Stabilization of Picea abies Spruce Bark Extracts within Ice-Templated Porous Dextran Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Picea abies SBE
2.3. Preparation of Dx Cryogels Containing Picea abies SBE
2.4. Characterization of Dx Cryogels Containing Picea abies SBE
2.5. The Release of SBE from the Dx Cryogels
2.6. Antimicrobial Assay
2.6.1. Bacterial Strains
2.6.2. Determination of Viable Bacterial Cell Numbers by Plate Count (Colony-Forming Units/mL or CFUs/mL)
2.7. Antioxidant Activity Evaluation
3. Results and Discussions
3.1. Preparation of Dx/SBE Cross-Linked Cryogels
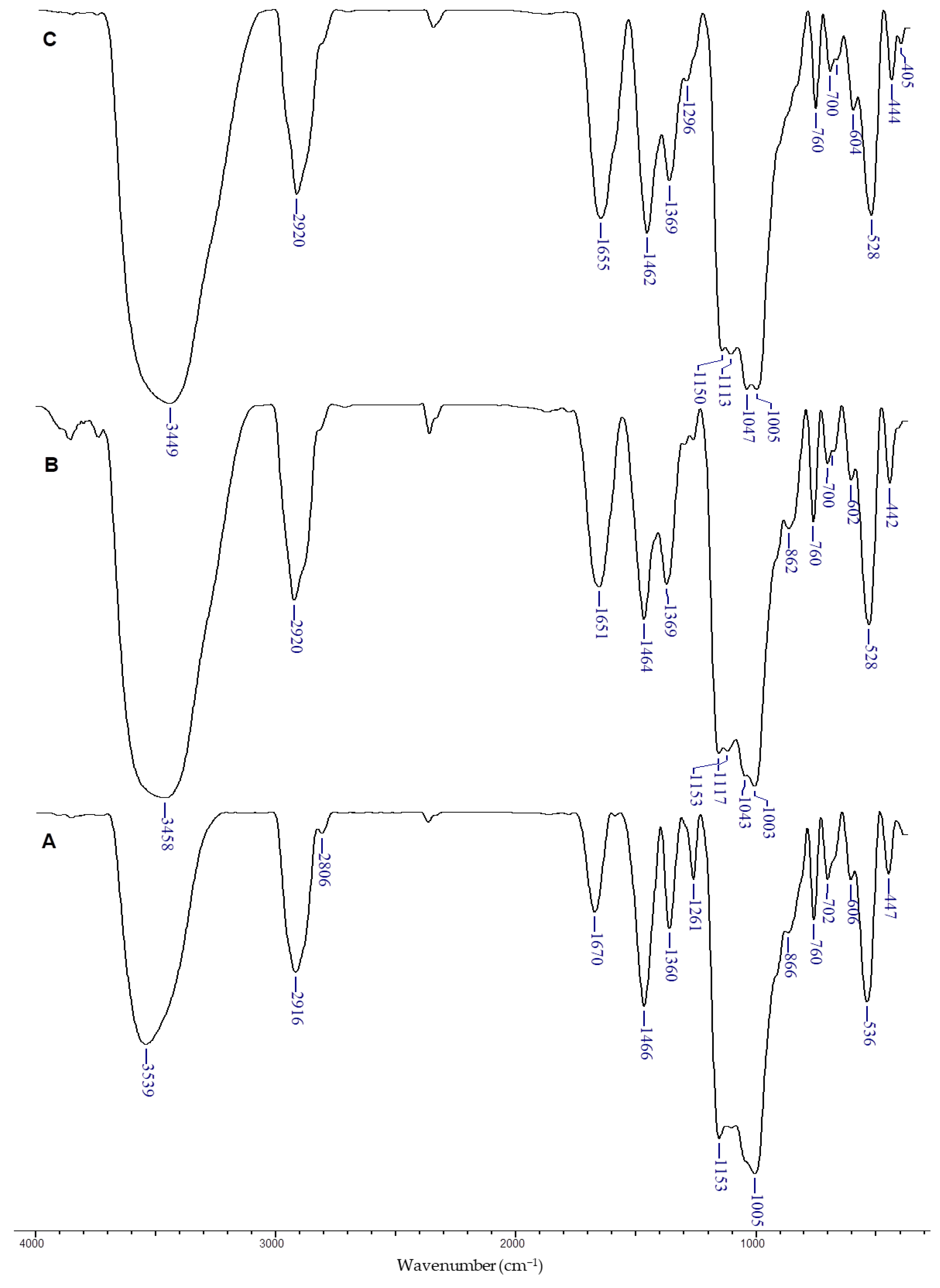
3.2. Structural and Elemental Characterization of Dx/SBE Cross-Linked Cryogels
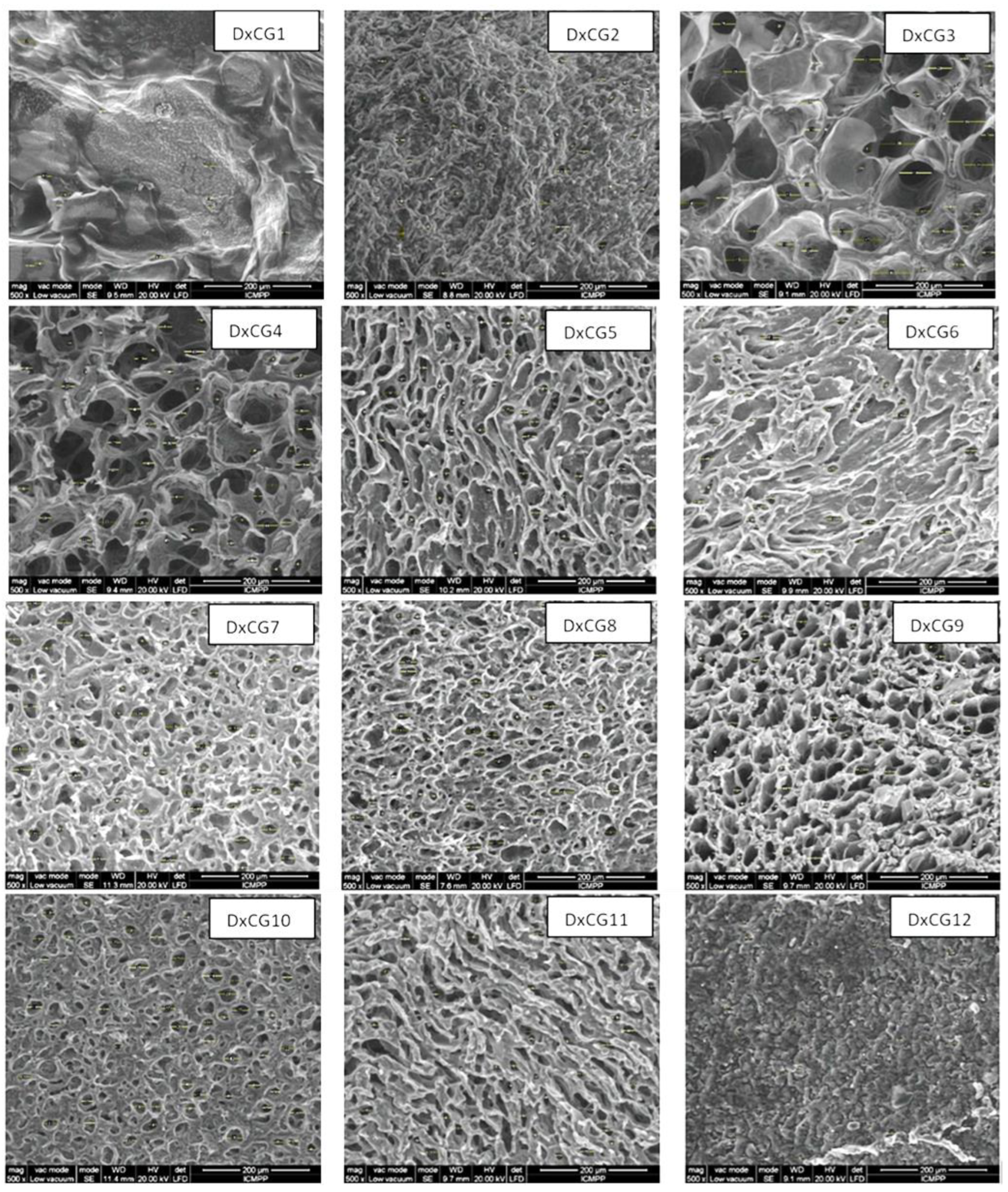
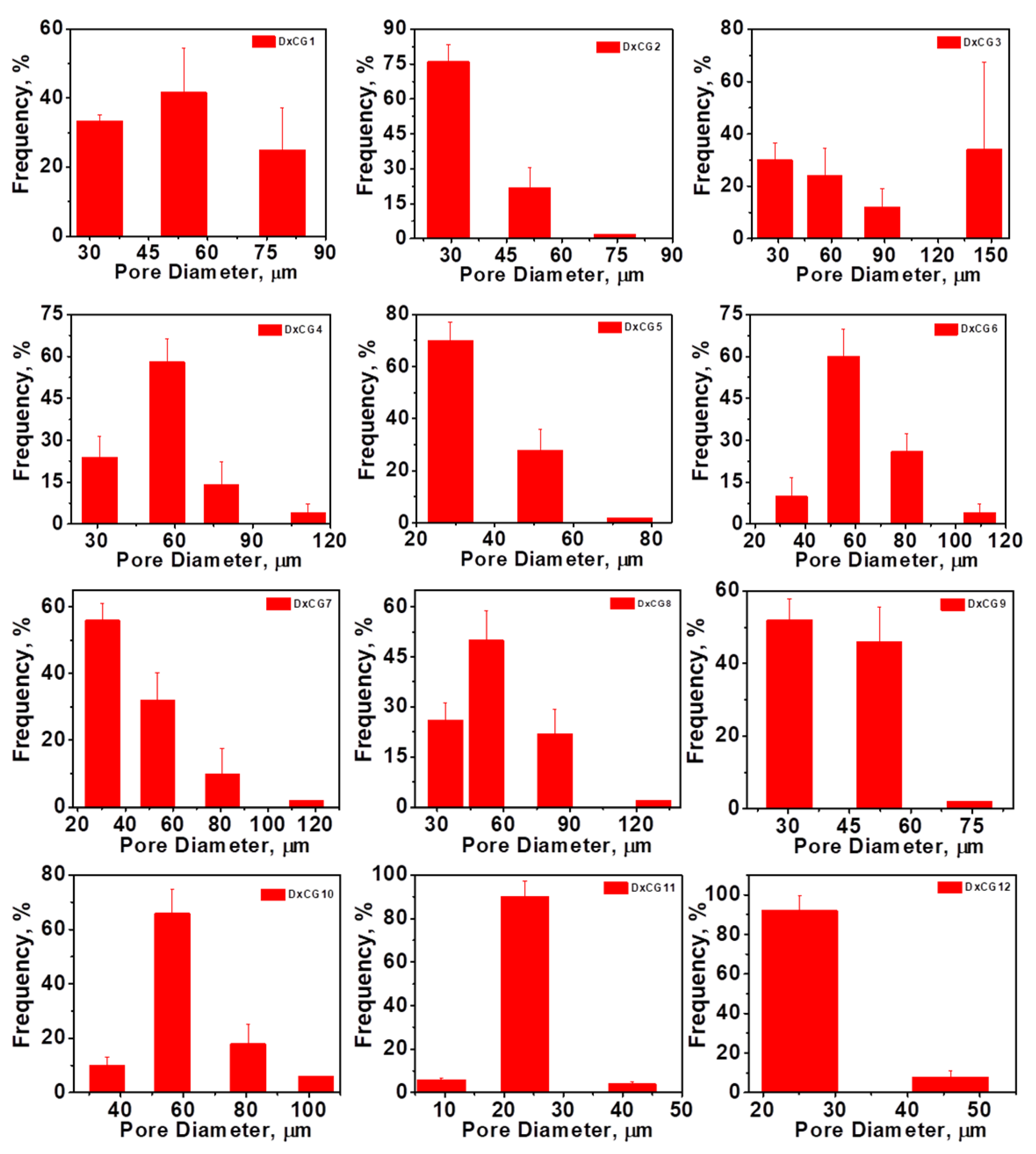
3.3. Morphology, Pore Sizes, and Porosity of Dx/SBE Cross-Linked Cryogels
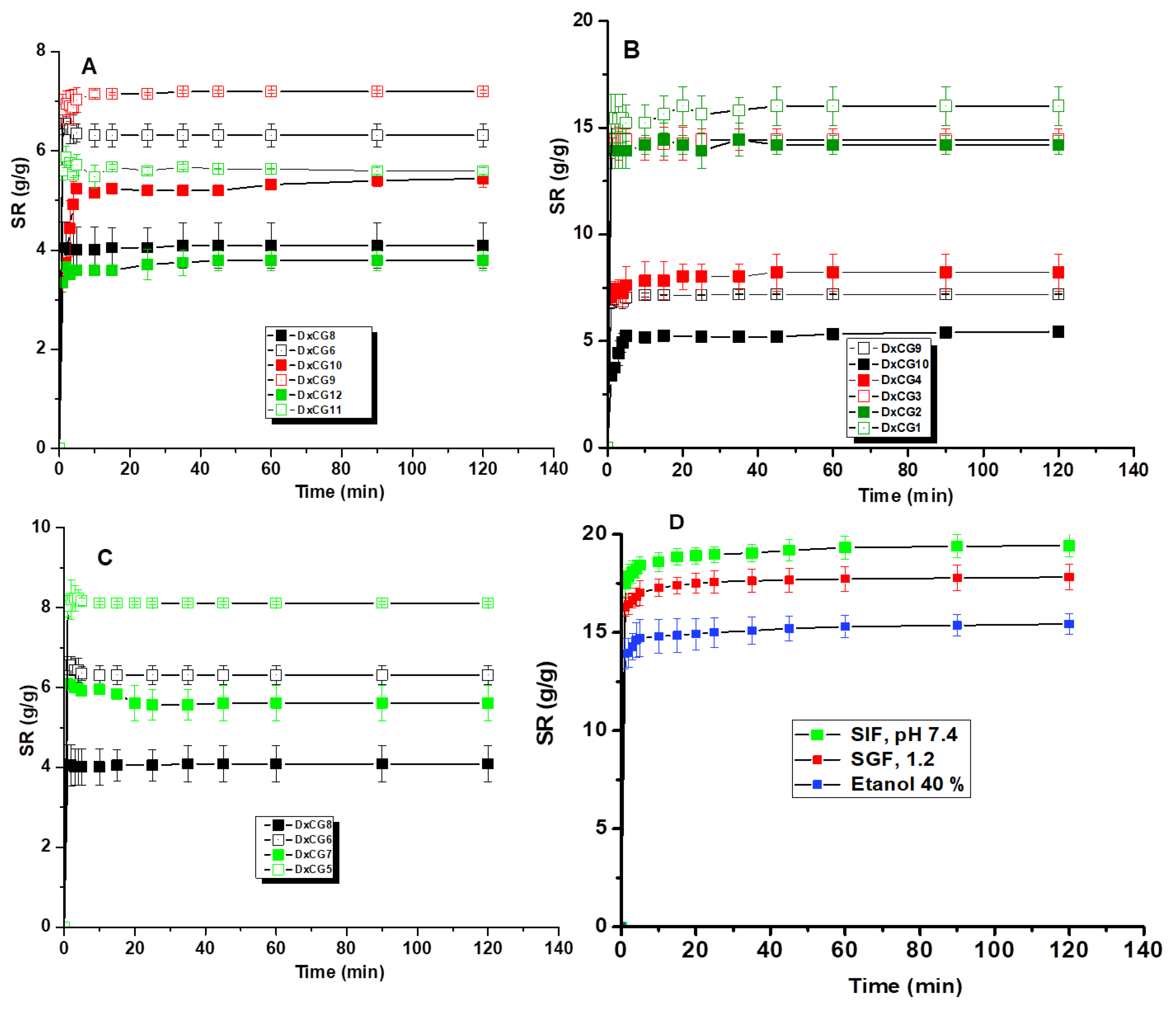
3.4. Swelling Behavior of Dx/SBE Cross-Linked Cryogels
3.5. Mechanical Properties of Dx/SBE Cross-Linked Cryogels
3.6. Stabilization of SBE within Dx-Based Hydrogels
3.7. Antimicrobial Activity
3.8. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Prooxidant Properties of Flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone Metal Complexes-Molecular Structure, Antioxidant Activity and Biological Effects. Chem. Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. Analytical Methods of Phenolic Compounds. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2061–2092. [Google Scholar] [CrossRef]
- Bǎlaş, A.; Popa, V.I. The Influence of Natural Aromatic Compounds on the Development of Lycopersicon Esculentum Plantlets. Bioresources 2007, 2, 363–370. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Volf, I.; Stîngu, A.; Popa, V.I. New Natural Chelating Agents with Modulator Effects on Copper Phytoextraction. Environ. Eng. Manag. J. 2012, 11, 487–491. [Google Scholar]
