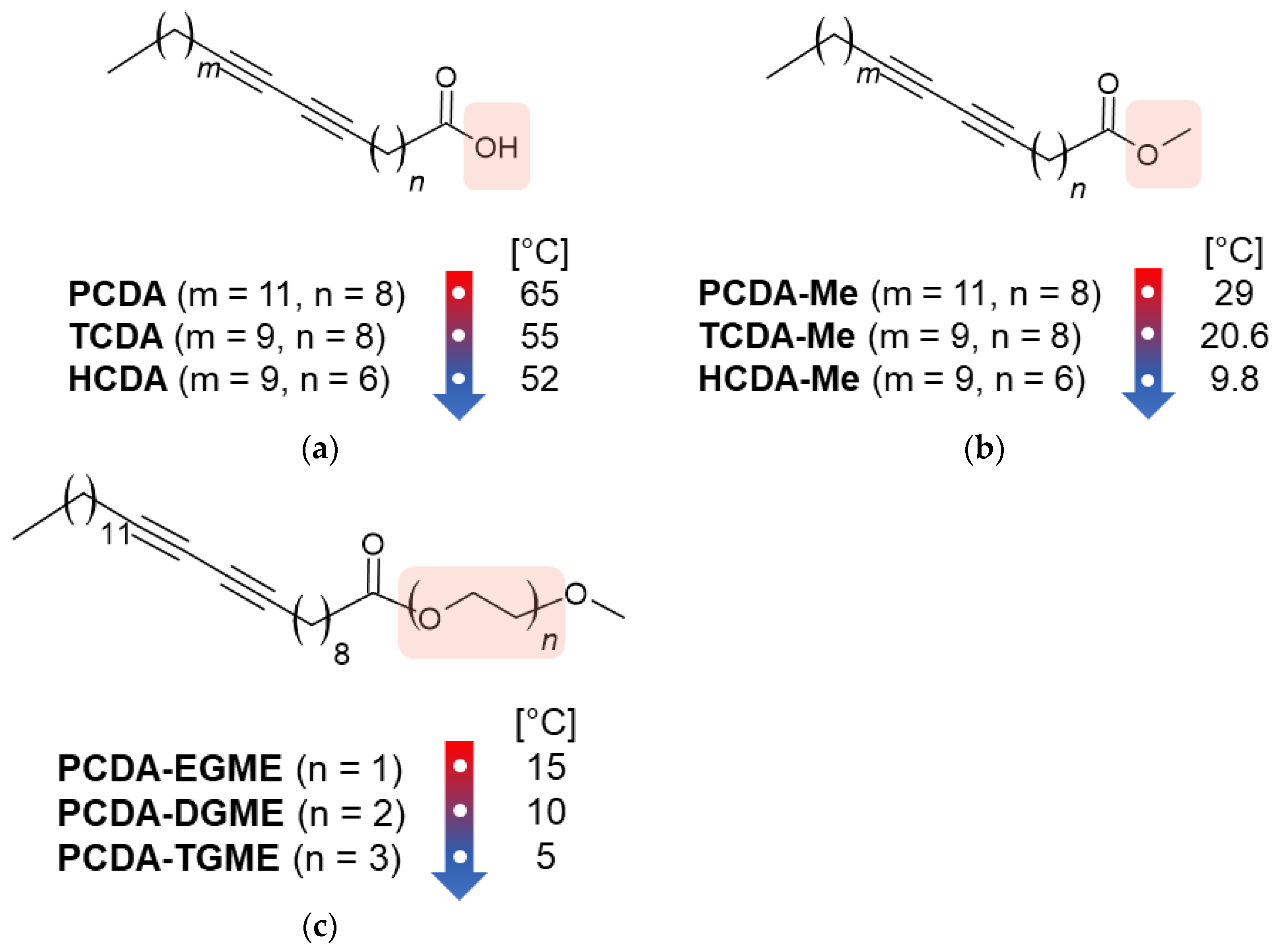
4. Influence of Side-Chain Length
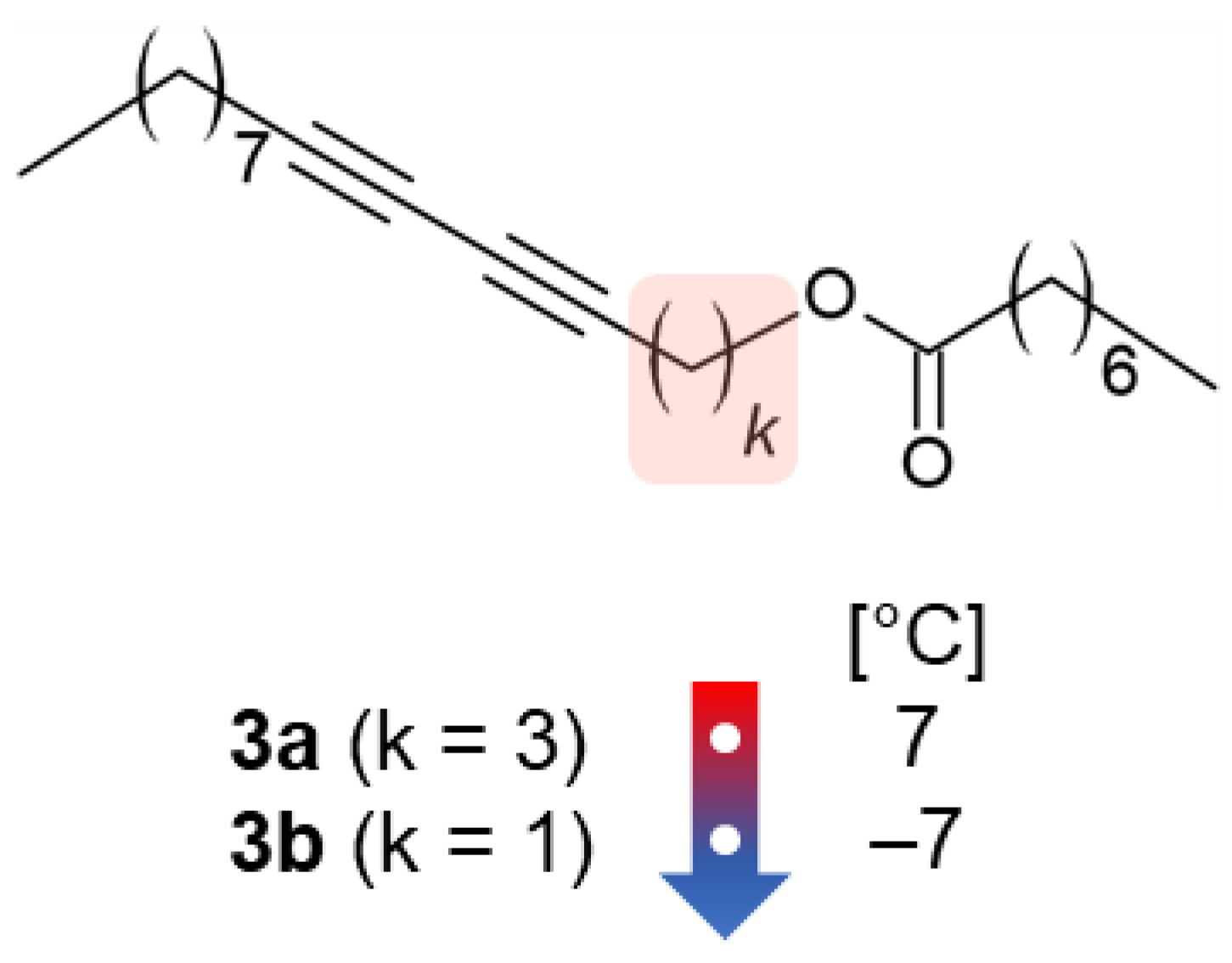
5. Influence of Methylene Groups Close to DA Moiety
6. Influence of Hydrogen-Bonding Interactions
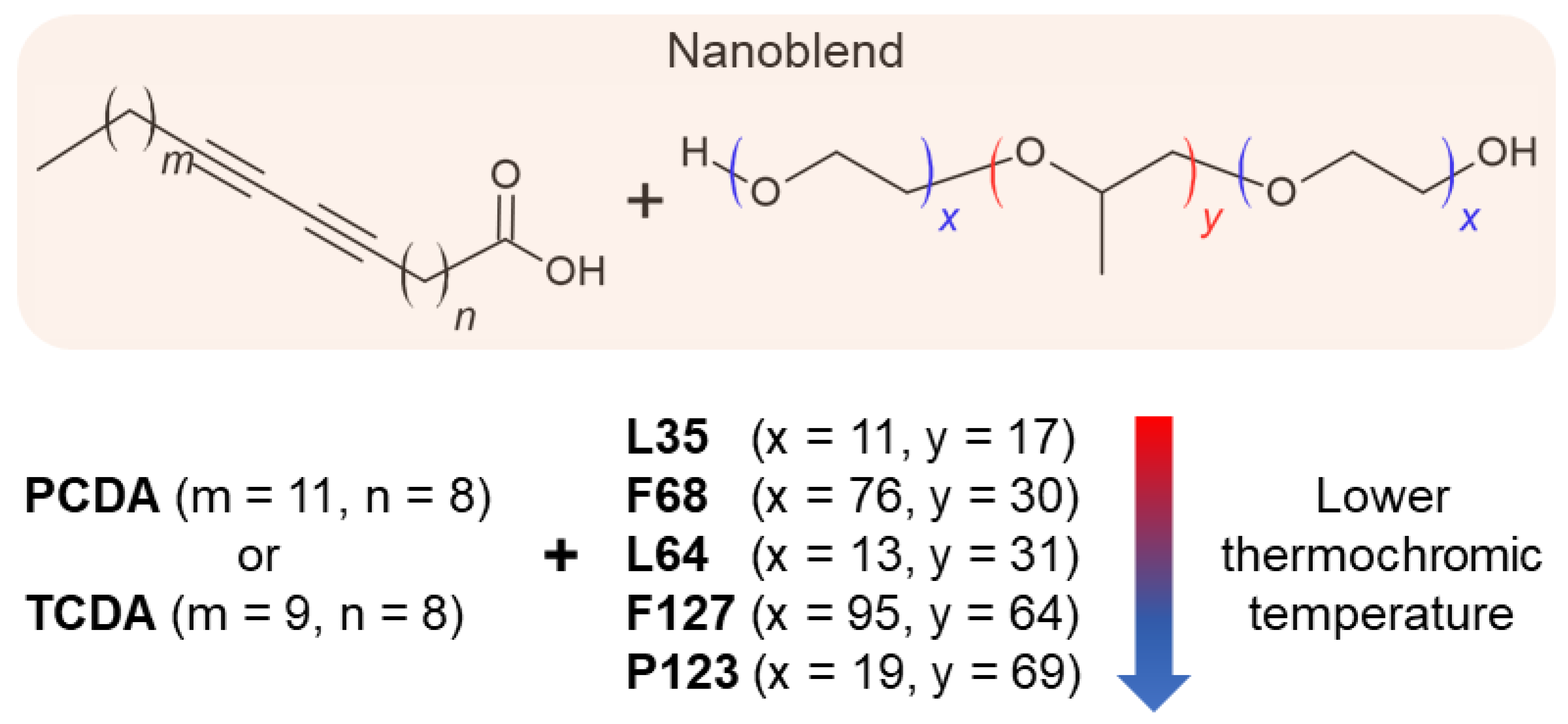
7. Influence of Copolymer
8. Applications of PDAs
9. Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Houston, E.J. On the Change of Color Produced in Certain Chemical Compounds by Heat. J. Frankl. Inst. 1871, 92, 115–127. [Google Scholar] [CrossRef]
- Hakami, A.; Srinivasan, S.S.; Biswas, P.K.; Krishnegowda, A.; Wallen, S.L.; Stefanakos, E.K. Review on Thermochromic Materials: Development, Characterization, and Applications. J. Coat. Technol. Res. 2022, 19, 377–402. [Google Scholar] [CrossRef]
- Sone, K.; Fukuda, Y. Inorganic Thermochromism; Inorganic Chemistry Concepts; Springer: Berlin/Heidelberg, Germany, 1987; Volume 10, ISBN 978-3-642-51019-9. [Google Scholar]
- Crano, J.C.; Guglielmetti, R.J. (Eds.) Organic Photochromic and Thermochromic Compounds: Volume 2: Physicochemical Studies, Biological Applications, and Thermochromism, 1st ed.; Topics in Applied Chemistry; Kluwer Academic Publishers: Boston, UK, 2002; ISBN 0-306-46912-X. [Google Scholar]
- Jia, Q.-Q.; Luo, Q.-F.; Ni, H.-F.; Su, C.-Y.; Fu, D.-W.; Xie, L.-Y.; Lu, H.-F. High-Sensitivity Organic–Inorganic Hybrid Materials with Reversible Thermochromic Property and Dielectric Switching. J. Phys. Chem. C 2022, 126, 1552–1557. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic Polymers—Function by Design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef] [PubMed]
- Strahl, W. Sympathetic Ink. U.S. Patent 3926645A, 16 December 1975. [Google Scholar]
- Aitken, D.; Burkinshaw, S.M.; Griffiths, J.; Towns, A.D. Textile Applications of Thennochromic Systems. Rev. Prog. Color. Relat. Top. 1996, 26, 1–8. [Google Scholar] [CrossRef]
- Bide, M. Hot Shirts. ChemMatters 1992, 8–11. [Google Scholar]
- Supian, A.B.M.; Asyraf, M.R.M.; Syamsir, A.; Najeeb, M.I.; Alhayek, A.; Al-Dala’ien, R.N.; Manar, G.; Atiqah, A. Thermochromic Polymer Nanocomposites for the Heat Detection System: Recent Progress on Properties, Applications, and Challenges. Polymers 2024, 16, 1545. [Google Scholar] [CrossRef]
- Kakade, V.U.; Lock, G.D.; Wilson, M.; Owen, J.M.; Mayhew, J.E. Accurate Heat Transfer Measurements Using Thermochromic Liquid Crystal. Part 1: Calibration and Characteristics of Crystals. Int. J. Heat. Fluid. Flow. 2009, 30, 939–949. [Google Scholar] [CrossRef]
