Aloe vera/Chitosan-Based Edible Film with Enhanced Antioxidant, Antimicrobial, Thermal, and Barrier Properties for Sustainable Food Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Aloe vera Gel Preparation
2.3. Preparation of Edible Film Solutions
2.3.1. The Viscosity, pH, and Particle Size Measurement of Edible Films Solutions
2.3.2. Antioxidant Activity of Edible Film Solutions
2.4. Preparation of Edible Films
2.4.1. Color, Opacity, Water Solubility (WS), and Water Vapor Permeability (WVP) of Edible Films
2.4.2. Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD) of Edible Films
2.4.3. Mechanical Properties of Edible Films
2.4.4. Thermal Properties of Edible Films
2.4.5. Fourier Transform Infrared (FTIR) Spectroscopy of Edible Films
2.5. Application of Edible Film Solutions on Fresh Fig Fruits
2.6. Statistical Analysis
3. Results
3.1. The Viscosity, pH, Zeta (ζ)-Potential, Polydispersity Index (PDI), and Particle Size
3.2. Antioxidant Property of Edible Film Solution
3.3. Color, Opacity, Water Solubility (WS), and Water Vapor Permeability (WVP) Properties of Edible Films
3.4. Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD) of Edible Films
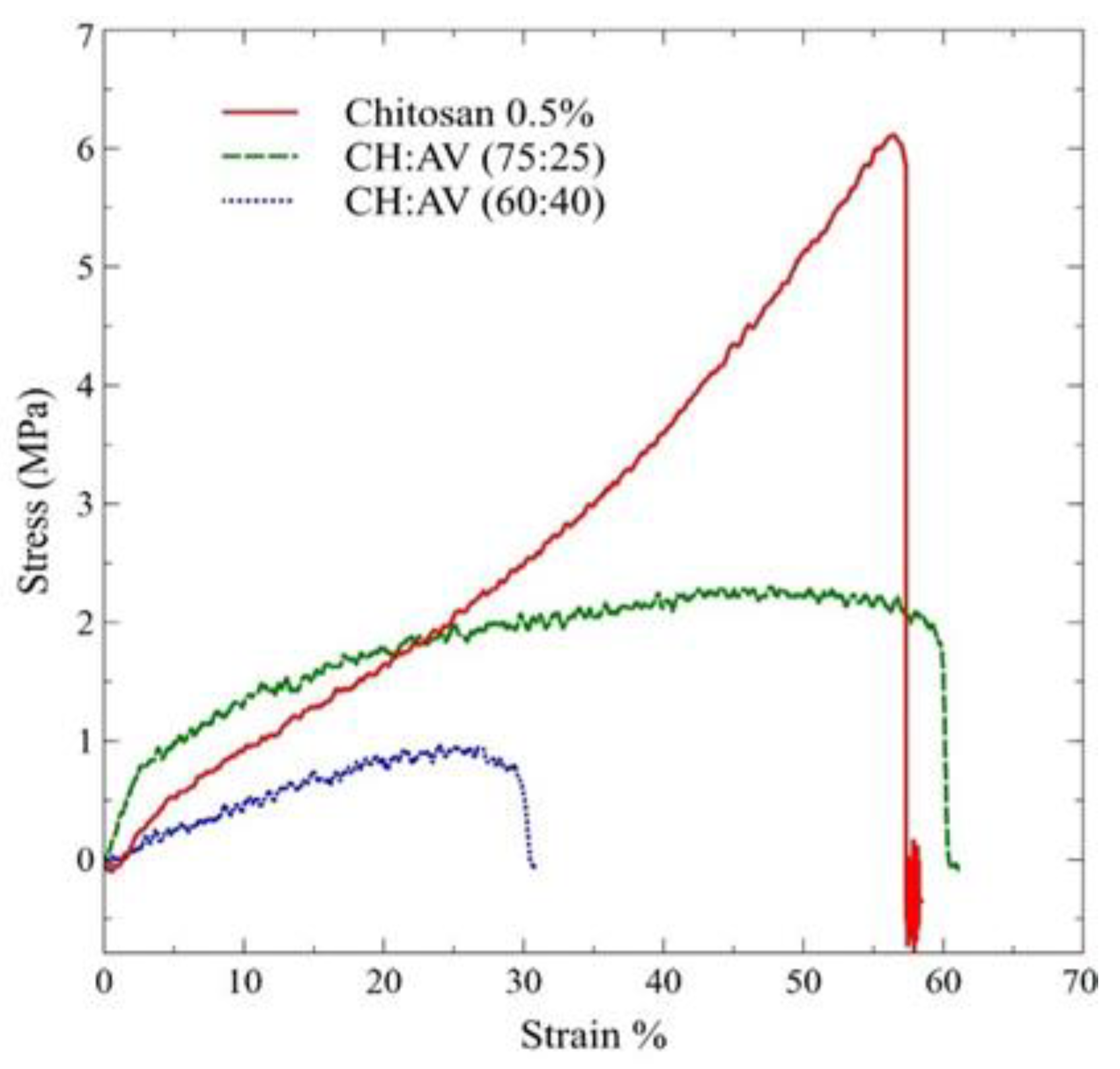
3.5. Mechanical Properties
3.6. Thermal Properties
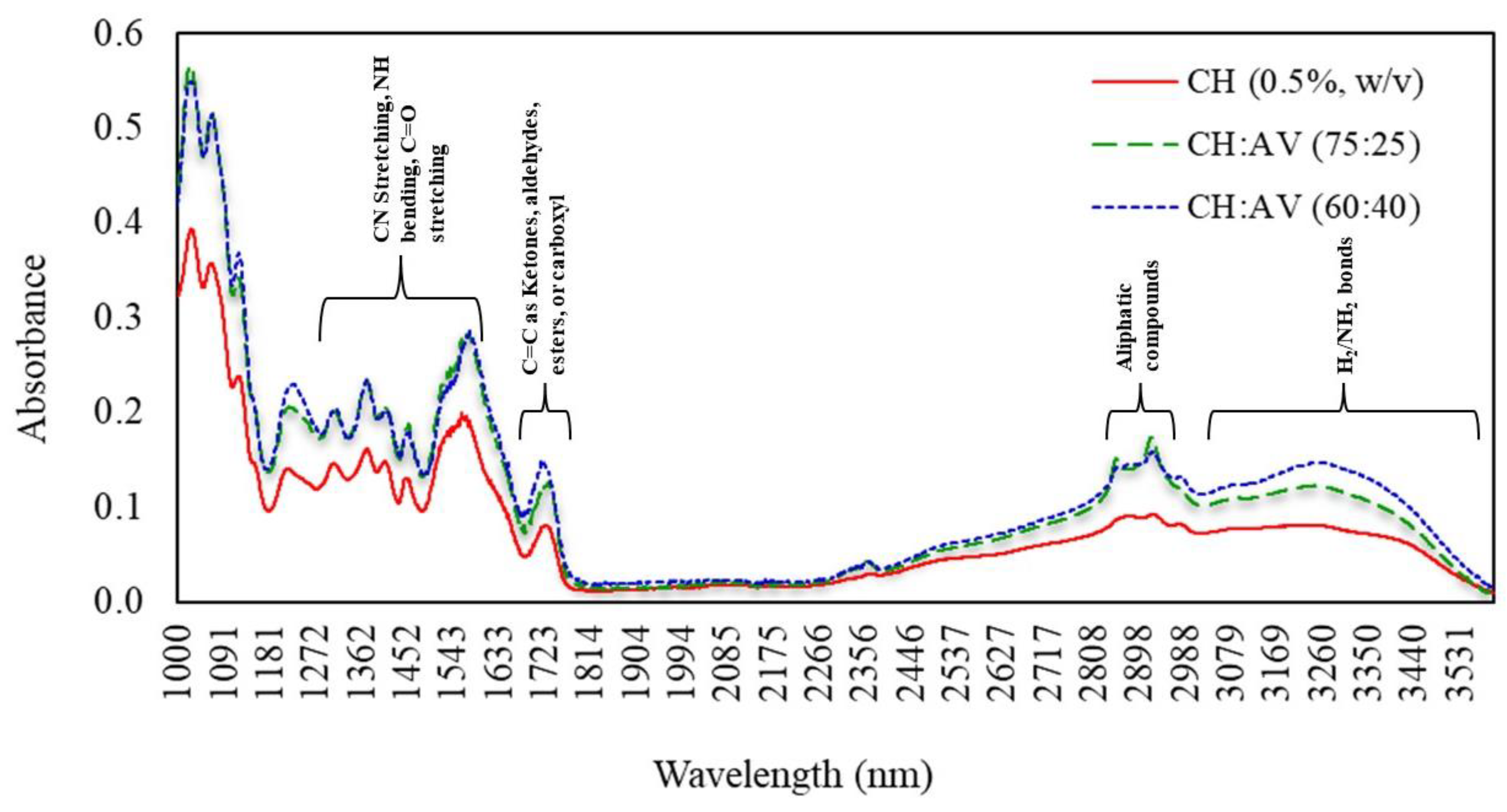
3.7. Fourier Transform Infrared (FTIR) Spectroscopy
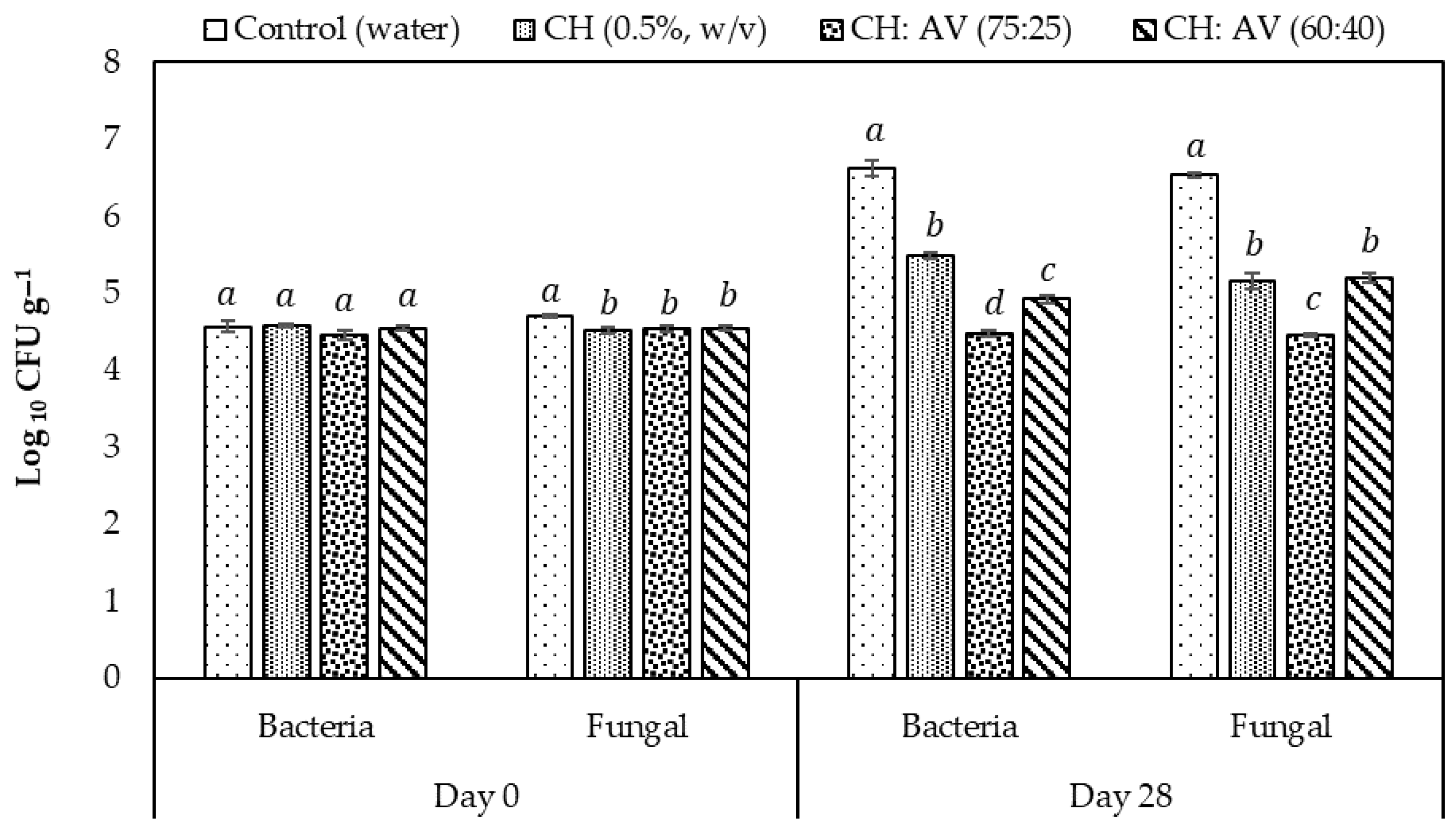
3.8. Microbial Load on Fig Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Jurić, S.; Bureš, M.S.; Vlahoviček-Kahlina, K.; Stracenski, K.S.; Fruk, G.; Jalšenjak, N.; Bandić, L.M. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem. X 2023, 17, 100575. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, H.; Fu, Y.; Chang, C.; Wu, J. Sodium alginate/gum arabic/glycerol multicomponent edible films loaded with natamycin: Study on physicochemical, antibacterial, and sweet potatoes preservation properties. Int. J. Biol. Macromol. 2022, 213, 1068–1077. [Google Scholar] [CrossRef]
- Herrera-Vázquez, S.E.; Dublán-García, O.; Arizmendi-Cotero, D.; Gómez-Oliván, L.M.; Islas-Flores, H.; Hernández-Navarro, M.D.; Ramírez-Durán, N. Optimization of the physical, optical and mechanical properties of composite edible films of gelatin, whey protein and chitosan. Molecules 2022, 27, 869. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Karam, L.; Mallah, A. Application of chitosan in active food packaging. In Chitosan: Novel Applications in Food Systems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–30. [Google Scholar] [CrossRef]
