Preparation and Characterization of Acrylic and Methacrylic Phospholipid-Mimetic Polymer Hydrogels and Their Applications in Optical Tissue Clearing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
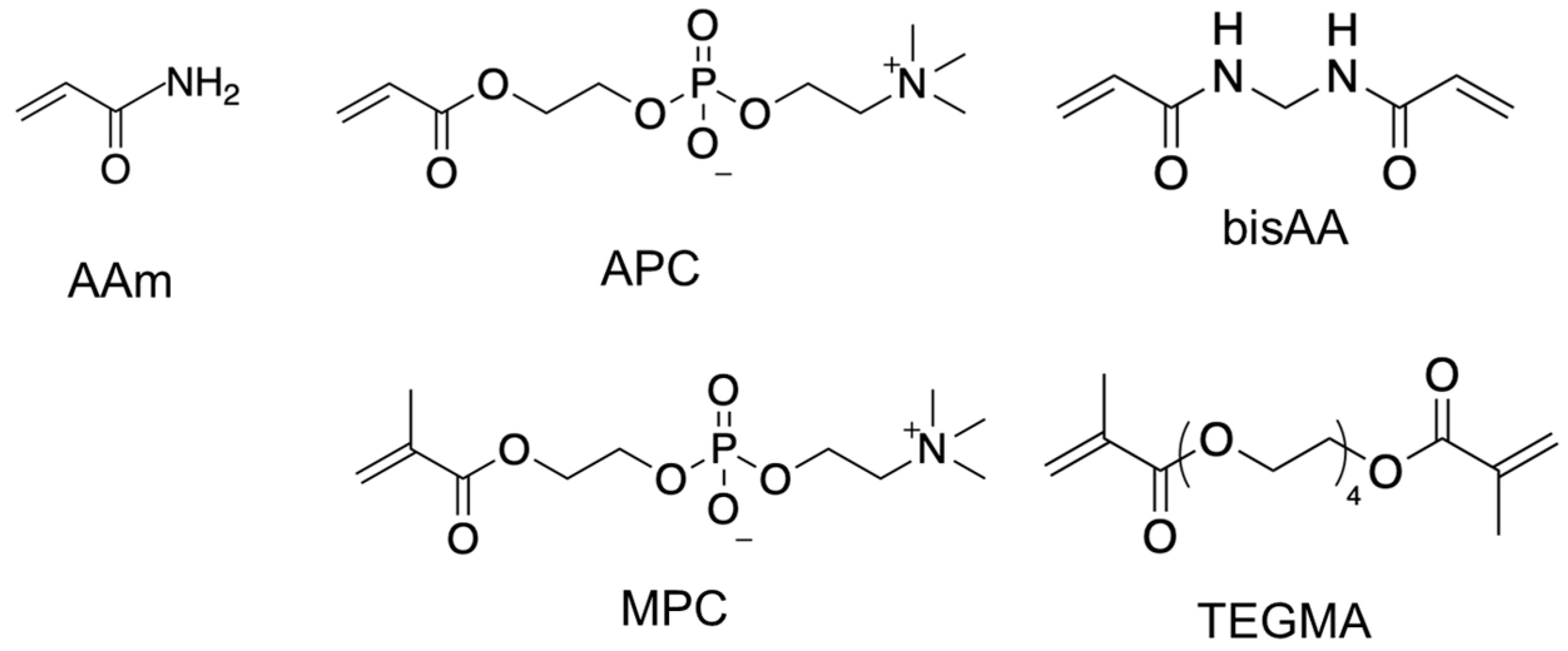
2.2. Polymer Synthesis and Characterization
2.3. Hydrogel Preparation and Characterization
2.4. Optical Clearing of Tumor Tissues
3. Results and Discussion
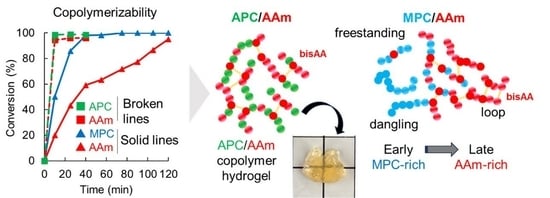
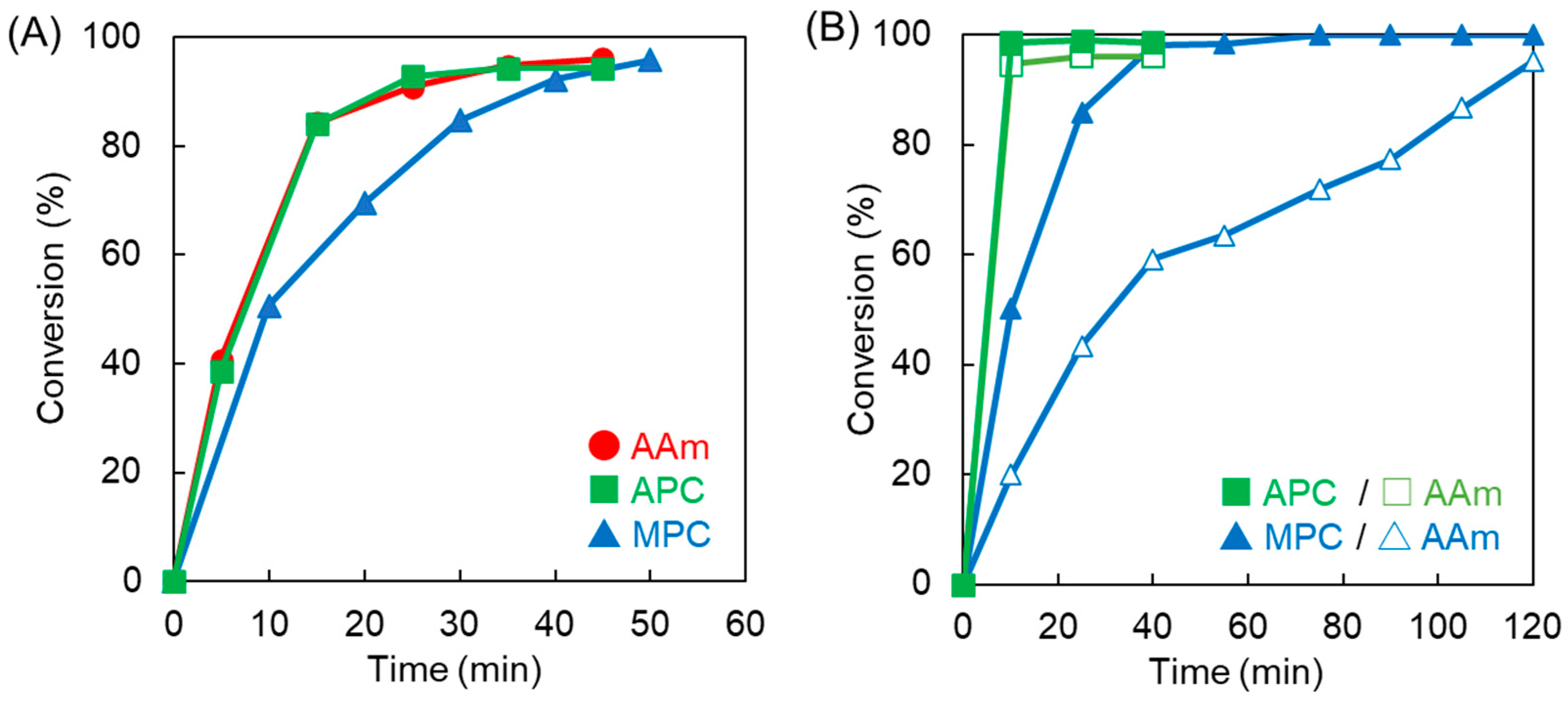
3.1. Synthesis and Characterization of Copolymers Containing MPC and APC
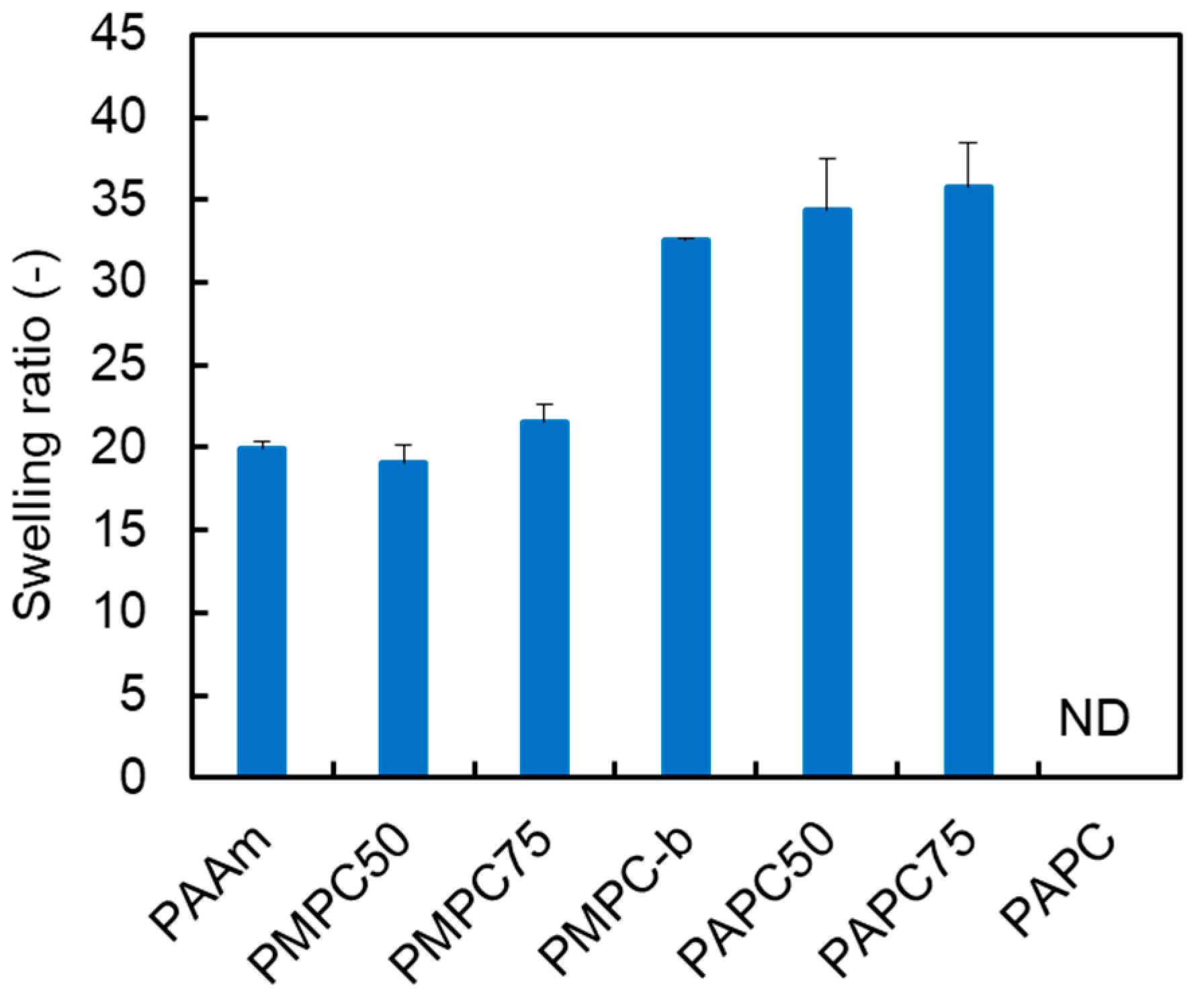
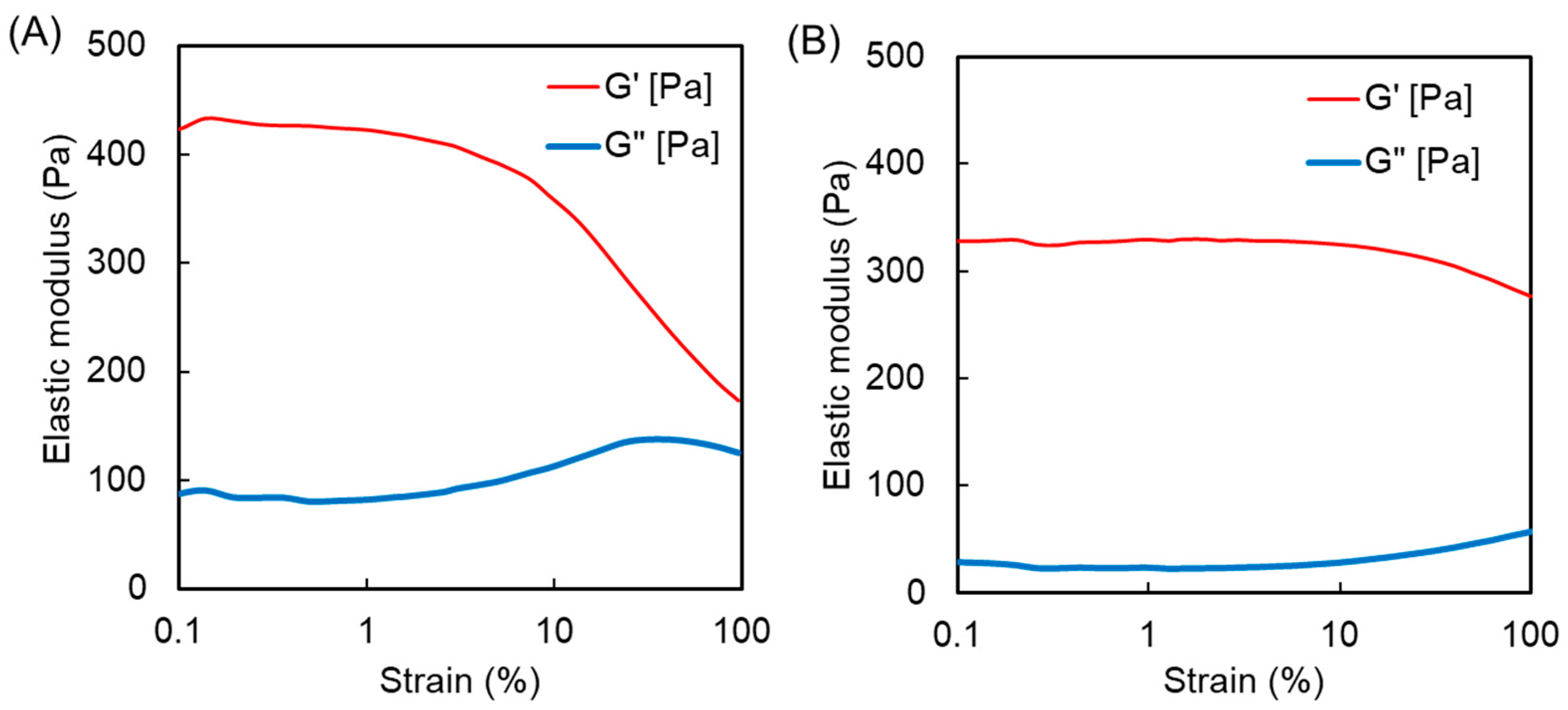
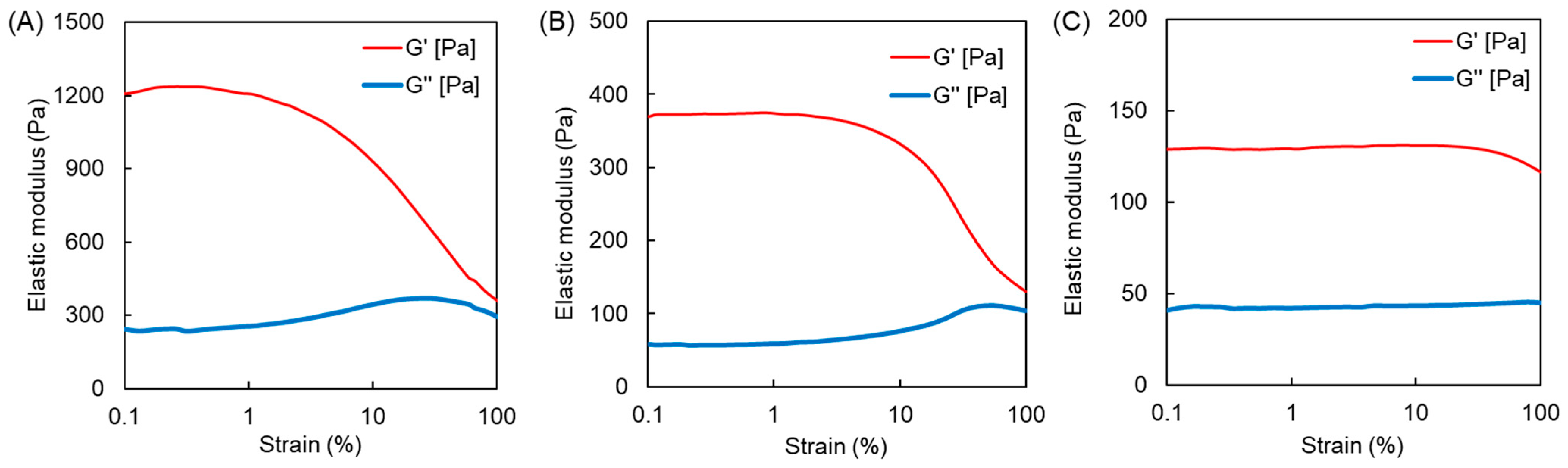
3.2. Synthesis and Characterization of Polymer Hydrogels Containing MPC and APC
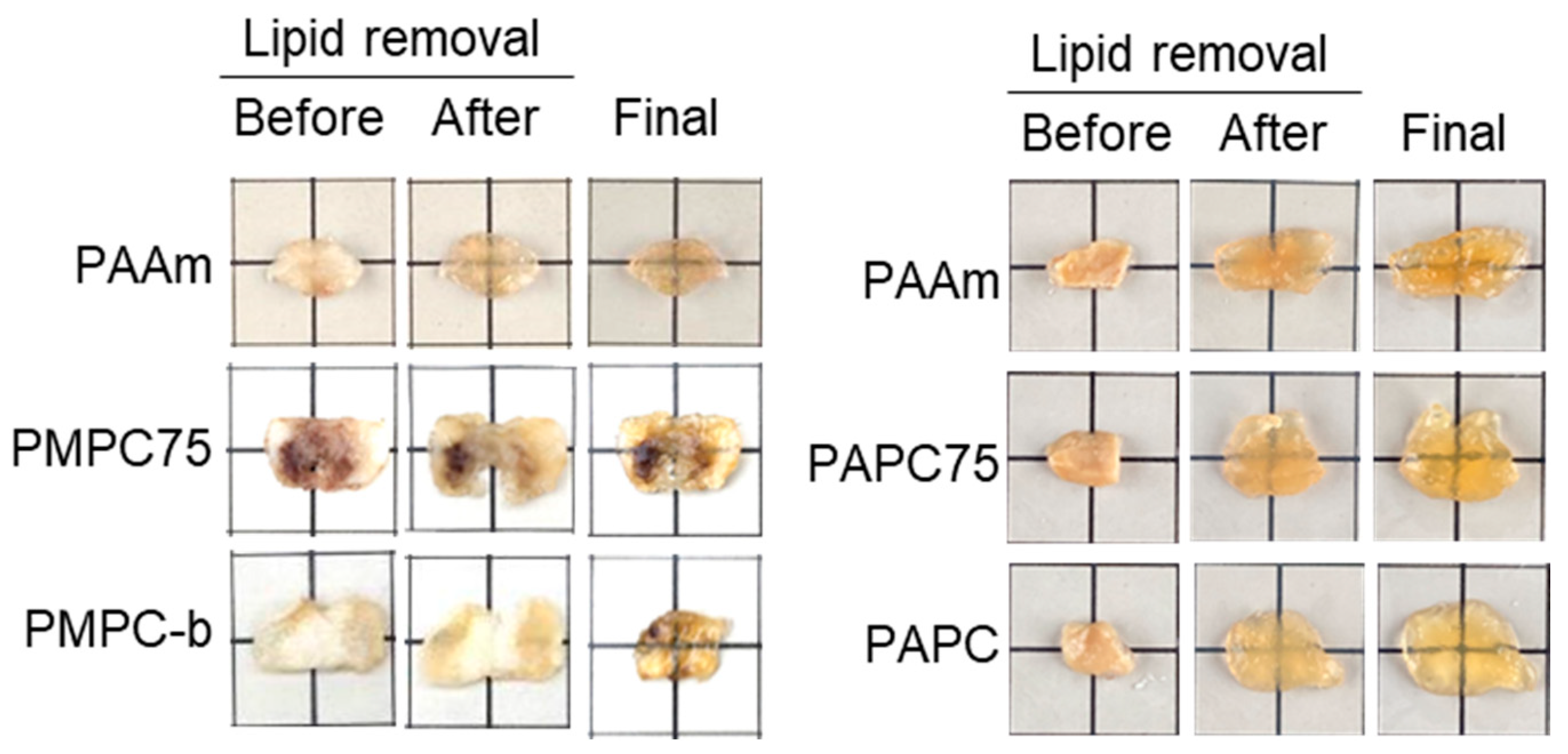
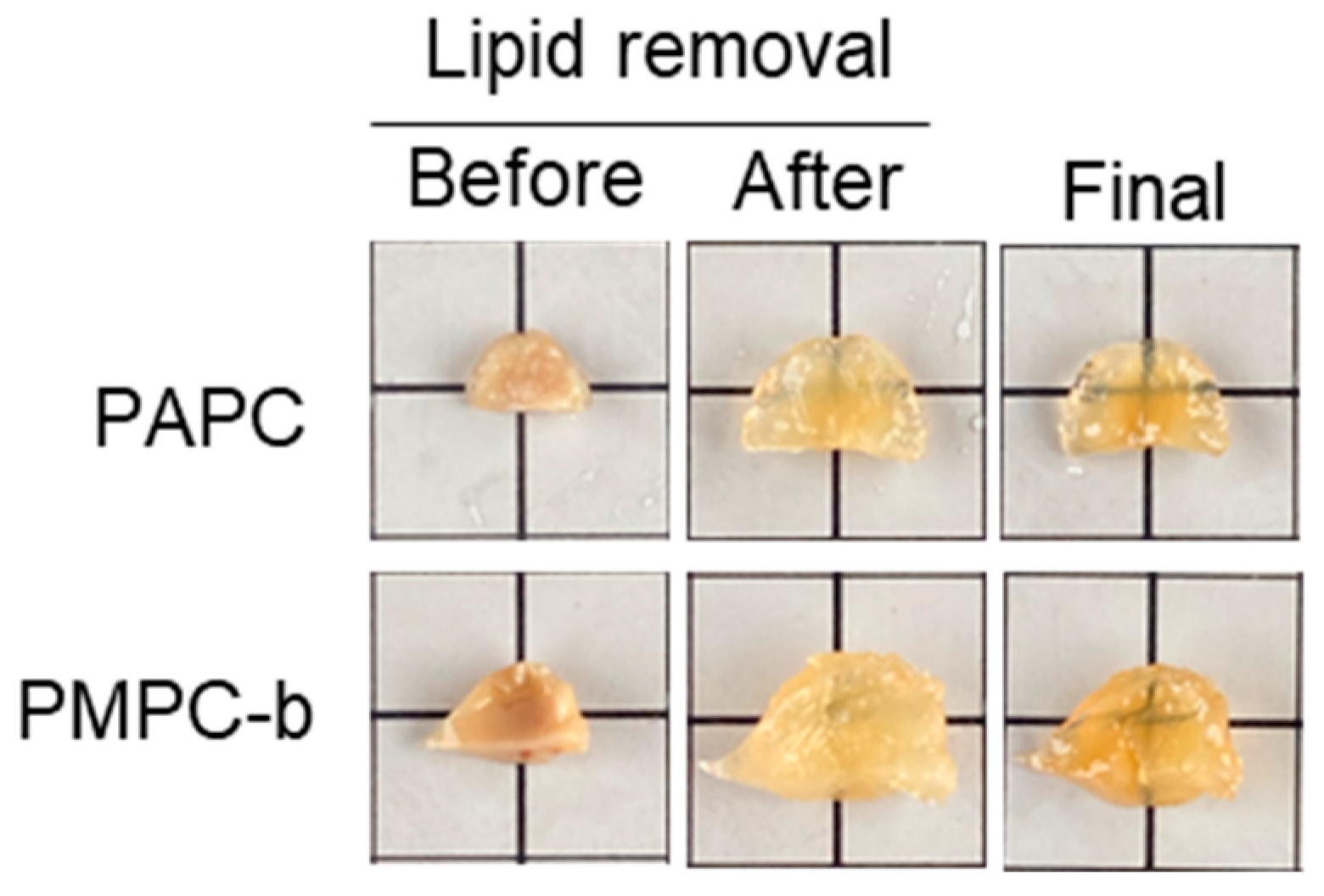
3.3. Optical Tissue Clearing Using APC- and MPC-Containing Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saha, P.; Ganguly, R.; Li, X.; Das, R.; Singha, N.K.; Pich, A. Zwitterionic nanogels and microgels: An overview on their synthesis and applications. Macromol. Rapid Commun. 2021, 42, 2100112. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, C.; Yang, J.; Zhou, X.; Zhu, Y.; Zheng, J.; Cheng, G.; Bai, J.; Xu, T.; Ji, J.; et al. Zwitterionic biomaterials. Chem. Rev. 2022, 122, 17073–17154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, P.; Xie, J.; Li, J. Recent advances of zwitterionic-based topological polymers for biomedical applications. J. Mater. Chem. B 2022, 10, 2338–2356. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Yong, W.F. Recent progress of zwitterionic materials as antifouling membranes for ultrafiltration, nanofiltration, and reverse osmosis. ACS Appl. Polym. Mater. 2021, 3, 4390–4412. [Google Scholar] [CrossRef]
- Ishihara, K. Biomimetic materials based on zwitterionic polymers toward human-friendly medical devices. Sci. Technol. Adv. Mater. 2022, 23, 498–524. [Google Scholar] [CrossRef] [PubMed]
- Seetasang, S.; Xu, Y. Recent progress and perspectives in applications of 2-methacryloyloxyethyl phosphorylcholine polymers in biodevices at small scales. J. Mater. Chem. B 2022, 14, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. A 2019, 107, 933–943. [Google Scholar] [CrossRef]
- Ishihara, K.; Shi, X.; Fukazawa, K.; Yamaoka, T.; Yao, G.; Wu, J.Y. Biomimetic-engineered silicone hydrogel contact lens materials. ACS Appl. Bio Mater. 2023, 6, 3600–3616. [Google Scholar] [CrossRef]