- Lv, L.-Y.; Cao, C.-F.; Qu, Y.-X.; Zhang, G.-D.; Zhao, L.; Cao, K.; Song, P.; Tang, L.-C. Smart Fire-Warning Materials and Sensors: Design Principle, Performances, and Applications. Mater. Sci. Eng. R. Rep. 2022, 150, 100690. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Stasiek, J.; Jewartowski, M.; Aleksander Kowalewski, T. The Use of Liquid Crystal Thermography in Selected Technical and Medical Applications—Recent Development. J. Cryst. Process Technol. 2014, 4, 46–59. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, A. Review of Different Temperatures for Biopreservation. Int. J. Refrig. 2024, 157, 53–59. [Google Scholar] [CrossRef]
- Ahmad, S.A.H.; Ab Rahman, M.N.; Muhamed, A.A. Optimal Temperature in Cold Storage for Perishable Foods. In Proceedings of the 5th NA International Conference on Industrial Engineering and Operations Management, Detroit, MI, USA, 10–14 August 2020; IEOM Society International: Southfield, MI, USA, 2020; pp. 3449–3456. [Google Scholar]
- Paludetti, L.F.; Kelly, A.L.; O’Brien, B.; Jordan, K.; Gleeson, D. The Effect of Different Precooling Rates and Cold Storage on Milk Microbiological Quality and Composition. J. Dairy Sci. 2018, 101, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Rojas, H.E.; Contreras, R.L.; Schnake, F.G.; Melín, P.; Marín, P.G.L.; Castillo, P.B. Factors Determining Meat Quality and Cold Preservation Methods to Extend Shelf Life. Open Access J. Biomed. Sci. 2022, 4, 1459–1470. [Google Scholar] [CrossRef]
- Suh, S.; Kim, Y.E.; Shin, D.; Ko, S. Effect of Frozen-Storage Period on Quality of American Sirloin and Mackerel (Scomber japonicus). Food Sci. Biotechnol. 2017, 26, 1077–1084. [Google Scholar] [CrossRef]
- Alsailawi, H.A.; Mudhafar, M.; Abdulrasool, M.M. Effect of Frozen Storage on the Quality of Frozen Foods—A Review. J. Chem. Chem. Eng. 2020, 14, 86–89. [Google Scholar] [CrossRef]
- Buyck, J.R.; Baer, R.J.; Choi, J. Effect of Storage Temperature on Quality of Light and Full-Fat Ice Cream. J. Dairy Sci. 2011, 94, 2213–2219. [Google Scholar] [CrossRef]
- Geeraedts, F.; Saluja, V.; ter Veer, W.; Amorij, J.-P.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.L.J.; Huckriede, A. Preservation of the Immunogenicity of Dry-Powder Influenza H5N1 Whole Inactivated Virus Vaccine at Elevated Storage Temperatures. AAPS J. 2010, 12, 215–222. [Google Scholar] [CrossRef]