- Kaur, N.; Shahwar, D.; Hassan, F.E.; Ahmed, Z.F.R. Antioxidant and antibacterial activities of date palm fruit (Phoenix dactylifera L.) in response to postharvest application with natural elicitors. Acta Hortic. 2023, 1364, 187–194. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Inamdar, M.S. Aqueous Behaviour of Chitosan. Int. J. Polym. Sci. 2010, 2010, 939536. [Google Scholar] [CrossRef]
- Fan, M.; Hu, Q.; Shen, K. Preparation and structure of chitosan soluble in wide pH range. Carbohydr. Polym. 2009, 78, 66–71. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Preharvest Applications of Chitosan, Salicylic Acid, and Calcium Chloride Have a Synergistic Effect on Quality and Storability of Date Palm Fruit (Phoenix dactylifera L.). HortScience 2022, 57, 422–430. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Chinthala, M.; Jacob, M.M.; Vo, D.-V.N.; Reddy, S.S.; Kunnel, E.S. Chitosan-based beads as sustainable adsorbents for wastewater remediation: A review. Environ. Chem. Lett. 2023, 21, 1881–1905. [Google Scholar] [CrossRef]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Do, N.H.N.; Lac, H.D.; Nguyen, P.L.N.; Le, P.K. Synthesis, properties, and applications of chitosan hydrogels as anti-inflammatory drug delivery system. J. Porous Mater. 2023, 30, 655–670. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Hou, H.; Li, Y.; Wang, J.; Fu, J.; Lu, L.; Gao, D.; Liu, Z.; Zhao, F.; et al. Review: Application of chitosan and its derivatives in medical materials. Int. J. Biol. Macromol. 2023, 240, 124398. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Butler, B.L.; Vergano, P.J.; Testin, R.F.; Bunn, J.M.; Wiles, J.L. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 1996, 61, 953–956. [Google Scholar] [CrossRef]
- Caner, C.; Vergano, P.J.; Wiles, J.L. Chitosan film mechanical and permeation properties as affected by acid, plasticizer, and storage. J. Food Sci. 1998, 63, 1049–1053. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Tang, H.; Hao, W.; Wu, J.; Sun, Y.; Sun, J.; Liu, Y.; Jiang, L. Development, characterization and application of intelligent/active packaging of chitosan/chitin nanofibers films containing eggplant anthocyanins. Food Hydrocoll. 2023, 139, 108496. [Google Scholar] [CrossRef]
