Preparation of High-Transparency Phosphenanthrene-Based Flame Retardants and Studies of Their Flame-Retardant Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Composite Materials
2.3. Characterization
3. Results and Discussion
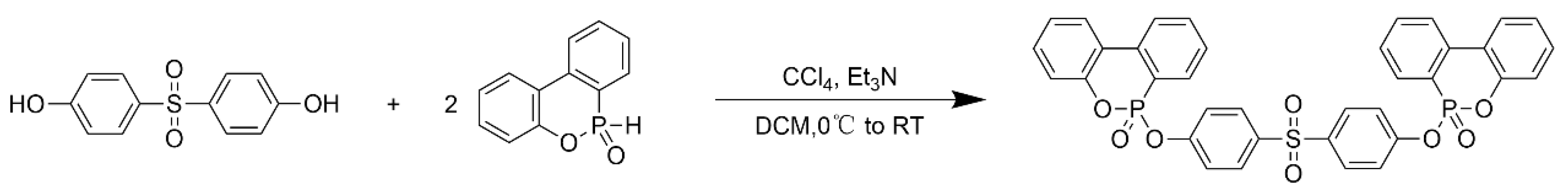
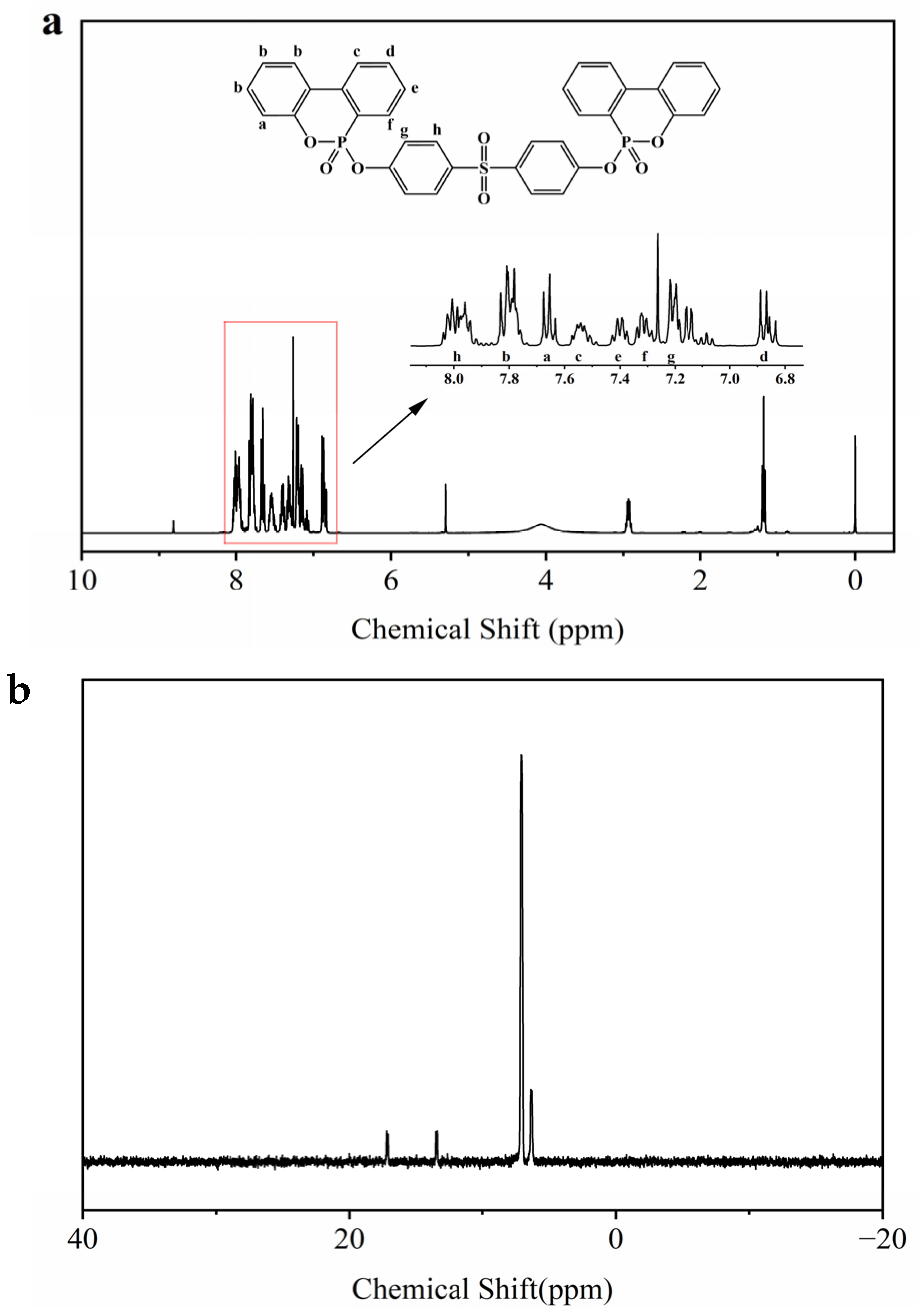
3.1. Characterization of SBPDOPO
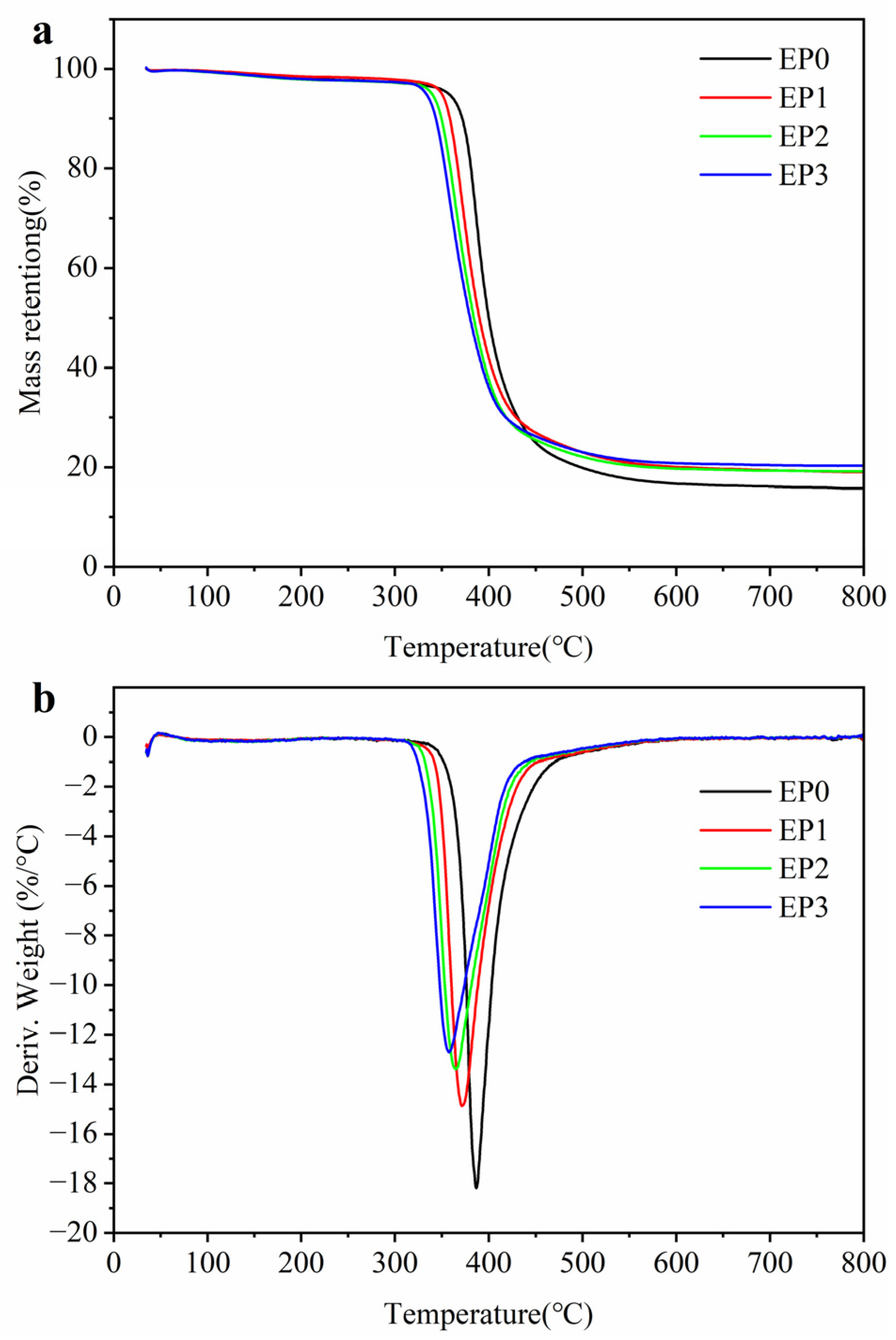
3.2. Thermal Stability
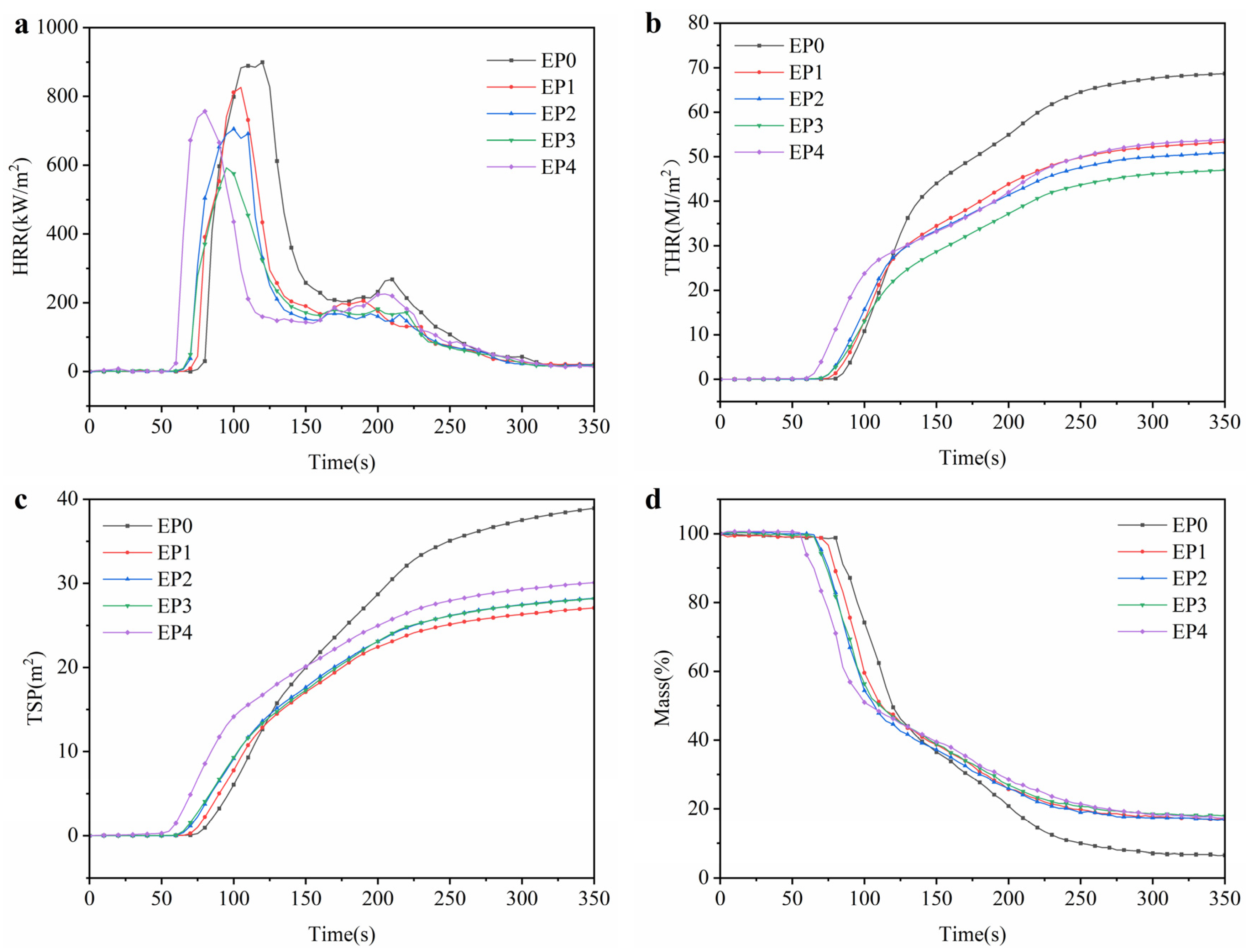
3.3. Flame-Retardant Properties
3.4. Gas-Phase Analysis
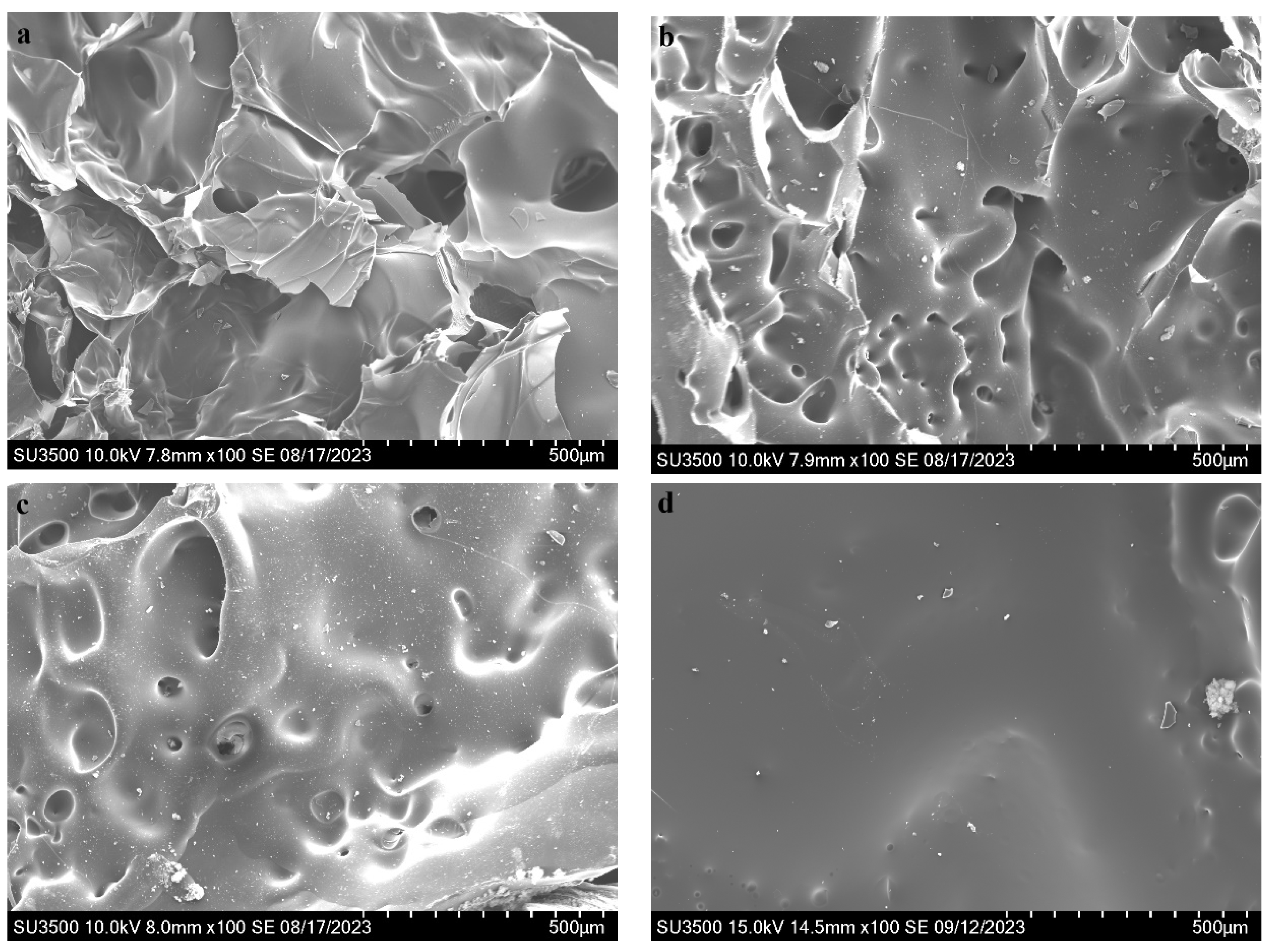
3.5. Residual Char Analysis
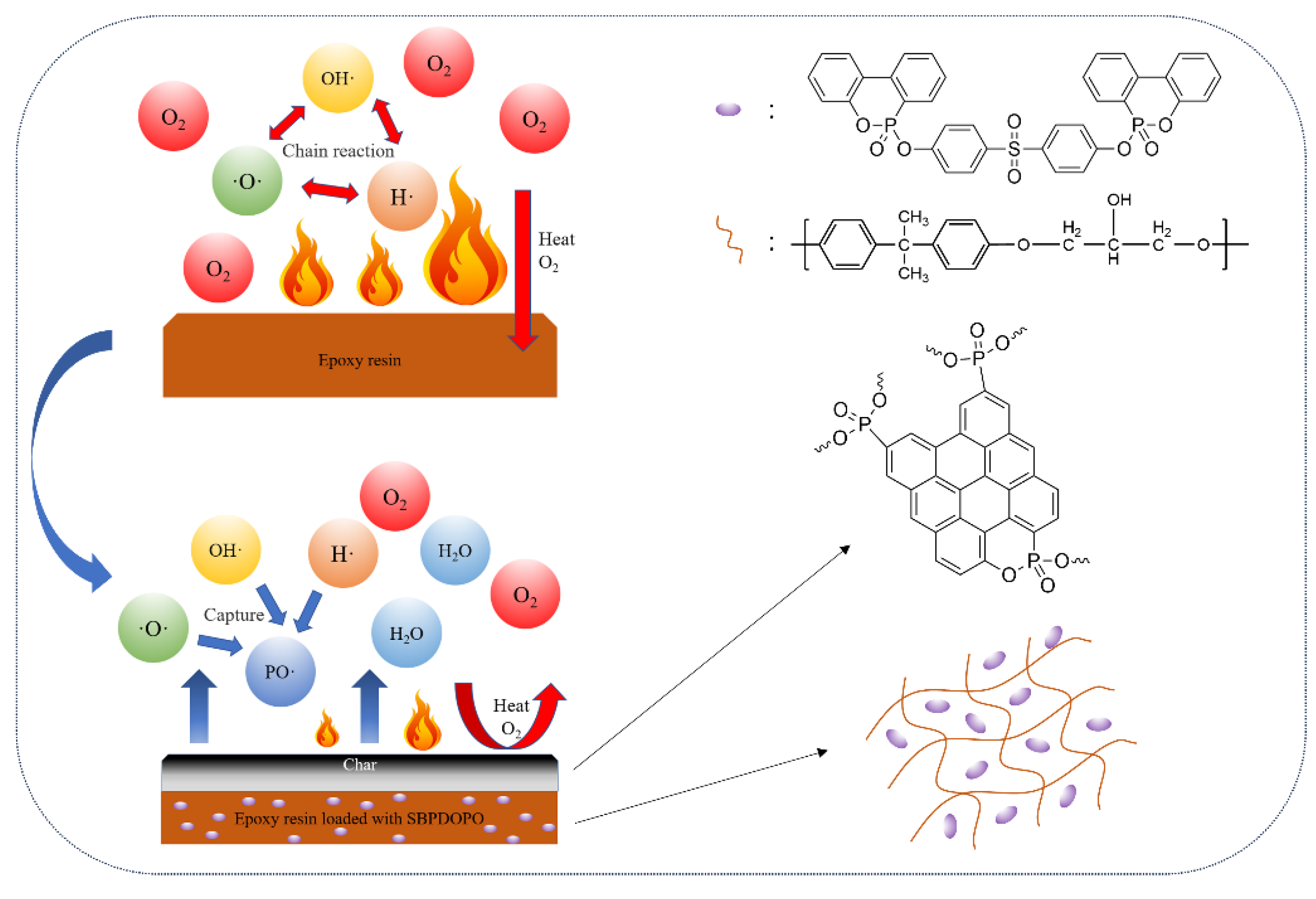
3.6. Flame-Retardant Mechanism
3.7. Mechanical Properties
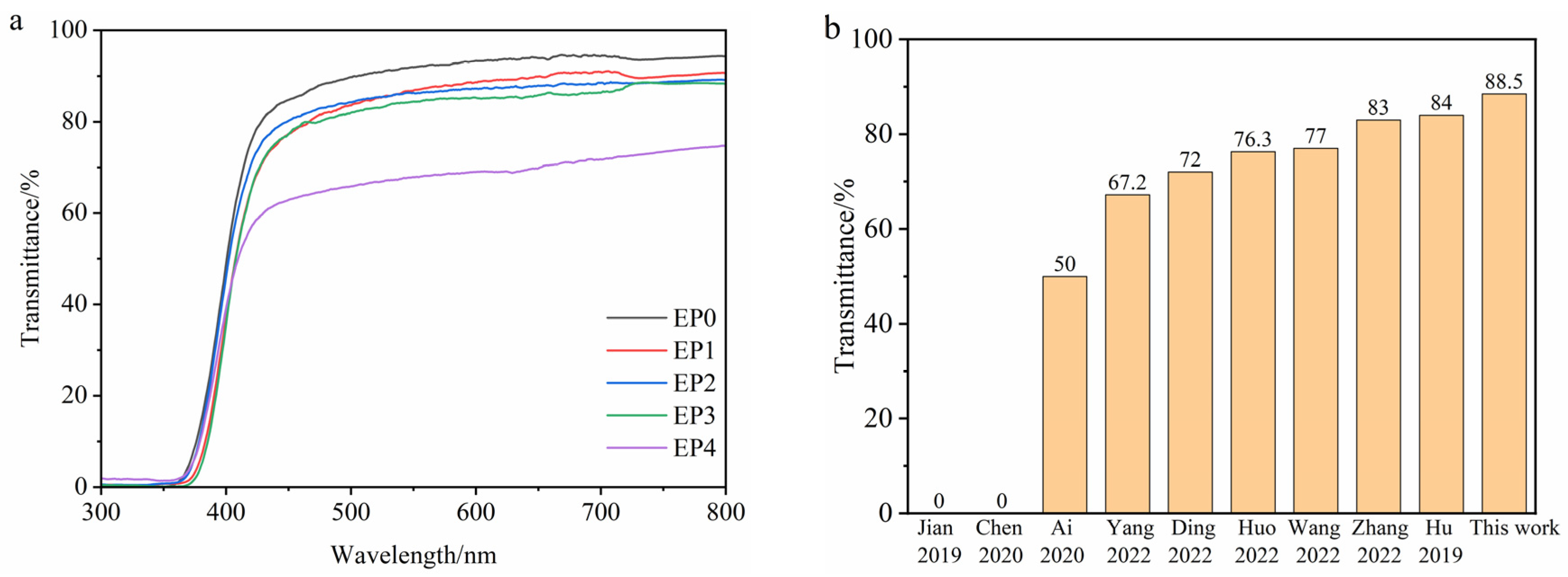
3.8. Transparency and Color Aberration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sukanto, H.; Raharjo, W.W.; Ariawan, D.; Triyono, J.; Kaavesina, M. Epoxy resins thermosetting for mechanical engineering. Open Eng. 2021, 11, 797–814. [Google Scholar] [CrossRef]
- Liu, X.-F.; Xiao, Y.-F.; Luo, X.; Liu, B.-W.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Flame-Retardant multifunctional epoxy resin with high performances. Chem. Eng. J. 2022, 427, 132031. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in epoxy resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Rosu, L.; Bifulco, A.; Rosu, D.; Mustata, F.; Gaan, S. Recent advances in flame retardant epoxy systems from reactive DOPO–based phosphorus additives. Polym. Degrad. Stab. 2022, 202, 110020. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame retardant epoxy composites on the road of innovation: An analysis with flame retardancy index for future development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.-F.; Xia, L.; Pang, F.-Q.; Xu, Y.-L.; Zhao, H.-B.; Jian, R.-K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Part B 2020, 193, 108019. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.; Wang, S.; Hu, J.; Liu, X. Hyperbranched flame retardant for epoxy resin modification: Simultaneously improved flame retardancy, toughness and strength as well as glass transition temperature. Chem. Eng. J. 2022, 428, 131226. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Shi, X.-H.; Lu, J.-H.; Qi, M.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Novel phosphorus-containing imidazolium as hardener for epoxy resin aiming at controllable latent curing behavior and flame retardancy. Compos. Part B 2020, 184, 107673. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, S.; Wang, J.; Cheng, J.; Zhang, Q.; Ding, G.; Hu, Y.; Huo, S. A DOPO based reactive flame retardant constructed by multiple heteroaromatic groups and its application on epoxy resin: Curing behavior, thermal degradation and flame retardancy. Polym. Degrad. Stab. 2019, 167, 10–20. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Li, Z.; Huang, G.; Wu, G. Efficient flame retardancy, good thermal stability, mechanical enhancement, and transparency of DOPO-conjugated structure compound on epoxy resin. Chem. Eng. J. 2022, 450, 138424. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.-Y.; Jian, R.-K.; Liu, Z.; Huang, G. Chemical structure construction of DOPO-containing compounds for flame retardancy of epoxy resin: A review. Prog. Org. Coat. 2023, 175, 107316. [Google Scholar] [CrossRef]
- Bifulco, A.; Varganici, C.D.; Rosu, L.; Mustata, F.; Rosu, D.; Gaan, S. Recent advances in flame retardant epoxy systems containing non-reactive DOPO based phosphorus additives. Polym. Degrad. Stab. 2022, 200, 109962. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q.; Qin, X.-Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Z.; Wu, G.; Zhang, X.; Zhao, C.; Lei, C. Synthesis of a novel nonflammable eugenol-based phosphazene epoxy resin with unique burned intumescent char. Chem. Eng. J. 2020, 390, 124620. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, Y.; Yao, M.; Cui, Y.; Meng, W.; Wang, S.; Qu, H.; Xu, J. Preparation of a MOF flame retardant containing phosphazene ring and its effect on the flame retardant of epoxy resin. React. Funct. Polym. 2023, 191, 105670. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- Tang, G.; Zhao, R.; Deng, D.; Yang, Y.; Chen, D.; Zhang, B.; Liu, X.; Liu, X. Self-extinguishing and transparent epoxy resin modified by a phosphine oxide-containing bio-based derivative. Front. Chem. Sci. Eng. 2021, 15, 1269–1280. [Google Scholar] [CrossRef]
- Liu, X.-F.; Liu, B.-W.; Luo, X.; Guo, D.-M.; Zhong, H.-Y.; Chen, L.; Wang, Y.-Z. A novel phosphorus-containing semi-aromatic polyester toward flame retardancy and enhanced mechanical properties of epoxy resin. Chem. Eng. J. 2020, 380, 122471. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Song, X.; Wang, T.-C.; Cui, J.; Ye, W.; Xu, B.; Wang, D.-Y. Facile Fabrication of organic zirconium/inorganic phosphorus complex for super-efficiently flame-retardant epoxy resin. Compos. Commun. 2022, 36, 101360. [Google Scholar] [CrossRef]
- Zou, B.; Qiu, S.; Ren, X.; Zhou, Y.; Zhou, F.; Xu, Z.; Zhao, Z.; Song, L.; Hu, Y.; Gong, X. Combination of black phosphorus nanosheets and MCNTs via phosphoruscarbon bonds for reducing the flammability of air stable epoxy resin nanocomposites. J. Hazard. Mater. 2020, 383, 121069. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, H.; Jiang, Y.; He, H.; Liu, H.; Huang, H.; Wan, C. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Chen, Y.; Ji, S.; Hu, R.; Ma, H. A novel phosphorus/nitrogen-containing polycarboxylic acid endowing epoxy resin with excellent flame retardance and mechanical properties. Chem. Eng. J. 2019, 375, 121916. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.; Li, J.; Tan, J.; Zhu, X. Enhancing toughness, flame retardant, hydrophobic and dielectric properties of epoxy resin by incorporating multifunctional additive containing phosphorus/silicon. Mater. Des. 2023, 225, 111529. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, S. A novel phosphorus− silicon containing epoxy resin with enhanced thermal stability, flame retardancy and mechanical properties. Polym. Degrad. Stab. 2019, 164, 36–45. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Song, L.; Hu, Y. Intrinsically flame retardant bio-based epoxy thermosets: A review. Compos. Part B 2019, 179, 107487. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Zhang, K.; Wang, J.; Zhang, S.; Liu, C.; Jian, X. Magnolol-based bio-epoxy resin with acceptable glass transition temperature, processability and flame retardancy. Chem. Eng. J. 2020, 387, 124115. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Kou, Y.; Song, L.; Li, J.; Wang, J.; Zhang, S.; Liu, C.; Jian, X. Synthesize and introduce bio-based aromatic s-triazine in epoxy resin: Enabling extremely high thermal stability, mechanical properties, and flame retardancy to achieve high-performance sustainable polymers. Chem. Eng. J. 2021, 406, 126881. [Google Scholar] [CrossRef]
- Jiang, G.; Xiao, Y.; Qian, Z.; Yang, Y.; Jia, P.; Song, L.; Hu, Y.; Ma, C.; Gui, Z. A novel phosphorus-, nitrogen-and sulfur-containing macromolecule flame retardant for constructing high-performance epoxy resin composites. Chem. Eng. J. 2023, 451, 137823. [Google Scholar] [CrossRef]