- Currie, L.M.; Harper, J.R.; Allan, H.; Connor, J. Inhibition of Cytokine Accumulation and Bacterial Growth during Storage of Platelet Concentrates at 4 °C with Retention of in Vitro Functional Activity. Transfusion 1997, 37, 18–24. [Google Scholar] [CrossRef]
- Khurana, G.; Gupta, V. Effect on Insulin upon Storage in Extreme Climatic Conditions (Temperature and Pressure) and Their Preventive Measures. J. Soc. Health Diabetes 2019, 07, 006–010. [Google Scholar] [CrossRef]
- Li, X.; An, J.; Dong, Z.J. Stability and Storage Conditions of Refrigerated and Frozen Drugs Commonly Used in Hospitals. China Pharm. 2016, 27, 983–986. [Google Scholar] [CrossRef]
- Cordsmeier, L.; Hahn, M.B. DNA Stability in Biodosimetry, Pharmacy and DNA Based Data-Storage: Optimal Storage and Handling Conditions. ChemBioChem 2022, 23, e202200391. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Ang, H.C.; Hoe, S.Y.; Lim, M.-L.; Tai, H.E.; Soh, R.C.H.; Syn, C.K.-C. Simple DNA Extraction of Urine Samples: Effects of Storage Temperature and Storage Time. Forensic Sci. Int. 2018, 287, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Shokri, S.; Shahkarami, M.K.; Shafyi, A.; Mohammadi, A.; Esna-ashari, F.; Hamta, A. Evaluation of the Thermal Stability of Live-Attenuated Rubella Vaccine (Takahashi strain) Formulated and Lyophilized in Different Stabilizers. J. Virol. Methods 2019, 264, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Long, X.F.; Liang, J.Y. Cryopreservation of Rare Blood Group Erythrocytes and Detection of Resuscitation Function. China Med. Eng. 2014, 22, 70–71. [Google Scholar]
- Sadoh, A.; Hossain, S.; Ravindra, N.M. Thermochromic Polymeric Films for Applications in Active Intelligent Packaging—An Overview. Micromachines 2021, 12, 1193. [Google Scholar] [CrossRef]
- Hossain, S.; Sadoh, A.; Ravindra, N.M. Principles, Properties and Preparation of Thermochromic Materials. Mater. Sci. Eng. Int. J. 2023, 7, 146–156. [Google Scholar] [CrossRef]
- White, M.A.; LeBlanc, M. Thermochromism in Commercial Products. J. Chem. Educ. 1999, 76, 1201. [Google Scholar] [CrossRef]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; van Bommel, M.R. Parameters That Affect the Photodegradation of Dyes and Pigments in Solution and on Substrate—An Overview. Dyes Pigment. 2023, 210, 110999. [Google Scholar] [CrossRef]