- Zarandona, I.; Minh, N.C.; Trung, T.S.; de la Caba, K.; Guerrero, P. Evaluation of bioactive release kinetics from crosslinked chitosan films with Aloe vera. Int. J. Biol. Macromol. 2021, 182, 1331–1338. [Google Scholar] [CrossRef]
- Bhan, C.; Asrey, R.; Meena, N.K.; Rudra, S.G.; Chawla, G.; Kumar, R.; Kumar, R. Guar gum and chitosan-based composite edible coating extends the shelf life and preserves the bioactive compounds in stored Kinnow fruits. Int. J. Biol. Macromol. 2022, 222, 2922–2935. [Google Scholar] [CrossRef] [PubMed]
- Gürler, N. Development of chitosan/gelatin/starch composite edible films incorporated with pineapple peel extract and aloe vera gel: Mechanical, physical, antibacterial, antioxidant, and sensorial analysis. Polym. Eng. Sci. 2023, 63, 426–440. [Google Scholar] [CrossRef]
- Hadi, A.; Nawab, A.; Alam, F.; Zehra, K. Sustainable food packaging films based on alginate and Aloe vera. Polym. Eng. Sci. 2022, 62, 2111–2118. [Google Scholar] [CrossRef]
- Jodhani, K.A.; Nataraj, M. Synergistic effect of Aloe gel (Aloe vera L.) and Lemon (Citrus Limon L.) peel extract edible coating on shelf life and quality of banana (Musa spp.). J. Food Meas. Charact. 2021, 15, 2318–2328. [Google Scholar] [CrossRef]
- Li, B.; Bao, Y.; Li, J.; Bi, J.; Chen, Q.; Cui, H.; Wang, Y.; Tian, J.; Shu, C.; Wang, Y. A sub-freshness monitoring chitosan/starch-based colorimetric film for improving color recognition accuracy via controlling the pH value of the film-forming solution. Food Chem. 2022, 388, 132975. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, L.; Ramezanian, A.; Tanaka, F.; Tanaka, F. Impact of Aloe vera gel coating enriched with basil (Ocimum basilicum L.) essential oil on postharvest quality of strawberry fruit. J. Food Meas. Charact. 2021, 15, 353–362. [Google Scholar] [CrossRef]
- Sarker, A.; Deltsidis, A.; Grift, T.E. Effect of Aloe vera gel-carboxymethyl cellulose composite coating on the degradation kinetics of cucumber. Biosyst. Eng. 2021, 46, 112–128. [Google Scholar] [CrossRef]
- Kouser, F.; Kumar, S.; Bhat, H.F.; Hassoun, A.; Bekhit, A.E.-D.A.; Bhat, Z.F. Aloe barbadensis Based Bioactive Edible Film Improved Lipid Stability and Microbial Quality of the Cheese. Foods 2023, 12, 229. [Google Scholar] [CrossRef]