- Tanase, C.; Stingu, A.; Asachi, G.; Popa, V.I.; Volf, I. The Effect of Spruce Bark Polyphenols Extract in Combination with Deuterium Depleted Water (DDW) on Glycine max L. and Helianthus annuus L. Development 2010, 12, 3. [Google Scholar]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food. Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Xiao, J. Recent advances on the stability of dietary polyphenols. eFood 2002, 3, e21. [Google Scholar] [CrossRef]
- Ozkan, G.; Ceyhan, T.; Çatalkaya, G.; Rajan, L.; Ullah, H.; Daglia, M.; Capanoglu, E. Encapsulated Phenolic Compounds: Clinical Efficacy of a Novel Delivery Method. Phytochem. Rev. 2024, 23, 781–819. [Google Scholar] [CrossRef]
- Bobrysheva, T.; Anisimov, G.; Zolotoreva, M.; Evdokimov, I.; Budkevich, R.; Muravyev, A. Encapsulated Polyphenols in Functional Food Production. Food. Raw Mater. 2024, 18–34. [Google Scholar] [CrossRef]
- Ghiorghita, C.A.; Platon, I.V.; Lazar, M.M.; Dinu, M.V.; Aprotosoaie, A.C. Trends in Polysaccharide-Based Hydrogels and Their Role in Enhancing the Bioavailability and Bioactivity of Phytocompounds. Carbohydr. Polym. 2024, 334, 122033. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Xiang, Y.; Qi, X.; Mao, R.; Cai, E.; Lan, Y.; Lu, H.; Shen, J.; Deng, H. Harnessing a biopolymer hydrogel reinforced by copper/tannic acid nanosheets for treating bacteria-infected diabetic wounds. Mater. Today Adv. 2022, 15, 100271. [Google Scholar] [CrossRef]
- Platon, I.-V.; Ghiorghita, C.-A.; Lazar, M.M.; Aprotosoaie, A.C.; Gradinaru, A.C.; Nacu, I.; Verestiuc, L.; Nicolescu, A.; Ciocarlan, N.; Dinu, M.V.; et al. Highly Compressible, Superabsorbent, and Biocompatible Hybrid Cryogel Constructs Comprising Functionalized Chitosan and St. John’s Wort Extract. Biomacromolecules 2024, 25, 5081–5097. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Mao, R.; Xiang, Y.; Cai, E.; Chen, Y.; Shen, J.; Dong, W.; Qi, X. Together is better: Poly(tannic acid) nanorods functionalized polysaccharide hydrogels for diabetic wound healing. Ind. Crop. Prod. 2022, 186, 115273. [Google Scholar] [CrossRef]