- Ishihara, K.; Oda, H.; Konno, T. Spontaneously and reversibly forming phospholipid polymer hydrogels as a matrix for cell engineering. Biomaterials 2020, 230, 119628. [Google Scholar] [CrossRef]
- Ertürk, A.; Bradke, F. High-resolution imaging of entire organs by 3-dimensional imaging of solvent cleared organs (3DISCO). Exp. Neurol. 2013, 242, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Susaki, E.A.; Ueda, H.R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: Toward organism-level systems biology in mammals. Cell Chem. Biol. 2016, 23, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Larin, K.V.; Luo, Q.; Tuchin, V.V. Recent progress in tissue optical clearing. Laser Photonics Rev. 2013, 7, 732–757. [Google Scholar] [CrossRef]
- Richardson, D.S.; Guan, W.; Matsumoto, K.; Pan, C.; Chung, K.; Ertürk, A.; Ueda, H.R.; Lichtman, J.W. Tissue clearing. Nat. Rev. Methods Primers 2021, 1, 84. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Yang, Z.; Li, X. Tissue clearing technique: Recent progress and biomedical applications. J. Anat. 2021, 238, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Ertürk, A.; Chung, K.; Gradinaru, V.; Chédotal, A.; Tomancak, P.; Keller, P.J. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 2020, 21, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Wallace, J.; Kim, S.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.K.; et al. Structural and molecular interrogation of intact biological systems. Nature 2013, 497, 332–337. [Google Scholar] [CrossRef]
- Tomer, R.; Ye, L.; Hsueh, B.; Deisseroth, K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 2014, 9, 1682–1697. [Google Scholar] [CrossRef]
- Kojima, C.; Koda, T.; Nariai, T.; Ichihara, J.; Sugiura, K.; Matsumoto, A. Application of zwitterionic polymer hydrogels to optical tissue clearing for 3D fluorescence imaging. Macromol. Biosci. 2021, 21, 2100170. [Google Scholar] [CrossRef]
- Ono, Y.; Nakase, I.; Matsumoto, A.; Kojima, C. Rapid optical tissue clearing using poly(acrylamide-co-styrenesulfonate) hydrogels for three-dimensional imaging. J. Biomed. Mater. Res. B 2019, 107, 2297–2304. [Google Scholar] [CrossRef]
- Koda, T.; Dohi, S.; Tachi, H.; Suzuki, Y.; Kojima, C.; Matsumoto, A. One-shot preparation of polyacrylamide/poly(sodium styrenesulfonate) double-network hydrogels for rapid optical tissue clearing. ACS Omega 2019, 4, 21083–21090. [Google Scholar] [CrossRef]