- Maan, A.A.; Ahmed, Z.F.R.; Khan, M.K.I.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Varidi, M.; Ahmadzadeh-Hashemi, S.; Nooshkam, M. Changes in fat uptake, color, texture, and sensory properties of Aloe vera gel-coated eggplant rings during deep-fat frying process. Food Sci. Nutr. 2023, 11, 2027–2035. [Google Scholar] [CrossRef]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of Aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Grace, O.M.; Buerki, S.; Symonds, M.R.E.; Forest, F.; van Wyk, A.E.; Smith, G.F.; Klopper, R.R.; Bjorå, C.S.; Neale, S.; Demissew, S. Evolutionary history and leaf succulence as explanations for medicinal use in aloes and the global popularity of Aloe vera. BMC Evol. Biol. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Kahramanoğlu, İ.; Chen, C.; Chen, J.; Wan, C. Chemical constituents, antimicrobial activity, and food preservative characteristics of Aloe vera gel. Agronomy 2019, 9, 831. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Naz, S.; Ejaz, S.; Maqbool, M.; Siddiqui, M.H.; Kalaji, H.M.; Wróbel, J.; Telesiński, A.; Auriga, A. Hydrogen Sulfide Mitigates Chilling Injury of Postharvest Banana Fruits by Regulating γ-Aminobutyric Acid Shunt Pathway and Ascorbate–Glutathione Cycle. Front. Plant Sci. 2022, 13, 941246. [Google Scholar] [CrossRef] [PubMed]
- Parven, A.; Sarker, M.R.; Megharaj, M.; Meftaul, I.M. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020, 265, 109228. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Nawaz, A.; Naz, S.; Maqbool, M.; Abbas, A.M. Aloe vera gel coating delays softening and maintains quality of stored persimmon (Diospyros kaki Thunb) Fruits. JFST 2022, 59, 3296–3306. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Hashmi, M.S. Chitosan–Aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Chin, S.S.; Lyn, F.H.; Hanani, Z.A.N. Effect of Aloe vera (Aloe barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Packag. Shelf Life 2017, 12, 128–134. [Google Scholar] [CrossRef]
- Alkaabi, S.; Sobti, B.; Mudgil, P.; Hasan, F.; Ali, A.; Nazir, A. Lemongrass essential oil and Aloe vera gel based antimicrobial coatings for date fruits. Appl. Food Res. 2022, 2, 100127. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Jeong, S.; Jang, A. Chemical-free scale inhibition method for seawater reverse osmosis membrane process: Air micro-nano bubbles. Desalination 2019, 461, 1–9. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Khoshgozaran-Abras, S.; Azizi, M.H.; Hamidy, Z.; Bagheripoor-Fallah, N. Mechanical, physicochemical and color properties of chitosan based-films as a function of Aloe vera gel incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Dutta, J.; Kumar, S. Chitosan-based active coating for pineapple preservation: Evaluation of antimicrobial efficacy and shelf-life extension. LWT 2022, 168, 113940. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Eelager, M.P.; Masti, S.P.; Chougale, R.B.; Hiremani, V.D.; Narasgoudar, S.S.; Dalbanjan, N.P.; Sk, P.K. Evaluation of mechanical, antimicrobial, and antioxidant properties of vanillic acid induced chitosan/poly (vinyl alcohol) active films to prolong the shelf life of green chilli. Int. J. Biol. Macromol. 2023, 232, 123499. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; De la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Macêdo, M.D.M.; Dantas, H.K.B.; de Souza, M.F.; Pedrosa, T.C.; de Azevedo, A.C.S.; Ferreira, V.P.; de Medeiros Lucena, B.; de Sousa, W.J.B.; Fook, M.V.L. Chitosan and Aloe vera gel formulations as wound healing agents in episiotomy. Soc. Dev. 2021, 10, e36310614895. [Google Scholar] [CrossRef]
- Jafari, A.; Vaghari, H.; Jafarizadeh-Malmiri, H. Development of Antimicrobial Films Based on Aloe vera and Fabricated AgNPs Using Propolis. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 95–103. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 9. [Google Scholar] [CrossRef]
- Lin, S.J.; Pascall, M.A. Incorporation of vitamin E into chitosan and its effect on the film forming solution (viscosity and drying rate) and the solubility and thermal properties of the dried film. Food Hydrocoll. 2014, 35, 78–84. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Garcia, O.R.; Villa, C.C. The influence of Aloe vera gel incorporation on the physicochemical and mechanical properties of banana starch-chitosan edible films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Suriati, L.; Utama, I.M.; Harjosuwono, B.A.; Gunam, I.B. Stability Aloe vera gel as edible coating. IOP Conf. Ser. Earth Environ. Sci. 2020, 411, 012053. [Google Scholar] [CrossRef]
- Melro, E.; Antunes, F.E.; da Silva, G.J.; Cruz, I.; Ramos, P.E.; Carvalho, F.; Alves, L. Chitosan films in food applications. tuning film properties by changing acidic dissolution conditions. Polymers 2020, 13, 1. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Servais, C.; Jones, R.; Roberts, I. The influence of particle size distribution on the processing of food. J. Food Eng. 2002, 51, 201–208. [Google Scholar] [CrossRef]
