Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications
Abstract
1. Introduction
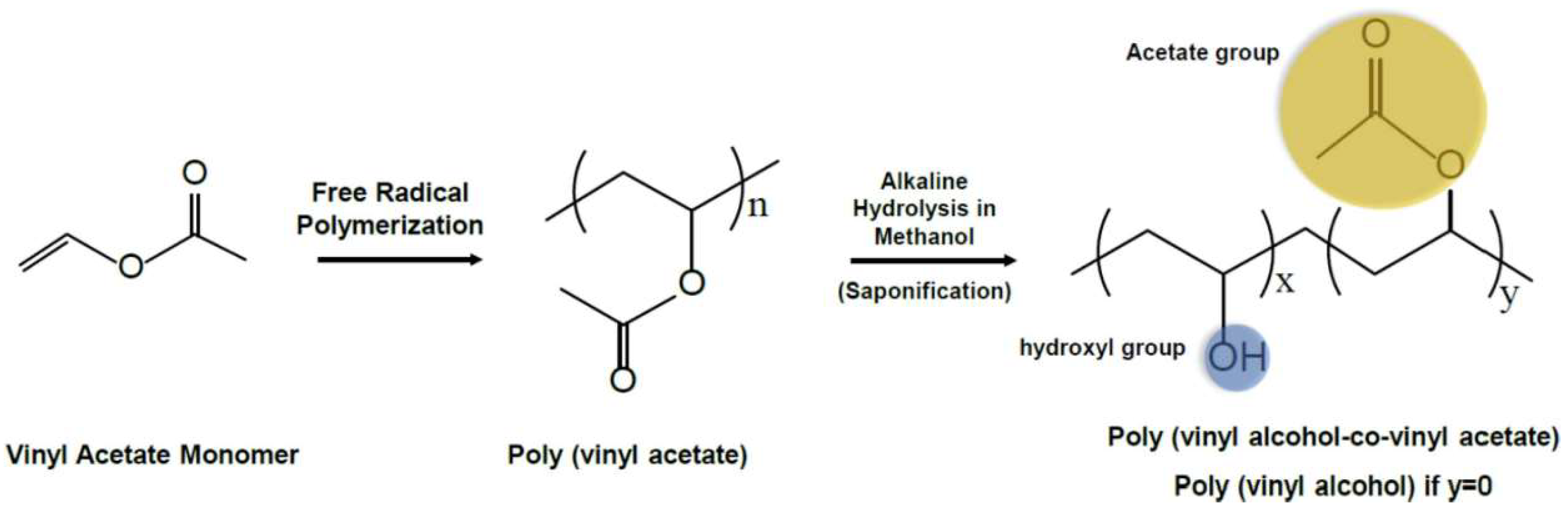
2. Fabrication and Enhancement of PVA-Based Hydrogels
2.1. Physical Cross-Linking Method of PVA-Based Hydrogels
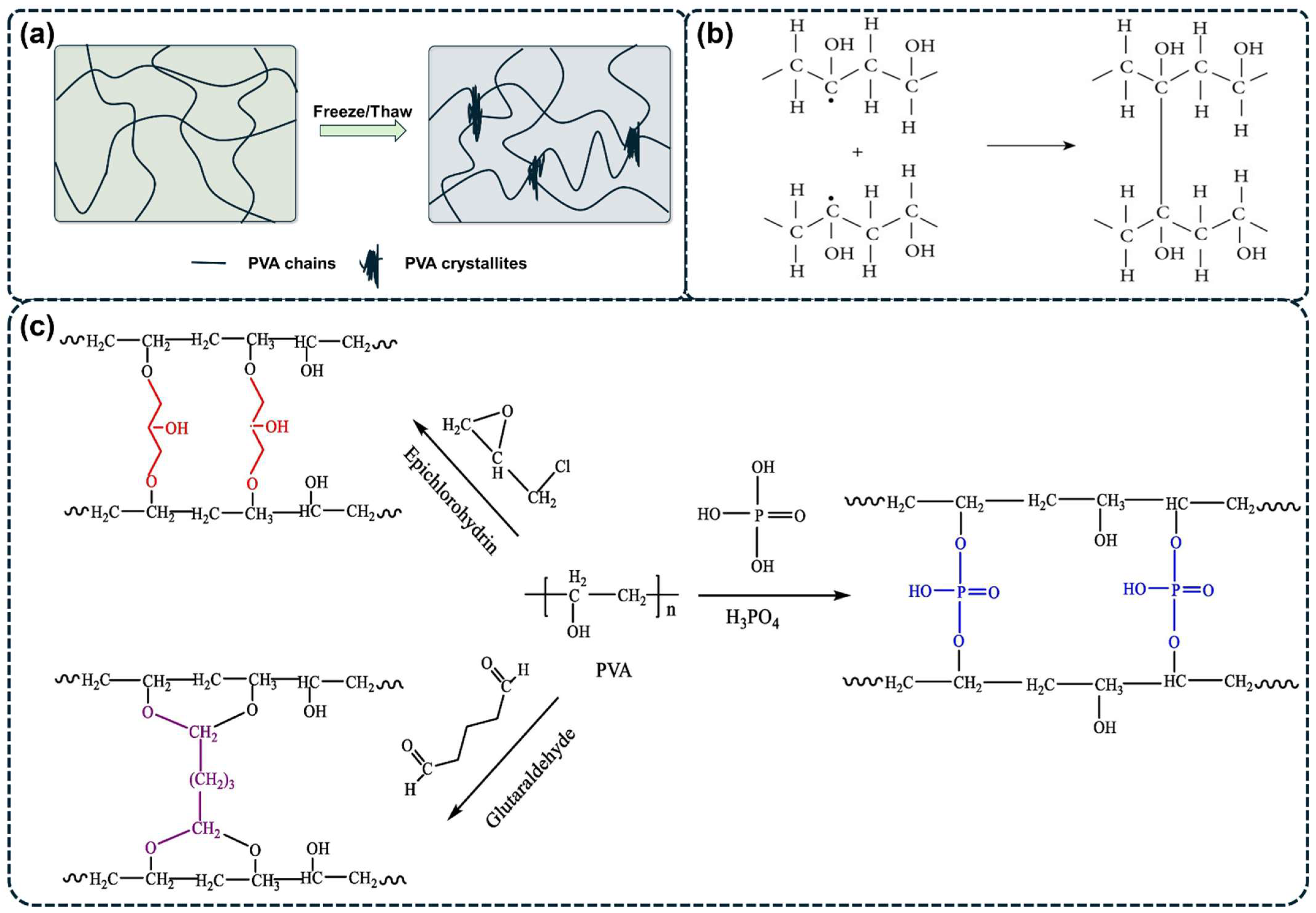
2.2. Irradiation Cross-Linking Methods
2.2.1. Irradiation Cross-Linking
2.2.2. Chemical Reagent Cross-Linking
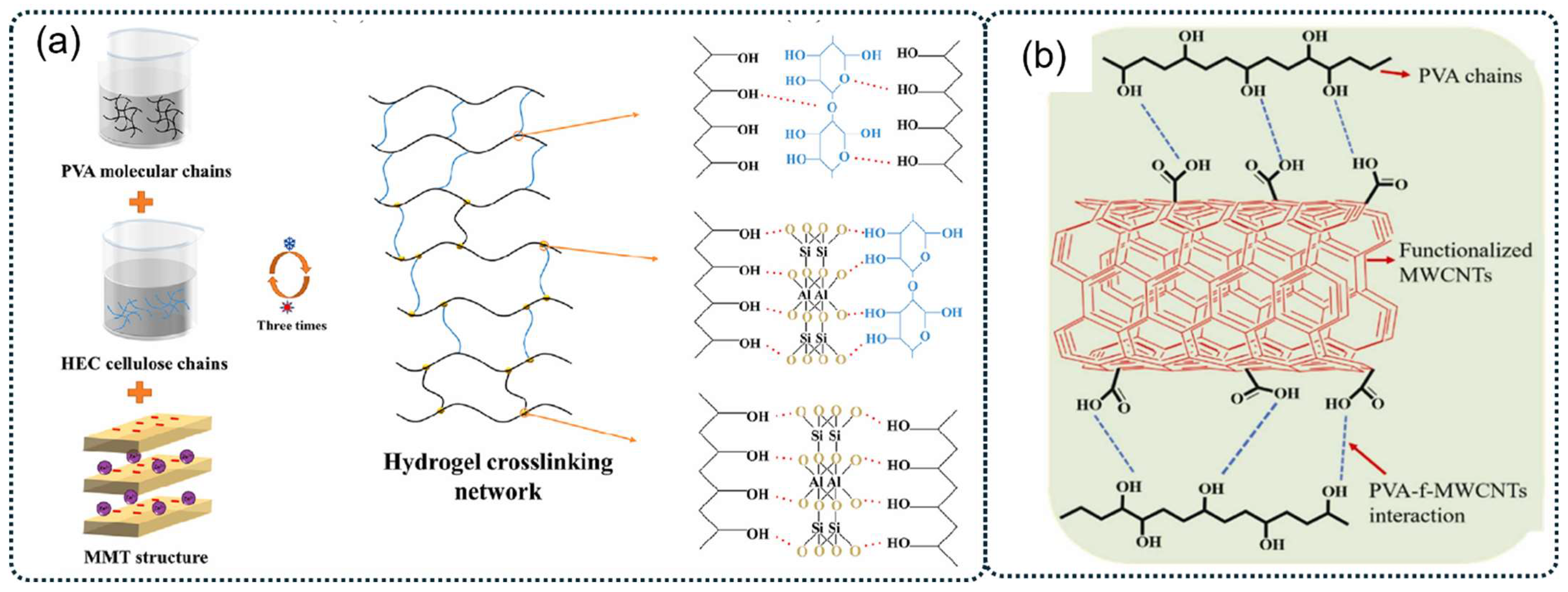
2.3. Enhancement of PVA-Based Hydrogels
3. Properties and Applications of PVA-Based Hydrogels
3.1. Biomedical Application of PVA-Based Hydrogels
3.1.1. Drug Delivery Systems
3.1.2. Wound Dressing
3.1.3. Tissue Engineering
| Hydrogel System | Fabrication Method | Results | Limitation | Application | Ref. |
|---|---|---|---|---|---|
| SA/PVA hydrogel | F/T cycle | 1. High swelling ratios achieved (up to 20 g/g in DI water). 2. Low drug release at pH 1.2; highest release (55%) at pH 8.0 after 6 h. 3. Release kinetics indicated non-Fickian diffusion mechanism. 4. Good mechanical properties and biocompatibility. | 1. Decreased stability at pH 8.0 after 5–6 h. 2. Only in vitro studies conducted. | Drug delivery carriers | [86] |
| β-cyclodextrin/CS-based (PVA-co-acrylic acid) hydrogels | Free radical grafting technique | 1. pH-sensitive swelling and drug release, peaking at pH 7.4. 2. Enhanced bioavailability of gallic acid with higher plasma concentrations than free drug solutions. 3. Good antioxidant and antibacterial properties. | 1. Tested only with gallic acid. 2. Long-term stability not evaluated. | Controlled drug delivery systems | [92] |
| Hydroxypropyl chitosan/PVA hydrogel (HOBP) | HPCS and PVA cross-linked with borax and OSA. | 1. Excellent injectability and self-healing properties. 2. High antimicrobial efficacy (E. coli: 86.18%, S. aureus: 85.69%). 3. High biocompatibility (cell viability >80%). 4. Favorable slow-release drug performance (168 h). | 1. Long-term in vivo stability and degradation not extensively studied. 2. Potential toxicity of borax. | Localized drug delivery | [77] |
| Conductive hydrogel (PVA/CS/GO) | F/T cycle | 1. Integration of electrical stimulation with drug delivery. 2. Dual effects: electronic drug release and tissue repair. 3. Low-voltage stimulation (2–5 V) enhances biological performance. | 1. High concentrations of GO may raise concerns regarding long-term biocompatibility. 2. Potential cytotoxicity at low CS concentration. | Controlled transdermal drug delivery | [93] |
| rGO-PDA@ZIF-8/PVA/CS composite hydrogel | Bidirectional freezing method and phase separation technique | 1. Excellent mechanical properties, low hemolysis rate, and water retention capabilities. 2. High biocompatibility and significant antibacterial effects against E. coli (99.1%) and S. aureus (99.0%). 3. Promoted wound healing effectively. | 1. Slight decrease in wound healing area under 808 nm light irradiation due to higher temperature. 2. Incorporation of rGO-PDA@ZIF-8 slightly reduced water retention compared to PVA/CS alone. | Wound healing | [87] |
| PVA/CNT hydrogel | Freeze-casting-assisted compression annealing and salting-out (FCAS) strategy | 1. Low hysteresis, good biocompatibility, and excellent mechanical properties (strength of 4.5 MPa and fatigue threshold of 1.5 kJ/m2). 2. High water content of 79.5%, comparable to natural ligaments. 3. Multifunctional properties (mechanical, electrical, and sensing). | 1. Potential water loss during long-term use. 2. Limited exploration of long-term stability in physiological conditions. | Artificial ligaments Wearable sensors Flexible electronics Tissue engineering | [88] |
| Slippery PVA hydrogel | Salting-out-after-syneresis | 1. Excellent optical transparency (98%). 2. Tribological coefficient down to 0.0081. 3. Excellent mechanical properties with tensile strength of 26.72 ± 1.05 MPa, modulus of 6.66 ± 0.29 MPa, and toughness of 55.21 ± 1.62 MJ/m3. | 1. Potential reduction in hydration of surface networks with higher crystallinity. | Artificial biological soft tissues Wearable electronics | [78] |
3.2. Smart and Responsive PVA-Based Hydrogels for Flexible Devices and Sensors
3.2.1. Supercapacitor
3.2.2. Flexible Sensor
3.2.3. Shape Memory Hydrogels
3.2.4. Actuator
3.2.5. Triboelectric Nanogenerator (TENG)
| Hydrogel System | Methodology | Results | Limitation | Application | Ref. |
|---|---|---|---|---|---|
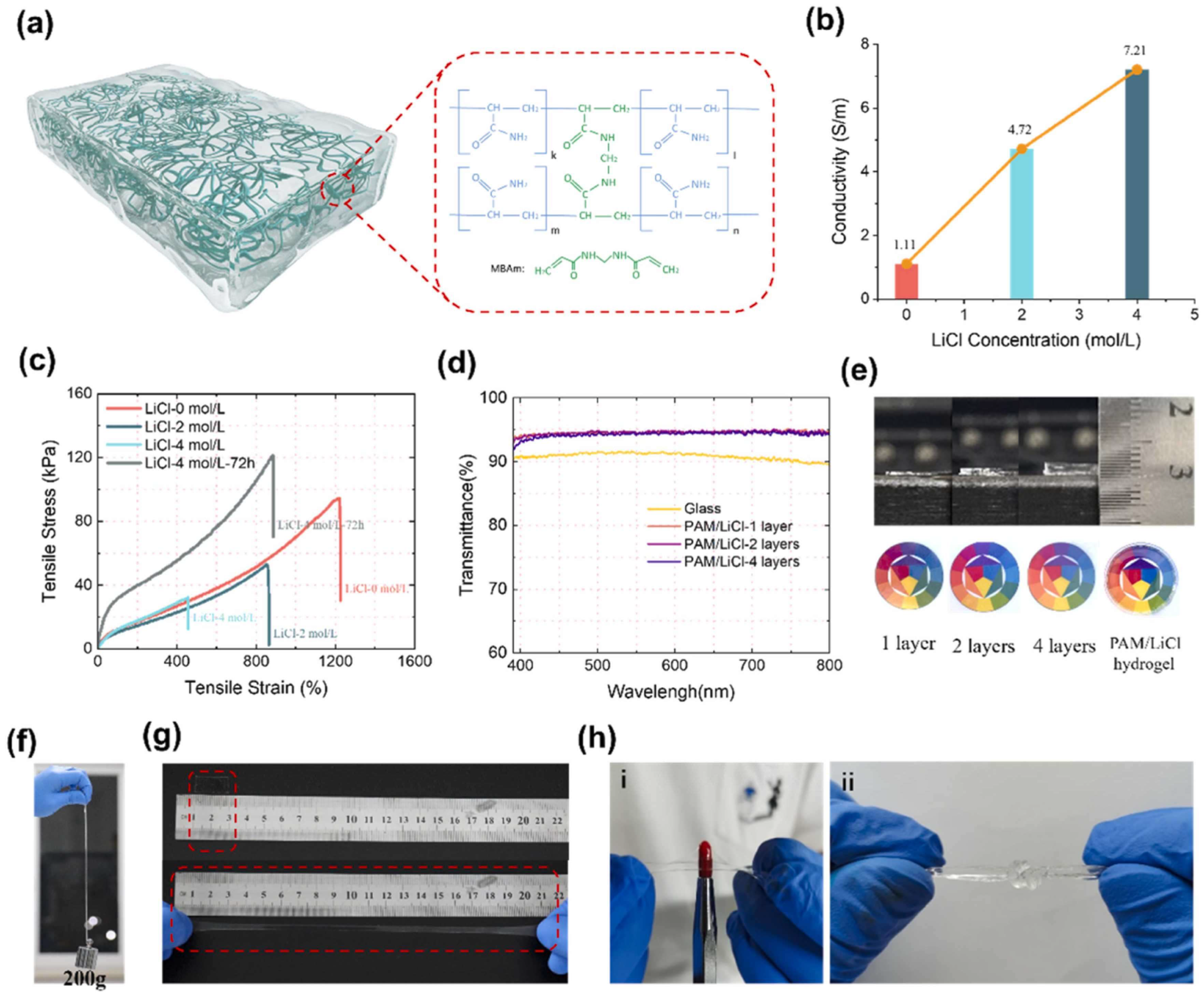
| PAM/PVA/LiTFSI hydrogel | One-step polymerization | 1. High stretchability (826%), High fracture stress (162.2 kPa) 2. High ionic conductivity (21.7 mS/cm); area specific capacitance of 383.4 mF/cm2 3. Good durability: 90.35% capacity retention after 10,000 cycles 4. Strain sensor with GF of 3.83 at 300–400% strain 5. High transparency (>90% transmittance) | 1. Potential toxicity of chemical cross-linker | 1. Flexible supercapacitors 2. Wearable sensors | [112] |
| SML/QCS/PVA | F/T method | 1. High ionic conductivity: 46.64 mS/cm 2. Excellent mechanical flexibility and stretchability tensile strain = 927.32%, compressive strain = 85% 3. Excellent performance in flexible supercapacitor application: specific capacitance: 192.6 F·g⁻1; energy density: 45.2 Wh·kg⁻1; and maintained 86.1% capacitance retention after 10,000 cycles | 1. Not extensively tested for performance at extreme temperatures and long-term stability in a variety of environments | 1. Flexible wearable devices 2. Portable energy storage devices 3. Flexible supercapacitors | [122] |
| B-PVA/kC hydrogel | F/T cycles | 1. Rapid sol–gel transition, good electrical conductivity 2. Good strain sensitivity (GF = 0.42 at 0–50% strain) 3. Enhanced mechanical properties after F/T cycles 4. Ability to rapidly form on curved surfaces or 3D-printed material | 1. Dissolution in water over time (swelling behavior) 2. Limited long-term stability in aqueous environments | 1. Flexible strain sensor | [130] |
| SPGL hydrogel | F/T cycles | 1. Outstanding anti-freezing properties (<70 °C) and excellent anti-drying properties (17.4% weight loss after 30 days) 2. Recyclability (84.7% conductivity retention after remolding); strain sensors exhibited a GF = 2.18 and rapid response time = 0.2 s 3. Supercapacitors demonstrated high specific capacity (110.8 mF/cm2) and favorable cycle stability (88.5% capacitance retention after 10,000 cycles) | 1. Relatively low ionic conductivity (52.63 mS /cm) compared to some other hydrogels | 1. Flexible strain sensors 2. Supercapacitors | [134] |
| ZBA hydrogel | Boronic ester dynamic bond cross-linking | 1. Longest reported shelf life for mammalian nucleated cells at refrigerated temperature 2. Dual protection against ROS overproduction and anoikis 3. Integration of smart hydrogel with computer-controlled system | 1. Some cell death still occurred over extended preservation periods | 1. Facilitation of cell-based clinical applications requiring extended storage or transport | [143] |
| OPNH | Physical cross-linking and orientation of the polymer chains | 1. Muscle-inspired design with multiscale oriented structure 2. Shape memory function from stretch-induced crystallization of natural rubber 3. Excellent mechanical properties (3.2 MPa tensile strength) 4. High shape fixity (≈80%) and recovery ratio (≈92%). Fast response time (≈2 s) and low response temperature (28 °C) 5. High actuation strength (206 kPa) and working capacity (105 kJ/m3) | 1. Complicated fabrication 2. CNT may not disperse evenly 3. Ensuring consistent stretch-drying and swelling is challenging | 1. Smart biomimetic muscles 2. Multistimulus-responsive devices 3. Biomedical robotics | [147] |