- Dohi, S.; Suzuki, Y.; Matsumoto, A. One-shot radical polymerization of vinyl monomers with different reactivity accompanying spontaneous delay of polymerization for the synthesis of double-network hydrogels. Polym. Int. 2020, 69, 954–963. [Google Scholar] [CrossRef]
- Roos, S.G.; Müller, A.H.E.; Matyjaszewski, K. Copolymerization of n-butyl acrylate with methyl methacrylate and PMMA macromonomers: Comparison of reactivity ratios in conventional and atom transfer radical copolymerization. Macromolecules 1999, 32, 8331–8335. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M. Rate coefficients of free-radical polymerization deduced from pulsed laser experiments. Prog. Polym. 2002, 27, 191–254. [Google Scholar] [CrossRef]
- Ruiz, L.; Hilborn, J.G.; Léonard, D.; Mathieu, H.J. Synthesis, structure and surface dynamics of phosphorylcholine functional biomimicking polymers. Biomaterials 1998, 19, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xia, Y.; Yang, S. Buckling, symmetry breaking, and cavitation in periodically micro-structured hydrogel membranes. Soft Matter 2014, 10, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Buback, M.; Feldermann, A.; Barner-Kowollik, C.; Lacík, I. Propagation rate coefficients of acrylate–methacrylate free-radical bulk copolymerizations. Macromolecules 2001, 34, 5439–5448. [Google Scholar] [CrossRef]
- Li, D.; Grady, M.C.; Hutchinson, R.A. High-temperature semibatch free radical copolymerization of butyl methacrylate and butyl acrylate. Ind. Eng. Chem. Res. 2005, 44, 2506–2517. [Google Scholar] [CrossRef]
- Lorenzo, D.F.; Seiffert, S. Nanostructural heterogeneity in polymer networks and gels. Polym. Chem. 2015, 6, 5515–5528. [Google Scholar] [CrossRef]
- Saimi, Y.; Ishihara, K.; Nakabayashi, N. Preparation and visible light polymerization of triethyleneglycol acrylate methacrylate. Polym. J. 1992, 24, 357–363. [Google Scholar] [CrossRef]
- Yang, B.; Treweek, J.B.; Kulkarni, R.P.; Deverman, B.E.; Chen, C.; Lubeck, E.; Shah, S.; Cai, L.; Gradinaru, V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 2014, 158, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Treweek, J.B.; Chan, K.Y.; Flytzanis, N.C.; Yang, B.; Deverman, B.E.; Greenbaum, A.; Lignell, A.; Xiao, C.; Cai, L.; Ladinsky, M.S.; et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc. 2015, 10, 1860–1896. [Google Scholar] [CrossRef] [PubMed]