- Sharifi, M.; Sharifi, A.; Abaee, M.S.; Mirzaei, M. A Review on Fluoran Compounds as Widely Used Leuco Dyes. Dyes Pigment. 2024, 221, 111783. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zheng, X.; Li, Y.; Luan, J. Microcapsule Encapsulated with Leuco Dye as a Visual Sensor for Concrete Damage Indication via Color Variation. RSC Adv. 2020, 10, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.T.; Roper, J.M.; Tsutsui, H. Polydiacetylene Supramolecules: Synthesis, Characterization, and Emerging Applications. Ind. Eng. Chem. Res. 2018, 57, 9037–9053. [Google Scholar] [CrossRef]
- Jelinek, R.; Ritenberg, M. Polydiacetylenes—Recent Molecular Advances and Applications. RSC Adv. 2013, 3, 21192. [Google Scholar] [CrossRef]
- Luo, C.-C.; Wang, X.-J.; Han, L.-J.; Jia, Y.-G.; Ying, S.-M.; Wang, J.-W. Preparation, Structure and Optical Properties of Thermochromic Liquid Crystal Compounds Containing (−)-Menthyl with Selective Reflection. J. Mol. Liq. 2019, 275, 241–250. [Google Scholar] [CrossRef]
- Yu, H.; Wei, Z.; Hao, Y.; Liang, Z.; Fu, Z.; Cai, H. Reversible Solid-State Thermochromism of a 2D Organic–Inorganic Hybrid Perovskite Structure Based on Iodoplumbate and 2-Aminomethyl-Pyridine. New J. Chem. 2017, 41, 9586–9589. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Dong, N.; Li, Z. Fabrication and Characterization of Reversible Thermochromic Wood Veneers. Sci. Rep. 2017, 7, 16933. [Google Scholar] [CrossRef]
- Saenjaiban, A.; Singtisan, T.; Suppakul, P.; Jantanasakulwong, K.; Punyodom, W.; Rachtanapun, P. Novel Color Change Film as a Time–Temperature Indicator Using Polydiacetylene/Silver Nanoparticles Embedded in Carboxymethyl Cellulose. Polymers 2020, 12, 2306. [Google Scholar] [CrossRef]
- Kingchok, S.; Nontasorn, P.; Laohhasurayotin, K.; Traiphol, N.; Traiphol, R. Reversible Thermochromic Polydiacetylene/Zinc-Aluminium Layered Double Hydroxides Nanocomposites for Smart Paints and Colorimetric Sensors: The Crucial Role of Zinc Ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125733. [Google Scholar] [CrossRef]
- Fang, F.; Meng, F.; Luo, L. Recent Advances on Polydiacetylene-Based Smart Materials for Biomedical Applications. Mater. Chem. Front. 2020, 4, 1089–1104. [Google Scholar] [CrossRef]
- Wegner, G. Topochemische Reaktionen von Monomeren Mit Konjugierten Dreifachbindungen/Tochemical Reactions of Monomers with Conjugated Triple Bonds. Z. Naturforsch. B 1969, 24, 824–832. [Google Scholar] [CrossRef]