| P(NIPAM-co-NMA)/PVA bilayer hydrogel | In situ photo polymerization and solvent exchange or F/T methods | 1. Exhibited self-strengthening behavior, with tensile strength increasing from 29.6 kPa to 45.8 kPa and fracture strain increasing from 95% to 104% after 100 cycles of mechanical training. 2. Programmable transformations and excellent mechanical properties. 3. Novel strategy of using size-differentiated PVA crystallites for asymmetric structure | 1. Potential damage from accumulated mechanical loading 2. Reduced mechanical strength at higher temperatures due to volume contraction | 1. Intelligent soft robotics 2. Biomimetic hydrogel systems 3. Potential use in wound dressings, tissue engineering, strain sensors | [157] |
| LM/PVA hydrogel | Chemical cross-linking | 1. High electrical performance: open circuit voltage of 250 V, short circuit current of 4 µA, and transferred charge of 120 nC 2. Excellent stability, recyclability, and self-healing capabilities. 3. Synergistic mechanism combining triboelectrification, ion transport, and streaming vibration potential (SVP) | 1. Performance decreased with excessive LM content (>2.0 g) due to aggregation | 1. Human motion detection 2. Handwriting recognition 3. Energy harvesting | [160] |
| MCGPP nanocomposite hydrogel | Assembly of MXene nanosheets and CNFs. The mixture was then cooled to form the hydrogel. | 1. High sensitivity (gauge factor of 3.37 at −20 °C and 3.62 at 60 °C) 2. Excellent mechanical properties at low and high temperatures 3. High conductivity in harsh environments (−20 °C to 60 °C) 4. Fast response time (100 ms) and low detection limit (150 mg) 5. Good anti-freezing and moisturizing properties | 1. Potential long-term stability issues not fully addressed | 1. Self-powered electronics in harsh environments 2. Wearable sensors for human motion detection | [163] |
| S-TENG based on ionic conductive hydrogel | Cross-linking PVA and CMC, followed by soaking in ionic solutions | 1. Maximum output: 584 V, 25 μA, and 120 μC/cm2. 2. Highly conductive, flexible, and stretchable 3. Stable performance over 15 days and long-term operation | 1. Potential water evaporation over very long periods 2. Performance dependent on environmental conditions | 1. Mechanical energy harvesting 2. Self-powered electronic displays 3. Smart touch sensors | [159] |
3.3. Environmental Treatment
3.3.1. Solar Water Purification and Seawater Desalination
3.3.2. Efficiently Removal Pollutants from Water
3.3.3. Oil/Water Separation
3.3.4. Air Purification
3.4. Civil Engineering
3.4.1. Improvement of Freezing/Thawing Resistance in Concrete
3.4.2. High Performance Concrete
3.4.3. Waterproofing Materials
3.4.4. Flame-Retardant Materials
3.5. Other Emerging Application of PVA-Based Hydrogels
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Sun, G.; Li, Z.; Liang, R.; Weng, L.; Zhang, L. Super stretchable hydrogel achieved by non-aggregated spherulites with diameters <5 nm. Nat. Commun. 2016, 7, 12095. [Google Scholar] [PubMed]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Takayuki, K.; Gong, J. Novel Developed Systems and Techniques Based on Double-Network Principle. Bull. Chem. Soc. Jpn. 2011, 84, 1295–1311. [Google Scholar] [CrossRef]
- Wanzke, C.; Tena-Solsona, M.; Rieß, B.; Tebcharani, L.; Boekhoven, J. Active droplets in a hydrogel release drugs with a constant and tunable rate. Mater. Horiz. 2020, 7, 1397–1403. [Google Scholar] [CrossRef]
- Ma, M. Actuating smart. Nat. Nanotechnol. 2019, 14, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, Y.; Zhao, F.; Yu, G. Hydrogels as an Emerging Material Platform for Solar Water Purification. Acc. Chem. Res. 2019, 52, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Dong, R. Physical dynamic double-network hydrogels as dressings to facilitate tissue repair. Nat. Protoc. 2023, 18, 3322–3354. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Wong, D.; Parasrampuria, J. Polyvinyl Alcohol. In Analytical Profiles of Drug Substances and Excipients; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 24, pp. 397–441. [Google Scholar]
- Daza Agudelo, J.I.; Ramirez, M.R.; Henquin, E.R.; Rintoul, I. Modelling of swelling of PVA hydrogels considering non-ideal mixing behaviour of PVA and water. J. Mater. Chem. B 2019, 7, 4049–4054. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(vinyl alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Liang, X.; Ding, H.; Wang, Q.; Wang, M.; Yin, B.; Sun, G. Nature-inspired semi-IPN hydrogels with tunable mechanical properties and multi-responsiveness. New J. Chem. 2021, 45, 861–871. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Rahman, N.; Dafader, N.C.; Sultana, S.; Sardar, M.N. Chapter 12—Chemical and radiation modification of gelatin. In Handbook Natural Polymers; Meyyarappallil Sadasivan, S., Ravindran, L., Goda, K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 267–300. [Google Scholar]
- Li, Z.; Liu, P.; Li, X.; Guan, S.; Chen, S.; Liu, S.; Cui, E.; Yu, Y.; Pan, W.; Tang, N.; et al. Design strategies for environmentally friendly polyvinyl alcohol hydrogel sensors: Research progress and Perspectives. Mater. Today Commun. 2024, 39, 109401. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray Diffraction Analysis of Poly(vinyl alcohol) Hydrogels, Obtained by Freezing and Thawing Techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Zhang, Y.; An, R.; Han, L.; Wang, X.; Shi, L.; Ran, R. Novel Self-Healing, Shape-Memory, Tunable Double-Layer Actuators Based on Semi-IPN and Physical Double-Network Hydrogels. Macromol. Mater. Eng. 2018, 303, 1800505. [Google Scholar] [CrossRef]
- Liao, M.; Liao, H.; Ye, J.; Wan, P.; Zhang, L. A Polyvinyl Alcohol Stabilized Liquid Metal Hydrogel for Wearable Transient Epidermal Sensors. ACS Appl. Mater. Interfaces 2019, 11, 47358–477364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Han, D.; Wang, G.; Xu, X.; Chen, J.; Lu, M.; Liu, X.; Zhang, L.; Lai, L. Ultra-stretchable, self-healing, bonding, and skin-inspired conductive triple network hydrogel for wearable strain sensors and friction nanogenerators. Polymer 2024, 305, 127169. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Peppast, N.A. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Peppas, N.A.; Scott, J.E. Controlled release from poly (vinyl alcohol) gels prepared by freezing-thawing processes. J. Control. Release 1992, 18, 95–100. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Rahmatolahzadeh, R. Influence of Cross-linking Agents on Drug Delivery Behavior of Magnetic Nanohydrogels Made of Polyvinyl Alcohol and Chitosan. BioNanoScience 2019, 9, 883–892. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C.; Han, Q. Molecular dynamic simulation on the state of water in poly(vinyl alcohol) hydrogel. Comput. Theor. Chem. 2017, 1102, 15–21. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Chen, H.; Cheng, D. Environmentally friendly hydrogel: A review of classification, preparation and application in agriculture. Sci. Total Environ. 2022, 846, 157303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Yang, B.; Li, S.; Yang, H.; Miao, Y.; Cong, Y.; Zhang, R.; Fu, J. Dynamic and structural studies on synergetic energy dissipation mechanisms of single-, double-, and triple-network hydrogels sequentially crosslinked by multiple non-covalent interactions. Polymer 2022, 250, 124868. [Google Scholar] [CrossRef]