- Rao, W.; Zhao, P.; Yu, C.; Zhao, H.-B.; Wang, Y.-Z. High strength, low flammability, and smoke suppression for epoxy thermoset enabled by a low-loading phosphorus-nitrogen-silicon compound. Compos. Part B 2021, 211, 108640. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, W.; Lu, H.; Song, K.; Geng, Z.; Ye, X.; Pan, Y.-T.; Zhang, W.; Yang, R. Multielement Flame-Retardant System Constructed with Metal POSS–Organic Frameworks for Epoxy Resin. ACS Appl. Mater. Interfaces 2022, 14, 49326–49337. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Liu, Z.; Wang, J. Thermal properties and flame retardancy of an intumescent flame-retarded epoxy system containing phosphaphenanthrene, triazine-trione and piperidine. J. Therm. Anal. Calorim. 2020, 139, 1099–1110. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Cui, Y.; Zhang, T.; Ji, P. Influence of the Chemical Structure on the Flame Retardant Mechanism and Mechanical Properties of Flame-Retardant Epoxy Resin Thermosets. Macromol. Mater. Eng. 2022, 307, 2200169. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Xu, B.; Qu, L.; Fang, D. Cross-linkable phosphorus/nitrogen-containing aromatic ethylenediamine endowing epoxy resin with excellent flame retardancy and mechanical properties. Compos. Part A 2022, 162, 107145. [Google Scholar] [CrossRef]
- Lv, Y.; Dai, J.; Xia, L.; Luo, L.; Xu, Y.; Dai, L. Smoke suppression and phosphorus-free condensed phase flame-retardant epoxy resin composites based on Salen-Ni. Polym. Degrad. Stab. 2022, 201, 109980. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. Application of chitosan and DOPO derivatives in fire protection of polyamide 66 textiles: Towards a combined gas phase and condensed phase activity. Polym. Degrad. Stab. 2020, 176, 109158. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Wang, J.; Pan, Z.; Zhou, H. Epoxy resin modified with chitosan derivatives and DOPO: Improved flame retardancy, mechanical properties and transparency. Polym. Degrad. Stab. 2022, 199, 109931. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Zhu, Z.; Yin, X.; Weng, Y.; Wang, Z.; Yang, F.; Zhan, J.; Wang, H.; Wang, L. High performance epoxy resin composites modified with multifunctional thiophene/phosphaphenanthrene-based flame retardant: Excellent flame retardance, strong mechanical property and high transparency. Compos. Part B 2021, 227, 109392. [Google Scholar] [CrossRef]
- Ding, H.; Luo, Z.; Wang, B. A phosphorus/sulfur-containing compound toward simultaneously endowing epoxy resin with good flame retardancy and high transparency. J. Appl. Polym. Sci. 2022, 139, e52431. [Google Scholar] [CrossRef]
- Ye, G.; Huo, S.; Wang, C.; Shi, Q.; Yu, L.; Liu, Z.; Fang, Z.; Wang, H. A novel hyperbranched phosphorus-boron polymer for transparent, flame-retardant, smoke-suppressive, robust yet tough epoxy resins. Compos. Part B 2021, 227, 109395. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, L.; Yuan, J.; Wang, L.; Wang, H.; Xiao, F.; Zhu, Z. Transparent, flame retardant, mechanically strengthened and low dielectric EP composites enabled by a reactive bio-based P/N flame retardant. Polym. Degrad. Stab. 2022, 204, 110106. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- ASTM D2863-19; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-like Combustion of Plastics (Oxygen Index). ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Zhang, Y.; Liu, R.; Yu, R.; Yang, K.; Guo, L.; Yan, H. Phosphorus-free hyperbranched polyborate flame retardant: Ultra-high strength and toughness, reduced fire hazards and unexpected transparency for epoxy resin. Compos. Part B 2022, 242, 110101. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wang, J.; Liu, J.; Long, S.; Wang, D. Synthesis of multifunctional flame retardant with toughening and transparency and its application in epoxy resin. React. Funct. Polym. 2022, 176, 105289. [Google Scholar] [CrossRef]