- Parra-Damas, A.; Saura, C.A. Tissue clearing and expansion methods for imaging brain pathology in neurodegeneration. Front. Neurosci. 2020, 14, 00914. [Google Scholar] [CrossRef]
- Asano, S.M.; Gao, R.; Wassie, A.T.; Tillberg, P.; Chen, F.; Boyden, E.S. Expansion microscopy: Protocols for imaging proteins and RNA in cells and tissues. Curr. Protoc. Cell Biol. 2018, 80, 30070431. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.J.; Carannante, V.; Kuhnigk, K.; Ring, H.; Tararuk, T.; Hallböök, F.; Blom, H.; Önfelt, B.; Brismar, H. High-resolution imaging of tumor spheroids and organoids enabled by expansion microscopy. Front. Mol. Biosci. 2020, 7, 7543521. [Google Scholar] [CrossRef]

| Name | Monomer (mol%) | Crosslinker | Gel Fraction (-) |
|---|---|---|---|
| PAAm | AAm | bisAA | 1 * |
| PAPC50 | APC/AAm (50/50) | bisAA | 0.6 |
| PAPC75 | APC/AAm (75/25) | bisAA | 0.6 |
| PAPC | APC | bisAA | 0 |
| PMPC50 | MPC/AAm (50/50) | bisAA | 0.8 * |
| PMPC75 | MPC/AAm (75/25) | bisAA | 0.9 * |
| PMPC-b | MPC | bisAA | 0.9 * |
| PMPC-t | MPC | TEGMA | 0 * |
| Polymer | Mn (/105) | Mw (/105) | Mw/Mn |
|---|---|---|---|
| PAAm | 1.1 | 4.8 | 4.4 |
| PAPC | 0.13 | 0.79 | 6.0 |
| PMPC | 2.6 | 8.4 | 3.2 |