- Alves, M.-H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(Vinyl Alcohol) Physical Hydrogels: New Vista on a Long Serving Biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, A.K.; Kailath, A.; Mishra, T.; Guha, A.; Nayar, S.; Sinha, A. Composition dependent structural modulations in transparent poly(vinyl alcohol) hydrogels. Colloids Surf. B Biointerfaces 2009, 74, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sinha, S.; Sinha, A. Composition dependent mechanical response of transparent poly(vinyl alcohol) hydrogels. Colloids Surf. B Biointerfaces 2010, 78, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Takeno, H.; Narita, T.; Hsieh, W.-C.; Saito, K.; Ku, Y.-T.; Su, Y.-C.; Inoguchi, H. Effects of repeated freeze-thaw cycles on the mechanical and structural properties of cellulose nanofiber and poly(vinyl alcohol) hydrogels. Cellulose 2024, 31, 7479–7492. [Google Scholar] [CrossRef]
- Takamura, A.; Ishii, F.; Hidaka, H. Drug release from poly(vinyl alcohol) gel prepared by freeze-thaw procedure. J. Control. Release 1992, 20, 21–27. [Google Scholar] [CrossRef]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Damshkaln, L.G. Study of cryostructuration of polymer systems. XVII. Poly(vinyl alcohol) cryogels: Dynamics of the cryotropic gel formation. J. Appl. Polym. Sci. 2000, 77, 2017–2023. [Google Scholar] [CrossRef]
- Gupta, S.; Webster, T.J.; Sinha, A. Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J. Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef]
- Yusong, P.; Jie, D.; Yan, C.; Qianqian, S. Study on mechanical and optical properties of poly(vinyl alcohol) hydrogel used as soft contact lens. Mater. Technol. 2016, 31, 266–273. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hyu, H.S. Development and evaluation of polyvinyl alcohol-hydrogels as an artificial atrticular cartilage for orthopedic implants. Materials 2010, 3, 2753–2771. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, C.; Peng, X.; Liu, T.; Shi, Y.; Liang, M.; Wang, H. Biomimetic anisotropic poly(vinyl alcohol) hydrogels with significantly enhanced mechanical properties by freezing–thawing under drawing. J. Mater. Chem. B 2019, 7, 3243–3249. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Wang, Z.; Wang, Z.; Lan, Y.; Ge, Q.; et al. Anisotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2021, 33, 2102011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, J.; Zhu, J.; He, C.; Wang, H. Anisotropic tough poly(vinyl alcohol) hydrogels. Soft Matter 2012, 8, 10439–10447. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef]
- El Salmawi, K.M. Gamma Radiation-Induced Crosslinked PVA/Chitosan Blends for Wound Dressing. J. Macromol. Sci. Part A 2007, 44, 541–545. [Google Scholar] [CrossRef]
- Hao, S.; Chen, Z.; Li, H.; Yuan, J.; Chen, X.; Sidorenko, A.; Huang, J.; Gu, Y. Skin-Inspired, Highly Sensitive, Broad-Range-Response and Ultra-Strong Gradient Ionogels Prepared by Electron Beam Irradiation. Small 2024, 20, 2309931. [Google Scholar] [CrossRef]
- Guo, W.; Yang, M.; Liu, S.; Zhang, X.; Zhang, B.; Chen, Y. Chitosan/polyvinyl alcohol/tannic acid multiple network composite hydrogel: Preparation and characterization. Iran. Polym. J. 2021, 30, 1159–1168. [Google Scholar] [CrossRef]
- Hiep, N.T.; Khon, H.C.; Niem, V.V.T.; Toi, V.V.; Ngoc Quyen, T.; Hai, N.D.; Ngoc Tuan Anh, M. Microwave-Assisted Synthesis of Chitosan/Polyvinyl Alcohol Silver Nanoparticles Gel for Wound Dressing Applications. Int. J. Polym. Sci. 2016, 2016, 1584046. [Google Scholar] [CrossRef]
- More, A.P.; Chapekar, S. Irradiation assisted synthesis of hydrogel: A Review. Polym. Bull. 2024, 81, 5839–5908. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Sheng, K.; Huang, J.; Fang, C.; Han, J. “Room Temperature Molten Salt”-Based Polymer Electrolyte Enabling a High-Rate and High-Thermal Stability Hybrid Li/Na-Ion Battery. ACS Appl. Energy Mater. 2022, 5, 6110–6117. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Shu, J.; Yuan, M.; Yan, W.; Bai, P.; He, L.; Shen, N.; Gong, S.; Zhang, D.; et al. Electron Beam Irradiation-Induced Formation of Defect-Rich Zeolites under Ambient Condition within Minutes. Angew. Chem. Int. Ed. 2021, 60, 14858–14863. [Google Scholar] [CrossRef]
- Burczak, K.; Fujisato, T.; Hatada, M.; Ikada, Y. Protein permeation through poly(vinyl alcohol) hydrogel membranes. Biomaterials 1994, 15, 231–238. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Koyama, T.; Hanabusa, K.; Shirai, H.; Ikeda, J.; Yoneno, H.; Itoh, T. Preparation and properties of highly phosphorylated poly(vinyl alcohol) hydrogels chemically crosslinked by glutaraldehyde. Polymer 1995, 36, 2297–2301. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, J.-W.; Ruckenstein, E. On the viscoelastic properties of poly(vinyl alcohol) and chemically crosslinked poly(vinyl alcohol). J. Appl. Polym. Sci. 2001, 82, 1816–1823. [Google Scholar] [CrossRef]
- Giménez, V.; Reina, J.A.; Mantecón, A.; Cádiz, V. Unsaturated modified poly(vinyl alcohol). Crosslinking through double bonds. Polymer 1999, 40, 2759–2767. [Google Scholar] [CrossRef]
- Paradossi, G.; Lisi, R.; Paci, M.; Crescenzi, V. New chemical hydrogels based on poly(vinyl alcohol). J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3417–3425. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Deng, J.; Luo, X. Effect of Glycerol on the Properties of the Cross-Linked Polyvinyl Alcohol Hydrogel Beads. ChemistrySelect 2018, 3, 467–470. [Google Scholar] [CrossRef]
- Miao, X.; Li, Z.; Hou, K.; Gao, Q.; Huang, Y.; Wang, J.; Yang, S. Bioinspired multi-crosslinking and solid–liquid composite lubricating MXene/PVA hydrogel based on salting out effect. Chem. Eng. J. 2023, 476, 146848. [Google Scholar] [CrossRef]
- Chen, J.; Shi, D.; Yang, Z.; Dong, W.; Chen, M. A solvent-exchange strategy to develop stiff and tough hydrogel electrolytes for flexible and stable supercapacitor. J. Power Sources 2022, 532, 231326. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Wan, H.; Wu, B.; Hou, L.; Wu, P. Amphibious Polymer Materials with High Strength and Superb Toughness in Various Aquatic and Atmospheric Environments. Adv. Mater. 2024, 36, 2307290. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Z.; Wang, H.; Ma, Z.; Yi, T.; Li, Y.; Yang, K.; Li, F.; Xue, B. Synergistic crosslinking effect of hydroxyethyl cellulose and montmorillonite on the improvement of mechanical and electrochemical properties of polyvinyl alcohol hydrogel. Mater. Today Chem. 2024, 37, 101996. [Google Scholar] [CrossRef]