- Hema, K.; Ravi, A.; Raju, C.; Sureshan, K.M. Polymers with Advanced Structural and Supramolecular Features Synthesized through Topochemical Polymerization. Chem. Sci. 2021, 12, 5361–5380. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Städler, B. Recent Developments in Polydiacetylene-Based Sensors. Chem. Mater. 2019, 31, 1196–1222. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, W.; Ai, K.; Liu, J. Highly Sensitive Polydiacetylene Ensembles for Biosensing and Bioimaging. Front. Chem. 2020, 8, 565782. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Jung, S. Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing. Molecules 2018, 23, 107. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D. Thermochromic and Thermotropic Materials, 1st ed.; Seeboth, A., Lötzsch, D., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2013; Volume 114, ISBN 978-981-4411-02-8. [Google Scholar]
- Mergu, N.; Son, Y.-A. Design and Synthesis of Polydiacetylenes, and Their Low Temperature Irreversible Thermochromic Properties. Dyes Pigment. 2021, 184, 108839. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, R.; Lin, G. Molecular-Level Design of Excellent Reversible Thermochromic Polydiacetylene Materials with the Simultaneous Enhancement of Multiple Performances. Mater. Chem. Front. 2021, 5, 7041–7050. [Google Scholar] [CrossRef]
- Ahn, D.J.; Lee, S.; Kim, J. Rational Design of Conjugated Polymer Supramolecules with Tunable Colorimetric Responses. Adv. Funct. Mater. 2009, 19, 1483–1496. [Google Scholar] [CrossRef]
- Foley, J.L.; Li, L.; Sandman, D.J. Polydiacetylenes with Long Wavelength Absorption. Chem. Mater. 1998, 10, 3984–3990. [Google Scholar] [CrossRef]
- Mino, N.; Tamura, H.; Ogawa, K. Analysis of Color Transitions and Changes on Langmuir-Blodgett Films of a Polydiacetylene Derivative. Langmuir 1991, 7, 2336–2341. [Google Scholar] [CrossRef]
- Park, I.S.; Park, H.J.; Jeong, W.; Nam, J.; Kang, Y.; Shin, K.; Chung, H.; Kim, J.-M. Low Temperature Thermochromic Polydiacetylenes: Design, Colorimetric Properties, and Nanofiber Formation. Macromolecules 2016, 49, 1270–1278. [Google Scholar] [CrossRef]
- Ogden, S.; Klintberg, L.; Thornell, G.; Hjort, K.; Bodén, R. Review on Miniaturized Paraffin Phase Change Actuators, Valves, and Pumps. Microfluid. Nanofluidics 2014, 17, 53–71. [Google Scholar] [CrossRef]
- Slovokhotov, Y.L.; Batsanov, A.S.; Howard, J.A.K. Molecular van Der Waals Symmetry Affecting Bulk Properties of Condensed Phases: Melting and Boiling Points. Struct. Chem. 2007, 18, 477–491. [Google Scholar] [CrossRef]