- Ghiorghita, C.A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-Based Composite Hydrogels as Sustainable Materials for Removal of Pollutants from Wastewater. Molecules 2022, 27, 8574. [Google Scholar] [CrossRef]
- Sowasod, N.; Nakagawa, K.; Charinpanitkul, T.; Tanthapanichakoon, W. Encapsulation of Curcumin Loaded Oil Droplets with Chitosan Based Cryogel: Influence of Freezing Condition on Nanocapsule Properties. Food Sci. Technol. Res. 2013, 19, 633–640. [Google Scholar] [CrossRef]
- Lazzari, L.K.; Ornaghi, H.L.; Neves, R.M.; Kerche, E.F.; Zattera, A.J.; Santana, R.M.C. Bio-based cellulose-biochar-peg cryogels for thermal insulation. Cellul. Chem. Tehnol. 2024, 58, 91–99. [Google Scholar] [CrossRef]
- Lazzari, L.K.; Zattera, A.J.; Santana, R.M.C. Cellulose/Graphene Nanoplatelets Cryogel for Adsorption of Dyes in an Aqueous Medium. Cellul. Chem. Tehnol. 2024, 58, 409–417. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the Basis of Natural and Synthetic Polymers: Preparation, Properties and Application. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for Biomedical Applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, J.; Srivastava, P.; Nebhani, L. Mechanically Robust and Highly Bactericidal Macroporous Polymeric Gels Based on Quaternized N,N-(Dimethylamino)Ethyl Methacrylate Possessing Varying Alkyl Chain Lengths. J. Mater. Chem. B 2023, 11, 2234–2248. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent Advances in Natural Polymer-based Drug Delivery Systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Takase, H.; Watanabe, N.; Shiomori, K.; Okamoto, Y.; Ciptawati, E.; Matsune, H.; Umakoshi, H. Preparation of Hydrophobic Monolithic Supermacroporous Cryogel Particles for the Separation of Stabilized Oil-in-Water Emulsion. Colloid. Interf. 2023, 7, 9. [Google Scholar] [CrossRef]