- Xue, R.; Xin, X.; Wang, L.; Shen, J.; Ji, F.; Li, W.; Jia, C.; Xu, G. A systematic study of the effect of molecular weights of polyvinyl alcohol on polyvinyl alcohol–graphene oxide composite hydrogels. Phys. Chem. Chem. Phys. 2015, 17, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems VII. Structure formation under freezing of poly(vinyl alcohol) aqueous solutions. Colloid Polym. Sci. 1986, 264, 19–24. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, Conductive Self-healing Organohydrogels with Stable Strain-Sensitivity at Subzero Temperatures. Angew. Chem. Int. Ed. 2017, 56, 14159–14163. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.-Y.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(vinyl alcohol) Hydrogels with Broad-Range Tunable Mechanical Properties via the Hofmeister Effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Tang, Y.; Pang, L.; Wang, D. Preparation and characterization of borate bioactive glass cross-linked PVA hydrogel. J. Non-Cryst. Solids 2017, 476, 25–29. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Chen, S.; Wang, B.; Liu, S.; Cui, E.; Li, F.; Yu, Y.; Pan, W.; Tang, N.; et al. Polyvinyl alcohol/chitosan based nanocomposite organohydrogel flexible wearable strain sensors for sports monitoring and underwater communication rescue. Int. J. Biol. Macromol. 2024, 258, 129054. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M.; Verma, H.R.; Kumar, S. Physically cross-linked PVA/f-MWCNTs nanocomposite hydrogel with enhanced thermal, mechanical, and dielectric properties. Mater. Today Commun. 2024, 40, 109400. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Liu, W.; Li, R.; Wang, D.; Zhang, N.; Chen, D.; Lee, S. Polyvinyl alcohol/chitosan-Fe3+/gelatin/tri-network composite hydrogel wound dressing with effective hemostasis and self-healing properties. Polymer 2024, 307, 127304. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, Y.; Wang, N.; Lei, Z. Facile synthesis of an acrylic acid-co-2-acrylamide-2-methylpropanesulfonic acid copolymer/polyvinylpyrrolidone semi-IPN hydrogel with excellent swelling and anti-leakage properties. Mater. Today Commun. 2024, 39, 109190. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- You, T.; You, Q.; Feng, X.; Li, H.; Yi, B.; Xu, H. A novel approach to wound healing: Green synthetic nano-zinc oxide embedded with sodium alginate and polyvinyl alcohol hydrogels for dressings. Int. J. Pharm. 2024, 654, 123968. [Google Scholar] [CrossRef]
- Wang, P.; Qian, L.; Liang, H.; Huang, J.; Jin, J.; Xie, C.; Xue, B.; Lai, J.; Zhang, Y.; Jiang, L.; et al. A Polyvinyl Alcohol/Acrylamide Hydrogel with Enhanced Mechanical Properties Promotes Full-Thickness Skin Defect Healing by Regulating Immunomodulation and Angiogenesis Through Paracrine Secretion. Engineering 2024, 37, 138–151. [Google Scholar] [CrossRef]
- Qiu, J.; Lan, J.; Xiang, Y.; Chen, L.; Xie, J.; Huang, T.; Tian, L.; Qiu, R.; Jiang, L. An injectable, self-healable, and antimicrobial hydroxypropyl chitosan/poly(vinyl alcohol) hydrogel for drug delivery systems. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134587. [Google Scholar] [CrossRef]
- Liu, D.; Cao, Y.; Jiang, P.; Wang, Y.; Lu, Y.; Ji, Z.; Wang, X.; Liu, W. Tough, Transparent, and Slippery PVA Hydrogel Led by Syneresis. Small 2023, 19, 2206819. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Wang, L.; Gao, G.; Zhou, Y.; Wang, R.; Xu, T.; Yin, J.; Fu, J. Flexible and wearable strain sensors based on tough and self-adhesive ion conducting hydrogels. J. Mater. Chem. B 2019, 7, 24–29. [Google Scholar] [CrossRef]
- Tiamduangtawan, P.; Kamkaew, C.; Kuntonwatchara, S.; Wimolmala, E.; Saenboonruang, K. Comparative mechanical, self-healing, and gamma attenuation properties of PVA hydrogels containing either nano- or micro-sized Bi2O3 for use as gamma-shielding materials. Radiat. Phys. Chem. 2020, 177, 109164. [Google Scholar] [CrossRef]
- Zha, X.-J.; Zhang, S.-T.; Pu, J.-H.; Zhao, X.; Ke, K.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Nanofibrillar Poly(vinyl alcohol) Ionic Organohydrogels for Smart Contact Lens and Human-Interactive Sensing. ACS Appl. Mater. Interfaces 2020, 12, 23514–23522. [Google Scholar] [CrossRef]
- Jiang, P.; Lin, P.; Yang, C.; Qin, H.; Wang, X.; Zhou, F. 3D Printing of Dual-Physical Cross-linking Hydrogel with Ultrahigh Strength and Toughness. Chem. Mater. 2020, 32, 9983–9995. [Google Scholar] [CrossRef]
- Wu, K.; Han, H.; Xu, L.; Gao, Y.; Yang, Z.; Jiang, Z.; De Schutter, G. The improvement of freezing–thawing resistance of concrete by cellulose/polyvinyl alcohol hydrogel. Constr. Build. Mater. 2021, 291, 123274. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Xia, M.; Cai, M.; Nie, Z.; Gao, J. Green multifunctional PVA composite hydrogel-membrane for the efficient purification of emulsified oil wastewater containing Pb2+ ions. Sci. Total Environ. 2023, 856, 159271. [Google Scholar] [CrossRef]
- Martínez-Gómez, F.; Guerrero, J.; Matsuhiro, B.; Pavez, J. In vitro release of metformin hydrochloride from sodium alginate/polyvinyl alcohol hydrogels. Carbohydr. Polym. 2017, 155, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Yang, H.; Xiong, Y.; Zeng, G.; Dong, F. Polyvinyl alcohol/chitosan quaternary ammonium salt composite hydrogel with directional macroporous structure for photothermal synergistic antibacterial and wound healing promotion. Int. J. Biol. Macromol. 2024, 267, 131549. [Google Scholar] [CrossRef]
- Han, S.; Wu, Q.; Zhu, J.; Zhang, J.; Chen, A.; Su, S.; Liu, J.; Huang, J.; Yang, X.; Guan, L. Tough hydrogel with high water content and ordered fibrous structures as an artificial human ligament. Mater. Horiz. 2023, 10, 1012–1019. [Google Scholar] [CrossRef]
- Peppas, N.A.; Mongia, N.K. Ultrapure poly(vinyl alcohol) hydrogels with mucoadhesive drug delivery characteristics. Eur. J. Pharm. Biopharm. 1997, 43, 51–58. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Zheng, X.; Yi, Y.; Chen, X.; Li, Y.; Sun, D.; Zhang, L. Multifunctional load-bearing hybrid hydrogel with combined drug release and photothermal conversion functions. NPG Asia Mater. 2020, 12, 18. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Naeem, A.; Yu, C.; He, T.; Zang, Z.; Zhu, W.; Guang, M. β-Cyclodextrin/chitosan-based (polyvinyl alcohol-co-acrylic acid) interpenetrating hydrogels for oral drug delivery. Int. J. Biol. Macromol. 2023, 242, 125149. [Google Scholar] [CrossRef]
- Xiong, S.; Ye, S.; Ni, P.; Zhong, M.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. Polyvinyl-alcohol, chitosan and graphene-oxide composed conductive hydrogel for electrically controlled fluorescein sodium transdermal release. Carbohydr. Polym. 2023, 319, 121172. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, C.; Wang, X.; Shi, G. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wang, S.; Li, W.; Jiang, Y.; Liu, X.; Huang, Q. A lignocellulose nanofibril-poly(vinyl alcohol) hydrogel with controlled drug delivery for wound healing. Ind. Crop. Prod. 2024, 220, 119234. [Google Scholar] [CrossRef]
- Rossari, F.; Birocchi, F.; Naldini, L.; Coltella, N. Gene-based delivery of immune-activating cytokines for cancer treatment. Trends Mol. Med. 2023, 29, 329–342. [Google Scholar] [CrossRef]
- Saharan, R.; Paliwal, S.K.; Tiwari, A.; Babu, M.A.; Tiwari, V.; Singh, R.; Beniwal, S.K.; Kumar, M.; Sharma, A.; Almalki, W.H.; et al. Beyond traditional hydrogels: The emergence of graphene oxide-based hydrogels in drug delivery. J. Drug Deliv. Sci. Technol. 2024, 94, 105506. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, N.; Zhang, H.; Sun, Y.; Lin, Z.; Hou, L. Preparation and characterization of poly(vinyl alcohol)/sodium alginate hydrogel with high toughness and electric conductivity. Carbohydr. Polym. 2018, 186, 377–383. [Google Scholar] [CrossRef] [PubMed]