- Rougeau, L.; Picq, D.; Rastello, M.; Frantz, Y. New Irreversible Thermochromic Polydiacetylenes. Tetrahedron 2008, 64, 9430–9436. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Alantary, D. Estimation of Melting Points of Organics. J. Pharm. Sci. 2018, 107, 1211–1227. [Google Scholar] [CrossRef]
- Goyal, S.; Sharma, D.; Kumar, K. Synthesis of Low-Temperature Irreversible Thermochromic Indicator Based on Functional Polydiacetylene for Food Storage Applications. J. Mater. Sci. 2024, 59, 7561–7573. [Google Scholar] [CrossRef]
- Park, I.S.; Park, H.J.; Kim, J.-M. A Soluble, Low-Temperature Thermochromic and Chemically Reactive Polydiacetylene. ACS Appl. Mater. Interfaces 2013, 5, 8805–8812. [Google Scholar] [CrossRef]
- Wrackmeyer, M.; O’Rourke, A.P.; Pugh, T.; Turner, M.L.; Webb, S.J. Effect of Varying Substituent on the Colour Change Transitions of Diacetylene Pigments. Dyes Pigment. 2021, 192, 109397. [Google Scholar] [CrossRef]
- Ferreira, G.M.D.; Ferreira, G.M.D.; do Carmo Hespanhol, M.; de Paula Rezende, J.; dos Santos Pires, A.C.; Ortega, P.F.R.; da Silva, L.H.M. A Simple and Inexpensive Thermal Optic Nanosensor Formed by Triblock Copolymer and Polydiacetylene Mixture. Food Chem. 2018, 241, 358–363. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric Drug Delivery System Based on Pluronics for Cancer Treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Watson, P.H.; Wilson-McManus, J.E.; Barnes, R.O.; Giesz, S.C.; Png, A.; Hegele, R.G.; Brinkman, J.N.; Mackenzie, I.R.; Huntsman, D.G.; Junker, A.; et al. Evolutionary Concepts in Biobanking—The BC BioLibrary. J. Transl. Med. 2009, 7, 95. [Google Scholar] [CrossRef]
- Avella-Oliver, M.; Morais, S.; Puchades, R.; Maquieira, Á. Towards Photochromic and Thermochromic Biosensing. TrAC Trends Anal. Chem. 2016, 79, 37–45. [Google Scholar] [CrossRef]
- Powell, S.; Molinolo, A.; Masmila, E.; Kaushal, S. Real-Time Temperature Mapping in Ultra-Low Freezers as a Standard Quality Assessment. Biopreservation Biobanking 2019, 17, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Espina, V.; Mueller, C.; Edmiston, K.; Sciro, M.; Petricoin, E.F.; Liotta, L.A. Tissue Is Alive: New Technologies Are Needed to Address the Problems of Protein Biomarker Pre-analytical Variability. Proteom. Clin. Appl. 2009, 3, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gould, T.D.; Yuan, P.; Manji, H.K.; Chen, G. Post-Mortem Interval Effects on the Phosphorylation of Signaling Proteins. Neuropsychopharmacology 2003, 28, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing Sample and MiRNA Profile Quality in Serum and Plasma or Other Biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef]