- Ari, B.; Demirci, S.; Ayyala, R.S.; Salih, B.; Sahiner, N. Superporous Poly(β-Cyclodextrin) Cryogels as Promising Materials for Simultaneous Delivery of Both Hydrophilic and Hydrophobic Drugs. Eur. Polym. J. 2022, 176, 111399. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A Review of Cryogels Synthesis, Characterization and Applications on the Removal of Heavy Metals from Aqueous Solutions. Adv. Colloid. Interf. Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Davidson-Rozenfeld, G.; Chen, X.; Qin, Y.; Ouyang, Y.; Sohn, Y.S.; Li, Z.; Nechushtai, R.; Willner, I. Stiffness-Switchable, Biocatalytic PH-Responsive DNA-Functionalized Polyacrylamide Cryogels and Their Mechanical Applications. Adv. Funct. Mater. 2024, 34, 2306586. [Google Scholar] [CrossRef]
- Doser, G.; Su, E.; Okay, O. Effects of Cryogenic Condition and Chemistry on the Properties of Synthetic and Biopolymer Cryogels. React. Funct. Polym. 2023, 190, 105635. [Google Scholar] [CrossRef]
- Platon, I.V.; Ghiorghita, C.A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. [Google Scholar] [CrossRef] [PubMed]
- Anghel, N.; Dinu, M.V.; Zaltariov, M.; Pamfil, D.; Spiridon, I. New Cellulose-Collagen-Alginate Materials Incorporated with Quercetin, Anthocyanins and Lipoic Acid. Int. J. Biol. Macromol. 2021, 181, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Do, N.H.N.; Truong, Q.T.; Le, P.K.; Ha, A.C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr. Polym. 2022, 294, 119726. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically Cross-Linked Chitosan/Dextrin Cryogels Entrapping Thymus Vulgaris Essential Oil with Enhanced Mechanical, Antioxidant and Antifungal Properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the delivery of plant-derived (poly)phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Ding, W.; Remon, J.; Xin, M.; Sun, T.; Wang, J. Fabrication, performance, and potential environmental impacts of polysaccharide-based food packaging materials incorporated with phytochemicals: A review. Int. J. Biol. Macromol. 2023, 249, 25922. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Dragan, E.S.; Ghiorghita, C.A.; Dinu, M.V.; Duceac, I.A.; Coseri, S. Fabrication of Self-Antibacterial Chitosan/Oxidized Starch Polyelectrolyte Complex Sponges for Controlled Delivery of Curcumin. Food Hydrocoll. 2023, 135, 108147. [Google Scholar] [CrossRef]