- King, I.C.C.; Sorooshian, P. Hyaluronan in skin wound healing: Therapeutic applications. J. Wound Care 2020, 29, 782–787. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Chen, H.; Wang, N.; Liu, X.; Sun, G.; Qiao, W. Effective wound dressing based on Poly (vinyl alcohol)/Dextran-aldehyde composite hydrogel. Int. J. Biol. Macromol. 2019, 132, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Tamahkar, E.; Özkahraman, B.; Süloğlu, A.K.; İdil, N.; Perçin, I. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Technol. 2020, 58, 101536. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharyya, S.; Uppaluri, R.; Das, C. Optimality of poly-vinyl alcohol/starch/glycerol/citric acid in wound dressing applicable composite films. Int. J. Biol. Macromol. 2020, 155, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Z.; Du, C.-C.; Xuan, Y.; Huang, D.; Qi, B.; Shi, Y.; Shen, X.; Zhang, Y.; Fu, Y.; Chen, Y.; et al. Bilirubin/morin self-assembled nanoparticle-engulfed collagen/polyvinyl alcohol hydrogel accelerates chronic diabetic wound healing by modulating inflammation and ameliorating oxidative stress. Int. J. Biol. Macromol. 2024, 261, 129704. [Google Scholar] [CrossRef]
- Li, N.; Yu, Q.; Duan, S.; Du, Y.; Shi, X.; Li, X.; Jiao, T.; Qin, Z.; He, X. Anti-Swelling, High-Strength, Anisotropic Conductive Hydrogel with Excellent Biocompatibility for Implantable Electronic Tendon. Adv. Funct. Mater. 2024, 34, 2309500. [Google Scholar] [CrossRef]
- Lei, L.; Cong, R.; Ni, Y.; Cui, X.; Wang, X.; Ren, H.; Wang, Z.; Liu, M.; Tu, J.; Jiang, L. Dual-Functional Injectable Hydrogel for Osteoarthritis Treatments. Adv. Healthc. Mater. 2024, 13, 2302551. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, M.; Zhang, W.; Guo, W.; Zhang, X.; Zhang, B. Facile Preparation of Irradiated Poly(vinyl alcohol)/Cellulose Nanofiber Hydrogels with Ultrahigh Mechanical Properties for Artificial Joint Cartilage. Materials 2024, 17, 4125. [Google Scholar] [CrossRef] [PubMed]
- Golabdar, A.; Adelnia, H.; Moshtzan, N.; Nasrollah Gavgani, J.; Izadi-Vasafi, H. Anti-bacterial poly(vinyl alcohol) nanocomposite hydrogels reinforced with in situ synthesized silver nanoparticles. Polym. Compos. 2019, 40, 1322–1328. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Liu, Z.; Wang, Y.; Lin, D.; Chen, L.; Dai, J.; Lin, K.; Shen, S.G. Polydopamine nanoparticles as dual-task platform for osteoarthritis therapy: A scavenger for reactive oxygen species and regulator for cellular powerhouses. Chem. Eng. J. 2021, 417, 129284. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Niu, J.; Wang, J.; Shi, Y.; Dong, Z.; Huang, T.; Dai, X.; Sha, W.; Long, Y.; Hu, W. Customizable, self-healing, and biocompatible microLED-hydrogel integration displays. Nano Energy 2024, 129, 110074. [Google Scholar] [CrossRef]
- Dong, X.; Chen, W.; Ge, X.; Li, S.; Xing, Z.; Zhang, Q.; Wang, Z.-X. Stretchable, self-adhesion and durable polyacrylamide/polyvinylalcohol dual-network hydrogel for flexible supercapacitor and wearable sensor. J. Energy Storage 2024, 89, 111793. [Google Scholar] [CrossRef]
- Patel, D.K.; Ganguly, K.; Dutta, S.D.; Patil, T.V.; Lim, K.-T. Multifunctional hydrogels of polyvinyl alcohol/polydopamine functionalized with carbon nanomaterials as flexible sensors. Mater. Today Commun. 2022, 32, 103906. [Google Scholar] [CrossRef]
- You, L.; Zheng, Z.; Xu, W.; Wang, Y.; Xiong, W.; Xiong, C.; Wang, S. Self-healing and adhesive MXene-polypyrrole/silk fibroin/polyvinyl alcohol conductive hydrogels as wearable sensor. Int. J. Biol. Macromol. 2024, 263, 130439. [Google Scholar] [CrossRef]
- Li, G.; Yan, Q.; Xia, H.; Zhao, Y. Therapeutic-Ultrasound-Triggered Shape Memory of a Melamine-Enhanced Poly(vinyl alcohol) Physical Hydrogel. ACS Appl. Mater. Interfaces 2015, 7, 12067–12073. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zheng, S.; Zhong, K.; Wang, F. Highly Bendable Ionic Electro-responsive Artificial Muscles Using Microfibrillated Cellulose Fibers Combined with Polyvinyl Alcohol. J. Bionic Eng. 2024, 21, 2313–2323. [Google Scholar] [CrossRef]
- Yan, Z.; Luo, S.; Li, Q.; Wu, Z.-S.; Liu, S. Recent Advances in Flexible Wearable Supercapacitors: Properties, Fabrication, and Applications. Adv. Sci. 2024, 11, 2302172. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, Z.; Zhao, W.; He, M.; Guo, N.; Weng, L.; Lin, Z.; Taleb, M.F.A.; Ibrahim, M.M.; Singh, M.V.; et al. Strategies in the preparation of conductive polyvinyl alcohol hydrogels for applications in flexible strain sensors, flexible supercapacitors, and triboelectric nanogenerator sensors: An overview. Adv. Compos. Hybrid Mater. 2023, 6, 203. [Google Scholar] [CrossRef]
- Rahim, A.A.; Shamsuri, N.A.; Adam, A.A.; Aziz, M.F.; Hamsan, M.H.; Rusdi, H.; Siong, S.O.J.; Noor, I.M.; Kadir, M.F.Z.; Shukur, M.F. Characterization of nanocomposite polyvinyl alcohol/cellulose acetate blend gel polymer electrolytes for supercapacitor application. J. Energy Storage 2024, 97, 112964. [Google Scholar] [CrossRef]
- Peng, H.; Gao, X.; Sun, K.; Xie, X.; Ma, G.; Zhou, X.; Lei, Z. Physically cross-linked dual-network hydrogel electrolyte with high self-healing behavior and mechanical strength for wide-temperature tolerant flexible supercapacitor. Chem. Eng. J. 2021, 422, 130353. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Wang, J.; Gai, L.; Ji, X.; Jiang, H.; Liu, L. Antifreezing Zwitterionic Hydrogel Electrolyte with High Conductivity of 12.6 mS cm−1 at −40 °C through Hydrated Lithium Ion Hopping Migration. Adv. Funct. Mater. 2021, 31, 2009438. [Google Scholar] [CrossRef]
- Sun, S.-F.; Zhao, X.-Y.; Gao, C.; Xiao, L.-P.; Sun, R.-C. Construction of amphoteric hydrogel electrolytes with charge modification: An investigation of ion migration mechanism and antibacterial property. Chem. Eng. J. 2024, 495, 153781. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Bi, W.; Yang, F.; Wang, X.; Wang, Y.; Zhao, H. Tough and conductive PVA-based double-network ionic hydrogels for flexible sensors. Polymer 2024, 309, 127465. [Google Scholar] [CrossRef]
- Wen, N.; Jiang, B.; Wang, X.; Shang, Z.; Jiang, D.; Zhang, L.; Sun, C.; Wu, Z.; Yan, H.; Liu, C.; et al. Overview of Polyvinyl Alcohol Nanocomposite Hydrogels for Electro-Skin, Actuator, Supercapacitor and Fuel Cell. Chem. Rec. 2020, 20, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, X.; Gu, H. Multi-Network Poly(β-cyclodextrin)/PVA/Gelatin/Carbon Nanotubes Composite Hydrogels Constructed by Multiple Dynamic Crosslinking as Flexible Electronic Devices. Macromol. Mater. Eng. 2022, 307, 2100724. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Pan, J.; He, H.; Wang, Z.; Deng, M.; Liu, X.; Fu, F. Silk fibroin enhanced double-network hydrogels with extreme stretchability, self-adhesive and biocompatibility for ultrasensitive strain sensors. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133035. [Google Scholar] [CrossRef]