- Li, J.Z.; Vawter, M.P.; Walsh, D.M.; Tomita, H.; Evans, S.J.; Choudary, P.V.; Lopez, J.F.; Avelar, A.; Shokoohi, V.; Chung, T.; et al. Systematic Changes in Gene Expression in Postmortem Human Brains Associated with Tissue pH and Terminal Medical Conditions. Hum. Mol. Genet. 2004, 13, 609–616. [Google Scholar] [CrossRef]
- Liu, N.; Luo, Y.; Zhu, Y.; Peng, H.; Zou, C.; Zhou, Z.; Chen, W.; Wang, H.; Liu, H.; Hu, Y.; et al. Effects of Warm Ischemia Time, Cryopreservation, and Grinding Methods on RNA Quality of Mouse Kidney Tissues. Biopreservation Biobanking 2021, 19, 306–311. [Google Scholar] [CrossRef]
- Sheedy, D.; Say, M.; Stevens, J.; Harper, C.G.; Kril, J.J. Influence of Liver Pathology on Markers of Postmortem Brain Tissue Quality. Alcohol. Clin. Exp. Res. 2012, 36, 55–60. [Google Scholar] [CrossRef]
- Tashjian, R.S.; Williams, R.R.; Vinters, H.V.; Yong, W.H. Autopsy Biobanking: Biospecimen Procurement, Integrity, Storage, and Utilization. In Biobanking: Methods and Protocols; Yong, W.H., Ed.; Springer: New York, NY, USA, 2019; pp. 77–87. [Google Scholar]
- Tas, R.P.; Sampaio-Pinto, V.; Wennekes, T.; van Laake, L.W.; Voets, I.K. From the Freezer to the Clinic. EMBO Rep. 2021, 22, e52162. [Google Scholar] [CrossRef]
- Shabihkhani, M.; Lucey, G.M.; Wei, B.; Mareninov, S.; Lou, J.J.; Vinters, H.V.; Singer, E.J.; Cloughesy, T.F.; Yong, W.H. The Procurement, Storage, and Quality Assurance of Frozen Blood and Tissue Biospecimens in Pathology, Biorepository, and Biobank Settings. Clin. Biochem. 2014, 47, 258–266. [Google Scholar] [CrossRef]
- Ji, X.; Wang, M.; Li, L.; Chen, F.; Zhang, Y.; Li, Q.; Zhou, J. The Impact of Repeated Freeze-Thaw Cycles on the Quality of Biomolecules in Four Different Tissues. Biopreservation Biobanking 2017, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Pham, A.-H.; Tubman, J.; Gao, Y.; Tjandra, A.D.; Chandrawati, R. Polydiacetylene-Based Sensors for Food Applications. Mater. Adv. 2022, 3, 4088–4102. [Google Scholar] [CrossRef]
- Weston, M.; Kuchel, R.P.; Ciftci, M.; Boyer, C.; Chandrawati, R. A Polydiacetylene-Based Colorimetric Sensor as an Active Use-by Date Indicator for Milk. J. Colloid. Interface Sci. 2020, 572, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Naficy, S.; McConchie, R.; Dehghani, F.; Chandrawati, R. Polydiacetylene-Based Sensors to Detect Food Spoilage at Low Temperatures. J. Mater. Chem. C 2019, 7, 1919–1926. [Google Scholar] [CrossRef]