- Chang, X.L.; Wang, C.; Feng, Y.; Liu, Z. Effects of heat treatments on the stabilities of polysaccharides substances and barbaloin in gel juice from Aloe vera Miller. J. Food Eng. 2006, 75, 245–251. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch-Stärke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Fajardo, P.; Martins, J.T.; Fuciños, C.; Pastrana, L.; Teixeira, J.A.; Vicente, A.A. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng. 2010, 101, 349–356. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinämäki, J.; Krogars, K.; Jörgensen, A.C.; Karjalainen, M.; Colarte, A.I.; Yliruusi, J. Solid-state and mechanical properties of aqueous chitosan-amylose starch films plasticized with polyols. Aaps Pharmscitech 2004, 5, 109–114. [Google Scholar] [CrossRef]
- Andonegi, M.; Irastorza, A.; Izeta, A.; de la Caba, K.; Guerrero, P. Physicochemical and Biological Performance of Aloe vera-Incorporated Native Collagen Films. Pharmaceutics 2020, 12, 1173. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.-H.I.; Hussain, A. Investigation on the mechanical behavior of polyester-scrap tire composites. Constr. Build. Mater. 2016, 127, 896–903. [Google Scholar] [CrossRef]
- Christy, J.V.; Arunachalam, R.; Mourad, A.-H.I.; Krishnan, P.K.; Piya, S.; Al-Maharbi, M. Processing, Properties, and Microstructure of Recycled Aluminum Alloy Composites Produced Through an Optimized Stir and Squeeze Casting Processes. J. Manuf. Process 2020, 59, 287–301. [Google Scholar] [CrossRef]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.-H.I. Thermo-mechanical characteristics of thermally aged polyethylene/polypropylene blends. Mater. Des. 2010, 31, 918–929. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Tafa, K.D.; Satheesh, N.; Abera, W. Mechanical properties of tef starch based edible films: Development and process optimization. Heliyon 2023, 9, e13160. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Álvarez, K. Physico-chemical properties and in vitro digestibility of edible films made from plantain flour with added Aloe vera gel. JFF 2016, 26, 750–762. [Google Scholar] [CrossRef]