- Chen, K.; Liang, K.; Liu, H.; Liu, R.; Liu, Y.; Zeng, S.; Tian, Y. Skin-Inspired Ultra-Tough Supramolecular Multifunctional Hydrogel Electronic Skin for Human–Machine Interaction. Nano-Micro Lett. 2023, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wu, S.; Su, H.; An, S.; Ruan, J.; Zeng, D. Research progress of PVA conductive hydrogel-based wearable biosensors in sweat detection. Chem. Eng. Sci. 2024, 300, 120620. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhang, G.; Chen, Z.; Ren, F.; Jin, Y.; Ren, P. Recyclable Organic Ionic Conductive Hydrogels for Flexible Wearable Sensors with Excellent Environmental Resistance. ACS Appl. Electron. Mater. 2024, 6, 6626–6639. [Google Scholar] [CrossRef]
- Feng, S.; Guo, J.; Guan, F.; Sun, J.; Song, X.; He, J.; Yang, Q. Preparation of 3D printable polyvinyl alcohol based conductive hydrogels via incorporating k-carrageenan for flexible strain sensors. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132141. [Google Scholar] [CrossRef]
- Fanan, W.; Tuanjie, D.; Ligang, Y.; Wenguang, Y. 3D printable and stretchable PVA-PAAm dual network hydrogel with conductivities for wearable sensors. J. Appl. Polym. Sci. 2022, 140, e53468. [Google Scholar]
- Shi, Y.; Guan, Y.; Liu, M.; Kang, X.; Tian, Y.; Deng, W.; Yu, P.; Ning, C.; Zhou, L.; Fu, R.; et al. Tough, Antifreezing, and Piezoelectric Organohydrogel as a Flexible Wearable Sensor for Human–Machine Interaction. ACS Nano 2024, 18, 3720–3732. [Google Scholar] [CrossRef]
- Li, Z.; Yin, F.; He, W.; Hang, T.; Li, Z.; Zheng, J.; Li, X.; Jiang, S.; Chen, Y. Anti-freezing, recoverable and transparent conductive hydrogels co-reinforced by ethylene glycol as flexible sensors for human motion monitoring. Int. J. Biol. Macromol. 2023, 230, 123117. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.Y.; Zhu, K.H.; Chen, H.M.; Ye, S.F.; Cui, P.X.; Dou, L.Y.; Ma, J.; Zhao, C.; He, J.; Feng, P.Z. Recyclable, anti-freezing and anti-drying silk fibroin-based hydrogels for ultrasensitive strain sensors and all-hydrogel-state super-capacitors. Mater. Today Chem. 2023, 32, 101624. [Google Scholar] [CrossRef]
- Wan, Z.; Qu, R.; Sun, Y.; Gao, Y.; Gao, G.; Chen, K.; Liu, T. Physically crosslinked polyvinyl alcohol, phytic acid and glycerol hydrogels for wearable sensors with biocompatibility, antimicrobial stability and anti-freezing. Eur. Polym. J. 2024, 211, 112974. [Google Scholar] [CrossRef]
- Chen, M.; Qian, X.; Cai, J.; Zhou, J.; Lu, A. Electronic skin based on cellulose/KCl/sorbitol organohydrogel. Carbohydr. Polym. 2022, 292, 119645. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Lv, A.; Tian, S.; Xie, T.; Sun, S. A tough and highly active catalyst carrier tailored by nanoparticles-encapsulation poly(ionic liquid) hydrogel: Synthesis and catalytic applications. Eur. Polym. J. 2023, 182, 111713. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Liu, R.; Qiao, C.; Liu, Q.; Liu, L.; Yao, J. Fabrication and Properties of Anti-freezing Gelatin Hydrogels Based on a Deep Eutectic Solvent. ACS Appl. Polym. Mater. 2023, 5, 4546–4553. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Song, F.; Yang, W.; Yang, B.; Ding, D.; Liu, Z.; Hui, L.; Zhang, F. A highly sensitive and anti-freezing conductive strain sensor based on polypyrrole/cellulose nanofiber crosslinked polyvinyl alcohol hydrogel for human motion detection. Int. J. Biol. Macromol. 2024, 257, 128800. [Google Scholar] [CrossRef]
- Huo, P.; Ding, H.; Tang, Z.; Liang, X.; Xu, J.; Wang, M.; Liang, R.; Sun, G. Conductive silk fibroin hydrogel with semi-interpenetrating network with high toughness and fast self-recovery for strain sensors. Int. J. Biol. Macromol. 2022, 212, 1–10. [Google Scholar] [CrossRef]
- Ding, H.; Liu, J.; Huo, P.; Ding, R.; Shen, X.; Mao, H.; Wen, Y.; Li, H.; Wu, Z.L. Ultra-stretchable and conductive polyacrylamide/carboxymethyl chitosan composite hydrogels with low modulus and fast self-recoverability as flexible strain sensors. Int. J. Biol. Macromol. 2023, 253, 127146. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, Y.; Kang, Y.; Tian, S.; Li, Q.; Zhang, L.; Yang, J. Zwitterionic-hydrogel-based sensing system enables real-time ROS monitoring for ultra-long hypothermic cell preservation. Acta Biomater. 2024, 186, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, Y.; Zhang, K.; Zhuo, S.; Fang, R.; Zhang, J.; Jiang, L.; Liu, M. Biphasic Synergistic Gel Materials with Switchable Mechanics and Self-Healing Capacity. Angew. Chem. Int. Ed. 2017, 56, 13464–13469. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liu, C.; Ma, S.; Wang, X.; Zhu, L.; Bao, C. Stimuli-Responsive Peptide Self-Assembly to Construct Hydrogels with Actuation and Shape Memory Behaviors. Adv. Funct. Mater. 2023, 33, 2300416. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Zheng, S.Y.; Wang, Q.; Li, Z.; Sun, G. Actuators assembled from hydrogel blocks of various shapes via condensation reactions. Mater. Chem. Phys. 2020, 253, 123332. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhou, S.; Zhang, J.; Zong, L. Biomimetic Multiscale Oriented PVA/NRL Hydrogel Enabled Multistimulus Responsive and Smart Shape Memory Actuator. Small 2024, 20, 2311240. [Google Scholar] [CrossRef]
- Guo, X.-R.; Sheng, P.-H.; Hu, J.-W.; Liu, J.; Wang, S.-L.; Ma, Q.; Yu, Z.-Z.; Ding, Y. Multistimuli-Responsive Shape-Memory Composites with a Water-Assisted Self-Healing Function Based on Sodium Carboxymethyl Cellulose/Poly(vinyl alcohol)/MXene. ACS Appl. Mater. Interfaces 2024, 16, 17981–17991. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Xu, Y.; Fu, L.; Yang, H. One-pot fabrication of triple shape memory hydrogel based on coordination bond, the dynamic borate ester bonds, and hydrogen bond. Soft Mater. 2019, 17, 342–349. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, J.; Li, S.; Wu, Z.; Kanti Mondal, A. Anti-swellable, stretchable, self-healable, shape-memory and supramolecular conductive TA-based hydrogels for amphibious motion sensors. Eur. Polym. J. 2024, 211, 113034. [Google Scholar] [CrossRef]
- Lee, T.H.; Jho, J.Y. Temperature-Responsive Actuators Fabricated with PVA/PNIPAAm Interpenetrating Polymer Network Bilayers. Macromol. Res. 2018, 26, 659–664. [Google Scholar] [CrossRef]
- Xie, T.; Gao, Y.; Li, Z.; Gao, W. A temperature-responsive dual network hydrogel for reversible smart actuator. J. Appl. Polym. Sci. 2024, 141, e55298. [Google Scholar] [CrossRef]
- Pahnavar, Z.; Ghaemy, M.; Naji, L.; Hasantabar, V. Bio-electronic muscular soft actuator based on double network κ-Carrageenan/PVA membrane and Vulcan carbon/ƒ-MWCNT electrode with remarkable performance. Sens. Actuators B Chem. 2024, 416, 136036. [Google Scholar] [CrossRef]
- Duan, J.; Fan, W.; Xu, Z.; Cui, L.; Wang, Z.; Nie, Z.; Sui, K. Polyelectrolyte-Mediated Modulation of Spatial Internal Stresses of Hydrogels for Complex 3D Actuators. Angew. Chem. Int. Ed. 2024, 136, e202410383. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Kim, J.; Jayaramudu, T.; Zhai, L.; Kim, H.C.; Agumba, D.O. Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application. Crystals 2020, 10, 969. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Fan, Y.; Liu, Z.; Li, R.; Li, X.; Yang, B.; Liu, Q. Self-strengthened hydrogel actuator based on the distribution of size-differentiated PVA crystallites. J. Polym. Sci. 2024, 62, 3346. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, Y.-H.; Chang, C.-C.; Rinawati, M.; Wang, P.-C.; Chang, L.-Y.; Yeh, M.-H. Enabling glucose adaptive self-healing hydrogel based triboelectric biosensor for tracking a human perspiration. Nano Energy 2023, 112, 108513. [Google Scholar] [CrossRef]