- Stanescu, M.D.; Gavrilas, S.; Ludwig, R.; Haltrich, D.; Lozinsky, V.I. Preparation of Immobilized Trametes Pubescens Laccase on a Cryogel-Type Polymeric Carrier and Application of the Biocatalyst to Apple Juice Phenolic Compounds Oxidation. Eur. Food Res. Technol. 2012, 234, 655–662. [Google Scholar] [CrossRef]
- Tak, U.N.; Rashid, S.; Kour, P.; Nazir, N.; Zargar, M.I.; Dar, A.A. Bergenia Stracheyi Extract-Based Hybrid Hydrogels of Biocompatible Polymers with Good Adhesive, Stretching, Swelling, Self-healing, Antibacterial and Antioxidant Properties. Int. J. Biol. Macromol. 2023, 234, 123718. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.; Akbari, T.; Hasani, M.; Sharifikolouei, E.; Raoufi, M.; Foroumadi, A.; Sharifzadeh, M.; Firoozpour, L.; Khoobi, M. Polysaccharide-Based Hydrogels Containing Herbal Extracts for Wound Healing Applications. Carbohydr. Polym. 2022, 294, 119808. [Google Scholar] [CrossRef] [PubMed]
- Yavaşer, R.; Karagözler, A.A. Covalent Immobilization of Papain onto Poly(Hydroxyethyl Methacrylate)-Chitosan Cryogels for Apple Juice Clarification. Food Sci. Technol. Int. 2020, 26, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Dey Chowdhury, S.; Babanejad, N. Cryogels: Advancing Biomaterials for Transformative Biomedical Applications. Pharmaceutics 2023, 15, 1836. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Perju, M.M.; Drǎgan, E.S. Porous Semi-Interpenetrating Hydrogel Networks Based on Dextran and Polyacrylamide with Superfast Responsiveness. Macromol. Chem. Phys. 2011, 212, 240–251. [Google Scholar] [CrossRef]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-Polyethylene Glycol Cryogels as Spongy Scaffolds for Drug Delivery. Int. J. Biol. Macromol. 2021, 166, 1292–1300. [Google Scholar] [CrossRef]