- Mohd Nizam, N.H.; Mohammad Rawi, N.F.; Mhd Ramle, S.F.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Mohamad Kassim, M.H. Physical, thermal, mechanical, antimicrobial and physicochemical properties of starch based film containing Aloe vera: A review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A. Optimized alginate and Aloe vera gel edible coating reinforced with nTiO2 for the shelf-life extension of tomatoes. Int. J. Biol. Macromol. 2020, 165, 2693–2701. [Google Scholar] [CrossRef]
- Akhila, V.; Badwaik, L.S. Recent advancement in improvement of properties of polysaccharides and proteins based packaging film with added nanoparticles: A review. Int. J. Biol. Macromol. 2022, 203, 515–525. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Shulga, O.; Chorna, A.; Kobylinskyi, S. Differential scanning calorimetry research of biodegradable films for confectionery and bakery products. Chem. Chem. Technol. 2017, 11, 492–496. Available online: https://dspace.nuft.edu.ua/handle/123456789/26596 (accessed on 10 May 2023). [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of Arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.M.P.; Pacheco, M.S.; de Moraes, M.A.; Lopes, P.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Effect of Chitosan and Aloe vera Extract Concentrations on the Physicochemical Properties of Chitosan Biofilms. Polymers 2021, 13, 1187. [Google Scholar] [CrossRef]
- Chalapud, M.A.G.; Caicedo, C.; Ruiz, E.M.; Valencia, M.F. Propiedades de conservación: Recubrimiento a base de quitosano y Aloe vera aplicado en papa criolla (Solanum phureja). Inf. Técnico 2016, 80, 9–19. [Google Scholar] [CrossRef][Green Version]
- Ramadan, M.F. Introduction to Fig (Ficus carica): Production, Processing, and Properties. In Fig (Ficus carica): Production, Processing, and Properties; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–8. [Google Scholar] [CrossRef]



| Formulations | Percentage of CH in Film Solution | Percentage of AV in Film Solution |
|---|---|---|
| CH (0.5%, w/v) | 0.5%, w/v | Nil |
| AV (100%) | Nil | 100% (Pure) |
| CH:AV (75:25) | 0.75%, w/v | 25%, v/v |
| CH:AV (60:40) | 0.60%, w/v | 40%, v/v |
| Formulations | Zeta Potential (mV) | Polydispersity Index (%) | Particle Size (µm) | Viscosity (cP) | pH |
|---|---|---|---|---|---|
| CH (0.5%, w/v) | 102.4 ± 0.70 a | 0.01 ± 0.00 b | 2.13 ± 0.00 a | 161.5 ± 1.49 a | 3.37 ± 0.05 b |
| AV (100%) | −42.95 ± 0.55 d | 0.95 ± 0.35 ab | 1.15 ± 0.12 b | 24.5 ± 0.50 d | 4.39 ± 0.03 a |