- Jian, R.K.; Ai, Y.F.; Xia, L.; Zhao, L.J.; Zhao, H.B. Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J. Hazard. Mater. 2019, 371, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Zhou, Z.; Jiang, J.; Sai, T.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Flame-retardant, transparent, mechanically-strong and tough epoxy resin enabled by high-efficiency multifunctional boron-based polyphosphonamide. Chem. Eng. J. 2022, 427, 131578. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Z.; Yu, X.; Tang, H.; Zhou, Y.; Zhou, H. Synthesis of chitosan-based flame retardant and its fire resistance in epoxy resin. Carbohydr. Polym. 2020, 245, 116530. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Pang, F.-Q.; Xu, Y.-L.; Jian, R.-K. Multifunctional Phosphorus-Containing Triazolyl Amine toward Self-Intumescent Flame-Retardant and Mechanically Strong Epoxy Resin with High Transparency. Ind. Eng. Chem. Res. 2020, 59, 11918–11929. [Google Scholar] [CrossRef]
- AlSaleh, S.; Labban, M.; AlHariri, M.; Tashkandi, E. Evaluation of self shade matching ability of dental students using visual and instrumental means. J. Dent. 2012, 40, e82–e87. [Google Scholar] [CrossRef]






| Samples | E-51 (g) | DDM (g) | SBPDOPO (g) | DOPO (g) | P (wt%) |
|---|---|---|---|---|---|
| EP0 | 40 | 9 | 0 | 0 | 0 |
| EP1 | 40 | 9 | 1.52 | 0 | 0.27 |
| EP2 | 40 | 9 | 2.58 | 0 | 0.46 |
| EP3 | 40 | 9 | 3.69 | 0 | 0.64 |
| EP4 | 40 | 9 | 0 | 1.52 | 0.43 |
| Samples | T5%/°C | Tmax/°C | Rmax/%min−1 | Residue/% |
|---|---|---|---|---|
| EP0 | 357.2 | 386.9 | 18.18 | 15.74 |
| EP1 | 349.7 | 371.7 | 14.88 | 19.02 |
| EP2 | 339.2 | 364.9 | 13.39 | 19.16 |
| EP3 | 333.2 | 357.7 | 12.72 | 20.31 |
| Samples | Average-t1/s | Average-t2/s | Dripping (Y/N) | Ignite Absorbent Cotton (Y/N) | Rating | LOI/% |