- Lazar, M.M.; Damaschin, R.P.; Volf, I.; Dinu, M.V. Deep Cleaning of Crystal Violet and Methylene Blue dyes from aqueous solution by dextran-based cryogel adsorbents. Gels 2024, 10, 546. [Google Scholar] [CrossRef]
- Ari, B.; Yetiskin, B.; Okay, O.; Sahiner, N. Preparation of Dextran Cryogels for Separation Processes of Binary Dye and Pesticide Mixtures from Aqueous Solutions. Polym. Eng. Sci. 2020, 60, 1890–1901. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Lazar, M.M.; Hitruc, G.E.; Dinu, M.V. Ice-Templated and Cross-Linked Xanthan-Based Hydrogels: Towards Tailor-Made Properties. Gels 2023, 9, 528. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The Comparison of Physic-Chemical Properties of Chitosan/Collagen/Hyaluronic Acid Composites with Nano-Hydroxyapatite Cross-Linked by Dialdehyde Starch and Tannic Acid. Polym. Test. 2017, 62, 171–176. [Google Scholar] [CrossRef]
- Popescu, I.; Turtoi, M.; Suflet, D.M.; Dinu, M.V.; Darie-Nita, R.N.; Anghelache, M.; Calin, M.; Constantin, M. Alginate/poloxamer hydrogel obtained by thiol-acrylate photopolymerization for the alleviation of the inflammatory response of human keratinocytes. Int. J. Biol. Macromol. 2021, 180, 418. [Google Scholar] [CrossRef] [PubMed]
- Ghiorghita, C.A.; Humelnicu, D.; Dinu, M.V.; Ignat, M.; Bonardd, S.; Diaz, D.D.; Dragan, E.S. Polyelectrolyte complex composite cryogels with self-antibacterial properties and wide window for simultaneous removal of multiple contaminants. Chem. Eng. J. 2023, 459, 141562. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food Text with EEA Relevance-EU Monitor. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vim53kh2pbw4 (accessed on 21 August 2024).
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- ISO 7218:2007; Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations. Available online: https://www.iso.org/standard/36534.html (accessed on 21 August 2024).