| PAPC50 | 0.50 | 1.7 | 3.4 |
| PMPC50 | 1.8 | 6.4 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dei, N.; Ishihara, K.; Matsumoto, A.; Kojima, C. Preparation and Characterization of Acrylic and Methacrylic Phospholipid-Mimetic Polymer Hydrogels and Their Applications in Optical Tissue Clearing. Polymers 2024, 16, 241. https://doi.org/10.3390/polym16020241
Dei N, Ishihara K, Matsumoto A, Kojima C. Preparation and Characterization of Acrylic and Methacrylic Phospholipid-Mimetic Polymer Hydrogels and Their Applications in Optical Tissue Clearing. Polymers. 2024; 16(2):241. https://doi.org/10.3390/polym16020241
Chicago/Turabian StyleDei, Nanako, Kazuhiko Ishihara, Akikazu Matsumoto, and Chie Kojima. 2024. "Preparation and Characterization of Acrylic and Methacrylic Phospholipid-Mimetic Polymer Hydrogels and Their Applications in Optical Tissue Clearing" Polymers 16, no. 2: 241. https://doi.org/10.3390/polym16020241
APA StyleDei, N., Ishihara, K., Matsumoto, A., & Kojima, C. (2024). Preparation and Characterization of Acrylic and Methacrylic Phospholipid-Mimetic Polymer Hydrogels and Their Applications in Optical Tissue Clearing. Polymers, 16(2), 241. https://doi.org/10.3390/polym16020241