|---|---|---|---|---|---|---|
| EP0 | >30 | / | Y | Y | NR | 24.9 |
| EP1 | 27.8 | 7.7 | N | N | NR | 29.7 |
| EP2 | 8.7 | 4 | N | N | V-1 | 30.9 |
| EP3 | 3.1 | 4.1 | N | N | V-0 | 32.1 |
| EP4 | 29.2 | 31.7 | N | N | NR | 27.5 |
| Samples | THR | PHRR | TSP | Residue/% | av-COY | av-CO2Y |
|---|---|---|---|---|---|---|
| EP0 | 68.7 | 899.3 | 39.2 | 6.6 | 0.12 | 1.32 |
| EP1 | 53.3 | 825.9 | 27.2 | 16.9 | 0.14 | 1.24 |
| EP2 | 51.0 | 705.4 | 28.4 | 17.0 | 0.16 | 1.23 |
| EP3 | 47.0 | 592.3 | 28.3 | 17.9 | 0.17 | 1.21 |
| EP4 | 53.7 | 756.8 | 30.1 | 17.3 | 0.17 | 1.25 |
| Samples | L* | ΔL* | a* | Δa* | b* | Δb* | ΔE |
|---|---|---|---|---|---|---|---|
| EP0 | 93.72 | / | −1.13 | / | 4.78 | / | / |
| EP1 | 94.80 | 1.08 | −1.79 | −0.49 | 5.86 | 1.08 | 1.60 |
| EP2 | 94.78 | 1.06 | −1.36 | −0.06 | 4.85 | 0.07 | 1.06 |
| EP3 | 94.15 | 0.43 | −1.74 | −0.44 | 6.29 | 1.51 | 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, Y. Preparation of High-Transparency Phosphenanthrene-Based Flame Retardants and Studies of Their Flame-Retardant Properties. Polymers 2023, 15, 4665. https://doi.org/10.3390/polym15244665
Zhang T, Liu Y. Preparation of High-Transparency Phosphenanthrene-Based Flame Retardants and Studies of Their Flame-Retardant Properties. Polymers. 2023; 15(24):4665. https://doi.org/10.3390/polym15244665
Chicago/Turabian StyleZhang, Tao, and Yong Liu. 2023. "Preparation of High-Transparency Phosphenanthrene-Based Flame Retardants and Studies of Their Flame-Retardant Properties" Polymers 15, no. 24: 4665. https://doi.org/10.3390/polym15244665
APA StyleZhang, T., & Liu, Y. (2023). Preparation of High-Transparency Phosphenanthrene-Based Flame Retardants and Studies of Their Flame-Retardant Properties. Polymers, 15(24), 4665. https://doi.org/10.3390/polym15244665