- Patnam, H.; Graham, S.A.; Manchi, P.; Paranjape, M.V.; Yu, J.S. Single-Electrode Triboelectric Nanogenerators Based on Ionic Conductive Hydrogel for Mechanical Energy Harvester and Smart Touch Sensor Applications. ACS Appl. Mater. Interfaces 2023, 15, 16768–16777. [Google Scholar] [CrossRef]
- Zhou, H.-W.; Zhao, C.; Zhao, Z.-Y.; Jiang, J.-C.; Jin, H.-L.; Wang, S.; Pan, S.; Xu, M.-Y.; Chen, Y.-H.; Jin, H.-M. Flexible and multifunctional triboelectric nanogenerator based on liquid metal/polyvinyl alcohol hydrogel for energy harvesting and self-powered wearable human–machine interaction. Rare Met. 2024, 43, 1186–1196. [Google Scholar] [CrossRef]
- Lu, D.; Liu, T.; Meng, X.; Luo, B.; Yuan, J.; Liu, Y.; Zhang, S.; Cai, C.; Gao, C.; Wang, J.; et al. Wearable Triboelectric Visual Sensors for Tactile Perception. Adv. Mater. 2023, 35, 2209117. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Wang, Z.; Zhang, X.-F.; Yao, J. Highly conductive and anti-freezing cellulose hydrogel for flexible sensors. Int. J. Biol. Macromol. 2023, 230, 123425. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, Z.; Zheng, S.; Liu, A.; Wang, Y.; Chen, L.; Miao, Q. High Ion-Conducting PVA Nanocomposite Hydrogel-Based Wearable Piezoelectric and Triboelectric Sensors for Harsh Environments. Biomacromolecules 2024, 25, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-Derived Hybrid Hydrogel Evaporators for Cost-Effective Solar Water Purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Han, L.; Yang, M.; Zhou, H.; Hong, C.; Li, J.; Ma, H.; Zhang, B. A photothermal hydrogel-fabric architected with polyvinyl alcohol decorated polypyrrole nanoparticles for efficient brine distillation. Appl. Surf. Sci. 2024, 662, 160110. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Q.; Xin, Q.; Lei, Z.; Hu, E.; Wang, H.; Wang, H.; Liang, F. Preparation of novel polyvinyl alcohol–carbon nanotubes containing imidazolyl ionic liquid/chitosan hydrogel for highly efficient uranium extraction from seawater. Int. J. Biol. Macromol. 2024, 258, 128751. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, P.; Zhong, L.; Zhang, R.; Hou, X.; Ren, X.; Wang, J.; Chu, X.; Lu, Y.; Zhou, Z. Chitosan-polyvinyl alcohol-diatomite hydrogel removes methylene blue from water. Int. J. Biol. Macromol. 2024, 254, 127886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, L.; Li, H.; Zhang, C.; Xue, J.; Wang, R.; Qiao, X.; Xue, Q. Enhancing oil-in-water emulsion separation performance of polyvinyl alcohol hydrogel nanofibrous membrane by squeezing coalescence demulsification. J. Membr. Sci. 2021, 630, 119324. [Google Scholar] [CrossRef]
- Tandorn, S.; Lamkhao, S.; Thiraphatchotiphum, C.; Rujijanagul, G.; Randorn, C. Fabrication of a bifunctionalized photocatalyst/hydrogel composite for the degradation of particulate matter (PM)-bound polycyclic aromatic hydrocarbons(PAHs). Chem. Eng. J. 2023, 457, 141190. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Sustainable implementation of innovative technologies for water purification. Nat. Rev. Chem. 2021, 5, 217–218. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, F.; Guo, Y.; Zhang, Y.; Yu, G. A hydrogel-based antifouling solar evaporator for highly efficient water desalination. Energy Environ. Sci. 2018, 11, 1985–1992. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Sheng, X.; Lin, P.; Tang, J.; Pan, L.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Solar-powered sustainable water production: State-of-the-art technologies for sunlight–energy–water nexus. ACS Nano 2021, 15, 12535–12566. [Google Scholar] [CrossRef]
- Zang, L.; Sun, L.; Zhang, S.; Finnerty, C.; Kim, A.; Ma, J.; Mi, B. Nanofibrous hydrogel-reduced graphene oxide membranes for effective solar-driven interfacial evaporation and desalination. Chem. Eng. J. 2021, 422, 129998. [Google Scholar] [CrossRef]
- Li, C.; Zhu, B.; Liu, Z.; Zhao, J.; Meng, R.; Zhang, L.; Chen, Z. Polyelectrolyte-based photothermal hydrogel with low evaporation enthalpy for solar-driven salt-tolerant desalination. Chem. Eng. J. 2022, 431, 134224. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Y.; Yin, Y.; Zou, L.; Chen, Q.; Liu, K.; Lin, P.; Su, H.; Chen, Y. Janus Polypyrrole Nanobelt@Polyvinyl Alcohol Hydrogel Evaporator for Robust Solar-Thermal Seawater Desalination and Sewage Purification. ACS Appl. Mater. Interfaces 2021, 13, 46717–46726. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, K.; Cai, B.; Zhang, J.; Wei, C.; Zhou, A. A magnetic nanostructure PAC@Fe3O4 driven design toward Janus hydrogel achieves highly efficient solar water evaporation. Chem. Eng. J. 2023, 465, 142944. [Google Scholar] [CrossRef]
- Zhou, H.; Han, L.; Yang, M.; Wu, X.; Li, J.; Ma, H.; Zhang, B. Architecting Janus hydrogel-fabric coupled evaporator for eliminating salt accumulation and highly efficient solar-driven brine desalination. Desalination 2023, 556, 116567. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, H.; Li, Z.; Huang, J.; Xu, Z.; Lai, Y.; Qian, X.; Zhang, S. Hydrogel materials for sustainable water resources harvesting & treatment: Synthesis, mechanism and applications. Chem. Eng. J. 2022, 439, 135756. [Google Scholar]
- Fan, X.; Wang, X.; Cai, Y.; Xie, H.; Han, S.; Hao, C. Functionalized cotton charcoal/chitosan biomass-based hydrogel for capturing Pb2+, Cu2+ and MB. J. Hazard. Mater. 2022, 423, 127191. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, Q.; Yan, T.; Jia, X.; Lu, D.; Ren, Y.; He, J. Enhanced removal efficiency of Cd2+ and Pb2+ from aqueous solution by H3PO4–modified tea branch biochar: Characterization, adsorption performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 112183. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.S.; Kumarathilaka, P.; Marchuk, A.; Bundschuh, J. Assessment and optimization of As(V) adsorption on hydrogel composite integrating chitosan-polyvinyl alcohol and Fe3O4 nanoparticles and evaluation of their regeneration and reusable capabilities in aqueous media. Sci. Total Environ. 2023, 855, 158877. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, H.; Zhou, H.; Xiang, A.; Deng, Y. Adsorption behaviors and mechanisms of porous polyvinyl alcohol/xanthan gum hydrogel for methylene blue and Pb2+. J. Environ. Chem. Eng. 2024, 12, 113461. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Wang, H.; Cavaco-Paulo, A.; Su, J. Co-immobilizing laccase-mediator system by in-situ synthesis of MOF in PVA hydrogels for enhanced laccase stability and dye decolorization efficiency. J. Environ. Manag. 2024, 353, 120114. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, W.; Zeng, Y.; Gao, Z.; Li, J.; Jia, H.; Ji, Q. Attachable, Self-Healing and Durable TiO2/rGO/PVA Photocatalytic Hydrogel Band for Dye Degradation. J. Mater. Chem. C 2024. [CrossRef]
- Hazeri, A.; Sirousazar, M.; Kheiri, F.; Jalilnejad, E.; Gozalzadeh, S. Adsorptive Removal of Methylene Blue Dye from Aqueous Solutions by Polyvinyl Alcohol/Activated Carbon Nanocomposite Hydrogels. J. Macromol. Sci. Part B 2022, 61, 1366–1394. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.; Ni, Z.; Ge, B.; Li, W.; Ren, G.; Miao, X.; Shao, X.; Liu, C. Construction of PVA hydrogel-based solar-driven interfacial distillation device and its performance research in selective adsorption of organic solvents and removal of Rh B. Sep. Purif. Technol. 2022, 295, 121274. [Google Scholar] [CrossRef]