- Raschip, I.E.; Fifere, N.; Dinu, M.V. A Comparative Analysis on the Effect of Variety of Grape Pomace Extracts on the Ice-Templated 3d Cryogel Features. Gels 2021, 7, 76. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Que, F.; Weiss, J.; Zhang, H. Characterization and Antioxidant Activity of Trilayer Gelatin/Dextran-Propyl Gallate/Gelatin Films: Electrospinning versus Solvent Casting. LWT 2020, 128, 109536. [Google Scholar] [CrossRef]
- Lu, X.; Xu, Y.; Zheng, C.; Zhang, G.; Su, Z. Ethylene glycol diglycidyl ether as a protein cross-linker: A case study for cross-linking of hemoglobin. J. Chem. Technol. Biot. 2006, 81, 767. [Google Scholar] [CrossRef]
- Díaz-Gonzalez, J.-C.M.; Escalona-Villalpando, R.A.; Arriaga, L.G.; Minteer, S.D.; Casanova-Moreno, J.R. Effects of the cross-linker on the performance and stability of enzymatic electrocatalytic films of glucose oxidase and dimethylferrocene-modified linear poly(ethyleneimine). Electrochim. Acta 2020, 337, 135782. [Google Scholar] [CrossRef]
- Enomoto-Rogers, Y.; Kimura, S.; Iwata, T. Soft, tough, and flexible curdlan hydrogels and organogels fabricated by covalent cross-linking. Polymer 2016, 100, 143. [Google Scholar] [CrossRef]
- Vargas, G.; Acevedo, J.L.; López, J.; Romero, J. Study of cross-linking of gelatin by ethylene glycol diglycidyl ether. Mater. Lett. 2008, 62, 3656. [Google Scholar] [CrossRef]
- Bratskaya, S.; Privar, Y.; Nesterov, D.; Modin, E.; Kodess, M.; Slobodyuk, A.; Marinin, D.; Pestov, A. Chitosan gels and cryogels cross-linked with diglycidyl ethers of ethylene glycol and polyethylene glycol in acidic media. Biomacromolecules 2019, 20, 1635. [Google Scholar] [CrossRef] [PubMed]
- Speit, G.; Neuss, S.; Schütz, P.; Fröhler-Keller, M.; Schmid, O. The genotoxic potential of glutaraldehyde in mammalian cells in vitro in comparison with formaldehyde. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 649, 146. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.; Larsson, A.; Okay, O. Preparation and physical properties of hyaluronic acid based cryogels. J. Appl. Polym. Sci. 2015, 132, 42194. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Varganici, C.-D.; Dinu, M.V. Development of antioxidant and antimicrobial xanthan-based cryogels with tuned porous morphology and controlled swelling features. Int. J. Biol. Macromol. 2020, 156, 608. [Google Scholar] [CrossRef]
- Kang, M.; Oderinde, O.; Liu, S.; Huang, Q.; Ma, W.; Yao, F.; Fu, G. Characterization of Xanthan Gum-Based Hydrogel with Fe3+ Ions Coordination and Its Reversible Sol-Gel Conversion. Carbohydr. Polym. 2019, 203, 139–147. [Google Scholar] [CrossRef]
- Park, Y.S.; Towantakavanit, K.; Kowalska, T.; Jung, S.T.; Ham, K.S.; Heo, B.G.; Cho, J.Y.; Yun, J.G.; Kim, H.J.; Gorinstein, S. Bioactive Compounds and Antioxidant and Antiproliferative Activities of Korean White Lotus Cultivars. J. Med. Food 2009, 12, 1057–1064. [Google Scholar] [CrossRef]
- Gorinstein, S.; Park, Y.S.; Heo, B.G.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Ham, K.S.; Cho, J.Y.; Kang, S.G. A Comparative Study of Phenolic Compounds and Antioxidant and Antiproliferative Activities in Frequently Consumed Raw Vegetables. Eur. Food Res. Technol. 2009, 228, 903–911. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Spruce Wood Bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-Ray (EDX) Microanalysis: A Powerful Tool in Biomedical Research and Diagnosis. Eur. J. Histochem. 2018, 62, 89–99. [Google Scholar] [CrossRef]
- Mrázková, M.; Sumczynski, D.; Orsavová, J. Influence of storage Conditions on stability of phenolic compounds and antioxidant activity values in nutraceutical mixtures with edible flowers as new dietary supplements. Antioxidants 2023, 12, 962. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Unalan, I.; Aguayo, C.R.; Fernández, K.; Boccaccini, A.R. Multifunctional Chitosan Scaffold Platforms Loaded with Natural Polyphenolic Extracts for Wound Dressing Applications. Biomacromolecules 2023, 24, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Cosarca, A.; Man, A.; Miklos, A.; Imre, S. Antibacterial Activities of Spruce Bark (Picea abies L.) Extract and Its Components against Human Pathogens. Rev.Chim. 2018, 69, 1462–1467. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Oroian, S.; Coşarcă, S.; Toma, F.; Mare, A.; Man, A. Antibacterial Activity of Spruce Bark (Picea abies L.) Extract against Escherichia Coli. Acta Biol. Marisiensis 2018, 1, 5–9. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial Activity of Phenolic Compounds Identified in Wild Mushrooms, SAR Analysis and Docking Studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic Acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell Longev. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- George, A.S.; Brandl, M.T. Plant Bioactive Compounds as an Intrinsic and Sustainable Tool to Enhance the Microbial Safety of Crops. Microorganisms 2021, 9, 2485. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases. 2015; World Health Organization: Geneva, Switzerland, 2020; p. 254.