| CH:AV (75:25) | 68.2 ± 1.26 b | 1.25 ± 0.16 a | 2.29 ± 0.06 a | 53.2 ± 1.38 b | 3.43 ± 0.08 b |
| CH:AV (60:40) | 56.6 ± 0.90 c | 0.08 ± 0.00 b | 1.43 ± 0.03 b | 43.4 ± 0.53 c | 3.41 ± 0.70 b |
| Formulations | L* | a* | b* | Chroma | Opacity (%) | WS (%) | WVP (gm−2 h−1 Pa−1 mm) |
|---|---|---|---|---|---|---|---|
| CH (0.5%, w/v) | 12.75 ± 1.53 c | −0.24 ± 0.01 a | 0.34 ± 0.97 b | 0.25 ± 0.96 c | 1.17 ± 0.64 c | 60.32 ± 0.52 a | 0.267 ± 0.07 a |
| CH:AV (75:25) | 17.68 ± 1.26 b | −0.07 + 0.05 c | 1.35 ± 0.06 a | 0.41 ± 0.83 b | 1.84 ± 0.57 b | 40.74 ± 0.65 b | 0.202 ± 0.17 b |
| CH:AV (60:40) | 23.31 ± 0.73 a | −0.18 ± 0.01 b | 0.19 ± 0.12 c | 1.35 ± 0.76 a | 2.36 ± 0.86 a | 39.10 ± 0.64 b | 0.082 ± 0.11 c |
| Formulations | Tensile Strength (Mpa) | Strain (%) | Young Modulus (Mpa) | Film Thickness (mm) |
|---|---|---|---|---|
| CH (0.5%, w/v) | 0.061 ± 0.41 a | 58.5 ± 0.01 a | 9.35 ± 0.59 a | 0.033 ± 0.001 c |
| CH:AV (75:25) | 0.016 ± 0.50 b | 63.0 ± 0.07 a | 5.60 ± 1.69 ab | 0.050 ± 0.002 a |
| CH:AV (60:40) | 0.006 ± 0.21 b | 33.0 ± 0.02 b | 3.61 ± 0.25 b | 0.042 ± 0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, N.; Somasundram, C.; Razali, Z.; Mourad, A.-H.I.; Hamed, F.; Ahmed, Z.F.R. Aloe vera/Chitosan-Based Edible Film with Enhanced Antioxidant, Antimicrobial, Thermal, and Barrier Properties for Sustainable Food Preservation. Polymers 2024, 16, 242. https://doi.org/10.3390/polym16020242
Kaur N, Somasundram C, Razali Z, Mourad A-HI, Hamed F, Ahmed ZFR. Aloe vera/Chitosan-Based Edible Film with Enhanced Antioxidant, Antimicrobial, Thermal, and Barrier Properties for Sustainable Food Preservation. Polymers. 2024; 16(2):242. https://doi.org/10.3390/polym16020242
Chicago/Turabian StyleKaur, Navjot, Chandran Somasundram, Zuliana Razali, Abdel-Hamid I. Mourad, Fathalla Hamed, and Zienab F. R. Ahmed. 2024. "Aloe vera/Chitosan-Based Edible Film with Enhanced Antioxidant, Antimicrobial, Thermal, and Barrier Properties for Sustainable Food Preservation" Polymers 16, no. 2: 242. https://doi.org/10.3390/polym16020242
APA StyleKaur, N., Somasundram, C., Razali, Z., Mourad, A.-H. I., Hamed, F., & Ahmed, Z. F. R. (2024). Aloe vera/Chitosan-Based Edible Film with Enhanced Antioxidant, Antimicrobial, Thermal, and Barrier Properties for Sustainable Food Preservation. Polymers, 16(2), 242. https://doi.org/10.3390/polym16020242