- Li, Q.; Ren, S.; Peng, Y.; Lv, Y.; Wang, W.; Wang, Z.; Gao, Z. A Colorimetric Strip for Rapid Detection and Real-Time Monitoring of Histamine in Fish Based on Self-Assembled Polydiacetylene Vesicles. Anal. Chem. 2020, 92, 1611–1617. [Google Scholar] [CrossRef]
- Wilk-Kozubek, M.; Potaniec, B.; Cybińska, J. Diacetylene Derivatives, Thermochromic Temperature Change Indicator Comprising these Derivatives, Method of Obtaining Thereof and Use Thereof. Patent Application P.445107; Poland, 2023. [Google Scholar]




| Application | Thermochromic Material | Mechanism | Reference |
|---|---|---|---|
| Invisible ink | Organic | Change in molecular structure | [7] |
| Heat-sensitive fax paper | Organic | Change in molecular structure | [8] |
| Hypercolor T-shirt | Organic | Change in molecular structure | [9] |
| Industrial heating device | Organic | Phase transition | [11] |
| Fire-resistant coating | Organic–inorganic | Change in coordination geometry and number | [12] |
| Monitoring of body temperature | Organic | Phase transition | [13] |
| Monitoring of blood circulation | Organic | Phase transition | [14] |
| Structural Parameter | Structural Modification | Thermochromic Temperature |
|---|---|---|
| Number of methylene units in side chain | Decrease | Decrease |
| Number of methylene units between DA moiety and any functional group | Decrease | Decrease |
| Number of hydrogen-bonding interactions | Decrease | Decrease |
| Number of PO segments in copolymer | Increase | Decrease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk-Kozubek, M.; Potaniec, B.; Gazińska, P.; Cybińska, J. Exploring the Origins of Low-Temperature Thermochromism in Polydiacetylenes. Polymers 2024, 16, 2856. https://doi.org/10.3390/polym16202856
Wilk-Kozubek M, Potaniec B, Gazińska P, Cybińska J. Exploring the Origins of Low-Temperature Thermochromism in Polydiacetylenes. Polymers. 2024; 16(20):2856. https://doi.org/10.3390/polym16202856
Chicago/Turabian StyleWilk-Kozubek, Magdalena, Bartłomiej Potaniec, Patrycja Gazińska, and Joanna Cybińska. 2024. "Exploring the Origins of Low-Temperature Thermochromism in Polydiacetylenes" Polymers 16, no. 20: 2856. https://doi.org/10.3390/polym16202856
APA StyleWilk-Kozubek, M., Potaniec, B., Gazińska, P., & Cybińska, J. (2024). Exploring the Origins of Low-Temperature Thermochromism in Polydiacetylenes. Polymers, 16(20), 2856. https://doi.org/10.3390/polym16202856