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Phytochemical Profile and Biological Effects of Spruce (Picea abies) Bark Subjected to Ultrasound Assisted and Microwave-Assisted Extractions. Plants 2021, 10, 870. [Google Scholar] [CrossRef]
- Shi, M.; Nie, Y.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Ultraviolet B (UVB) photosensitivities of tea catechins and the relevant chemical conversions. Molecules 2016, 21, 1345. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Neagoe, I.B. The Relationships Between Biological Activities and Structure of Flavan-3-Ols. Int. J. Mol. Sci. 2011, 12, 9342. [Google Scholar] [CrossRef] [PubMed]



| Sample Code | Dx, wt.% | SBE, g | H2O, mL | a EGDGE, g/mL | bGFY, % | cEE, % | dCLDx, % | eP, % |
|---|---|---|---|---|---|---|---|---|
| DxCG1 | 5 | 0 | 2 | 0.28 | 83.00 | - | 11.16 | 78.9 |
| DxCG2 | 5 | 1.4 | - | 0.28 | 41.9 | 6.22 | 13.86 | 77.31 |
| DxCG3 | 10 | 0 | 2 | 0.28 | 80.66 | - | 12.47 | 72.96 |
| DxCG4 | 10 | 1.4 | - | 0.28 | 60.63 | 10.85 | 11.95 | 83.52 |
| DxCG5 | 20 | 0 | 1 | 0.42 | 94.23 | - | 2.88 | 48.36 |
| DxCG6 | 20 | 0 | 2 | 0.42 | 96.4 | - | 2.05 | 56.25 |
| DxCG7 | 20 | 0.7 | - | 0.42 | 75.97 | 33.14 | 8.72 | 55.01 |
| DxCG8 | 20 | 1.4 | - | 0.42 | 62.19 | 29.21 | 9.01 | 57.23 |
| DxCG9 | 20 | 0 | 2 | 0.28 | 87.78 | - | 5.63 | 58.74 |
| DxCG10 | 20 | 1.4 | - | 0.28 | 57.34 | 12.74 | 11.08 | 96.36 |
| DxCG11 | 20 | 0 | 2 | 0.56 | 91.1 | - | 3.46 | 54.27 |
| DxCG12 | 20 | 1.4 | - | 0.56 | 69.62 | 36.51 | 5.37 | 94.24 |
| Sample Code | Compressive Nominal Stress, kPa | Strain % | Compressive Elastic Modulus, kPa | R2 |
|---|---|---|---|---|
| DxCG6 | 679.72 | 90.90 | 15.40 | 0.997 |
| DxCG8 | 404.43 | 84.58 | 5.43 | 0.998 |
| DxCG11 | 1003.93 | 97.73 | 25.22 | 0.997 |
| DxCG12 | 383.64 | 86.61 | 8.18 | 0.998 |
| Microorganisms | Inhibition of Bacterial Growth (%) | ||||
|---|---|---|---|---|---|
| DxCG6 | DxCG7 | DxCG8 | SBE | ||
| Gram(+) | Listeria monocytogenes ATCC 7644 | 51 | 100 | 100 | 100 |
| Gram(−) | Escherichia coli ATCC 25922 | 27 | 100 | 100 | 100 |
| Gram(−) | Salmonella typhymurium ATCC 14028 | 67 | 100 | 100 | 100 |
| Samples | DPPH Radical Inhibition (%) | IC50 (mg/mL) |
|---|---|---|
| DxCG6 | 0 | - |
| DxCG7 | 30.37 ± 0.11 | - |
| DxCG8 | 42.19 ± 0.29 | - |
| SBE 3.33 mg/mL | 78.17 ± 0.19 | 1.76 ± 0.05 |
| SBE 1.66 mg/mL | 54.12 ± 0.12 | |
| SBE 0.83 mg/mL | 17.11 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damaschin, R.P.; Lazar, M.M.; Ghiorghita, C.-A.; Aprotosoaie, A.C.; Volf, I.; Dinu, M.V. Stabilization of Picea abies Spruce Bark Extracts within Ice-Templated Porous Dextran Hydrogels. Polymers 2024, 16, 2834. https://doi.org/10.3390/polym16192834
Damaschin RP, Lazar MM, Ghiorghita C-A, Aprotosoaie AC, Volf I, Dinu MV. Stabilization of Picea abies Spruce Bark Extracts within Ice-Templated Porous Dextran Hydrogels. Polymers. 2024; 16(19):2834. https://doi.org/10.3390/polym16192834
Chicago/Turabian StyleDamaschin, Roxana Petronela, Maria Marinela Lazar, Claudiu-Augustin Ghiorghita, Ana Clara Aprotosoaie, Irina Volf, and Maria Valentina Dinu. 2024. "Stabilization of Picea abies Spruce Bark Extracts within Ice-Templated Porous Dextran Hydrogels" Polymers 16, no. 19: 2834. https://doi.org/10.3390/polym16192834
APA StyleDamaschin, R. P., Lazar, M. M., Ghiorghita, C.-A., Aprotosoaie, A. C., Volf, I., & Dinu, M. V. (2024). Stabilization of Picea abies Spruce Bark Extracts within Ice-Templated Porous Dextran Hydrogels. Polymers, 16(19), 2834. https://doi.org/10.3390/polym16192834