- Gao, J.; Cai, M.; Nie, Z.; Zhang, J.; Chen, Y. Superwetting PVDF membrane prepared by in situ extraction of metal ions for highly efficient oil/water mixture and emulsion separation. Sep. Purif. Technol. 2021, 275, 119174. [Google Scholar] [CrossRef]
- Myint, K.T.T.; Ge, J.G.; Niu, H.J.Y.; Chen, J.; Jiao, Z. A separation-free and pizza-structure PAM/GCN/PAA composite hydrogel (PCH) in wastewater treatment at visible light or solar light. Sci. Total Environ. 2020, 705, 135821. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Yu, S.; Du, J.; Hu, X.; Bai, G.; Wang, Z. Environment-friendly dual-network hydrogel dust suppressant based on xanthan gum, polyvinyl alcohol and acrylic acid. J. Environ. Manag. 2021, 295, 113139. [Google Scholar] [CrossRef]
- Guo, H.; Tang, Z.; Liu, Q.; Xu, J.; Wang, M.; Liang, R.; Sun, G. Ultra-stable anti-washout cement grout achieved by super water absorbing villus-like nanocomposite hydrogel. Constr. Build. Mater. 2021, 301, 124035. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Zhao, P.; Dai, N.; Zhai, Z.; Ai, T. Improving cracking resistance of cement mortar by thermo-sensitive poly N-isopropyl acrylamide (PNIPAM) gels. J. Clean. Prod. 2018, 176, 1292–1303. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Du, J.; Liu, Z.; Li, P.; Ren, X.; Feng, Y. Development of Ca2+-based, ion-responsive superabsorbent hydrogel for cement applications: Self-healing and compressive strength. J. Colloid Interface Sci. 2019, 538, 397–403. [Google Scholar] [CrossRef]
- Litvan, G.G. Phase Transitions of Adsorbates: IV, Mechanism of Frost Action in Hardened Cement Paste. J. Am. Ceram. Soc. 1972, 55, 38–42. [Google Scholar] [CrossRef]
- Yan, M.-L.; Xie, J.; Yan, J.-B. Experimental study on size effect and durability properties of PVA reinforced ice at Arctic low temperatures. J. Build. Eng. 2023, 65, 105757. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Wu, Z.; Pan, H.; Huang, P.; Tang, J.; She, W. Biomimetic Mechanical Robust Cement-Resin Composites with Machine Learning-Assisted Gradient Hierarchical Structures. Adv. Mater. 2024, 36, 2405183. [Google Scholar] [CrossRef]
- Kong, X.; Emmerling, S.; Pakusch, J.; Rueckel, M.; Nieberle, J. Retardation effect of styrene-acrylate copolymer latexes on cement hydration. Cem. Concr. Res. 2015, 75, 23–41. [Google Scholar] [CrossRef]
- Baueregger, S.; Perello, M.; Plank, J. Influence of carboxylated styrene–butadiene latex copolymer on Portland cement hydration. Cem. Concr. Compos. 2015, 63, 42–50. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Zhou, Y.; Zhang, W.; Li, W.; She, W.; Liu, J.; Miao, C. Multi-layered cement-hydrogel composite with high toughness, low thermal conductivity, and self-healing capability. Nat. Commun. 2023, 14, 3438. [Google Scholar] [CrossRef]
- Su, J.; Bloodworth, A. Numerical calibration of mechanical behaviour of composite shell tunnel linings. Tunn. Undergr. Space Technol. 2018, 76, 107–120. [Google Scholar] [CrossRef]
- Moon, J.R.; Jeon, Y.S.; Kim, Y.J.; Kim, J.-H. Adhesive, self-healing and antibacterial properties of Cu-coordinated soft gel based on histamine-conjugated polyaspartamide. J. Polym. Res. 2018, 26, 12. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Chang, S.-H.; Choi, S.-W.; Park, B.; Lee, C. Numerical approach to assessing the contact characteristics of a polymer-based waterproof membrane. Tunn. Undergr. Space Technol. 2018, 79, 242–249. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; He, H.; Ma, S.; Yao, J. Flame-retardant PNIPAAm/sodium alginate/polyvinyl alcohol hydrogels used for fire-fighting application: Preparation and characteristic evaluations. Carbohydr. Polym. 2021, 255, 117485. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Z.; Huang, Y.; Hai, Y.; Zhong, X.; Xiao, S.; Jiang, S. Influence of phytic acid on flame retardancy and adhesion performance enhancement of poly (vinyl alcohol) hydrogel coating to wood substrate. Prog. Org. Coat. 2021, 161, 106453. [Google Scholar] [CrossRef]
- Guo, C.; Tang, H.; Wang, P.; Xu, Q.; Pan, H.; Zhao, X.; Fan, F.; Li, T.; Zhao, D. Radiative cooling assisted self-sustaining and highly efficient moisture energy harvesting. Nat. Commun. 2024, 15, 6100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, F.; Wang, X.; Fang, S.; Tan, J.; Chu, W.; Rong, R.; Yin, J.; Zhang, Z.; Liu, Y.; et al. Hydrovoltaic technology: From mechanism to applications. Chem. Soc. Rev. 2022, 51, 4902–4927. [Google Scholar] [CrossRef]
- Lambertini, L.; Coccarelli, G.; Toto, E.; Santonicola, M.G.; Laurenzi, S. Poly(vinyl alcohol) gels cross-linked by boric acid for radiation protection of astronauts. Acta Astronaut. 2024, 221, 142–154. [Google Scholar] [CrossRef]
- Gupta, S.C.; Baheti, G.L.; Gupta, B.P. Application of hydrogel system for neutron attenuation. Radiat. Phys. Chem. 2000, 59, 103–107. [Google Scholar] [CrossRef]
- KP, A.A.; Saeed, P.A.; Manholi, S.; Sujith, A. Polyvinyl alcohol-soy protein isolate hydrogels: Controlled release of fertilizer and matrix nutrients for sustainable agriculture. J. Clean. Prod. 2024, 451, 141827. [Google Scholar] [CrossRef]
- Seeponkai, N.; Khammuang, K.; Fuggate, P.; Seephonkai, P. Physical properties and ion permeability of crosslinking hydrogel membrane based on poly(vinyl alcohol) for soilless cultivation. J. Appl. Polym. Sci. 2023, 140, e53311. [Google Scholar] [CrossRef]
- Torres-Figueroa, A.V.; de los Santos-Villalobos, S.; Rodríguez-Félix, D.E.; Moreno-Salazar, S.F.; Pérez-Martínez, C.J.; Chan-Chan, L.H.; Ochoa-Meza, A.; del Castillo-Castro, T. Physically and Chemically Cross-Linked Poly(vinyl alcohol)/Humic Acid Hydrogels for Agricultural Applications. ACS Omega 2023, 8, 44784–44795. [Google Scholar] [CrossRef]
- Fabian, D.R.C.; Durpekova, S.; Dusankova, M.; Hanusova, D.; Bergerova, E.D.; Sedlacik, M.; Skoda, D.; Sedlarik, V. Renewable whey-based hydrogel with polysaccharides and polyvinyl alcohol as a soil amendment for sustainable agricultural application. Int. J. Biol. Macromol. 2024, 259, 129056. [Google Scholar] [CrossRef]
- Carretti, E.; Natali, I.; Matarrese, C.; Bracco, P.; Weiss, R.G.; Baglioni, P.; Salvini, A.; Dei, L. A new family of high viscosity polymeric dispersions for cleaning easel paintings. J. Cult. Herit. 2010, 11, 373–380. [Google Scholar] [CrossRef]
- Carretti, E.; Grassi, S.; Cossalter, M.; Natali, I.; Caminati, G.; Weiss, R.G.; Baglioni, P.; Dei, L. Poly(vinyl alcohol)−Borate Hydro/Cosolvent Gels: Viscoelastic Properties, Solubilizing Power, and Application to Art Conservation. Langmuir 2009, 25, 8656–8662. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, C.; Severini, L.; Domenici, F.; Toumia, Y.; Mazzotta, F.; Micheli, L.; Titubante, M.; Di Napoli, B.; Paradossi, G.; Palleschi, A. Polyvinyl alcohol based hydrogels as new tunable materials for application in the cultural heritage field. Colloids Surf. B Biointerfaces 2020, 188, 110777. [Google Scholar] [CrossRef]
- Al-Emam, E.; Soenen, H.; Caen, J.; Janssens, K. Characterization of polyvinyl alcohol-borax/agarose (PVA-B/AG) double network hydrogel utilized for the cleaning of works of art. Herit. Sci. 2020, 8, 106. [Google Scholar] [CrossRef]

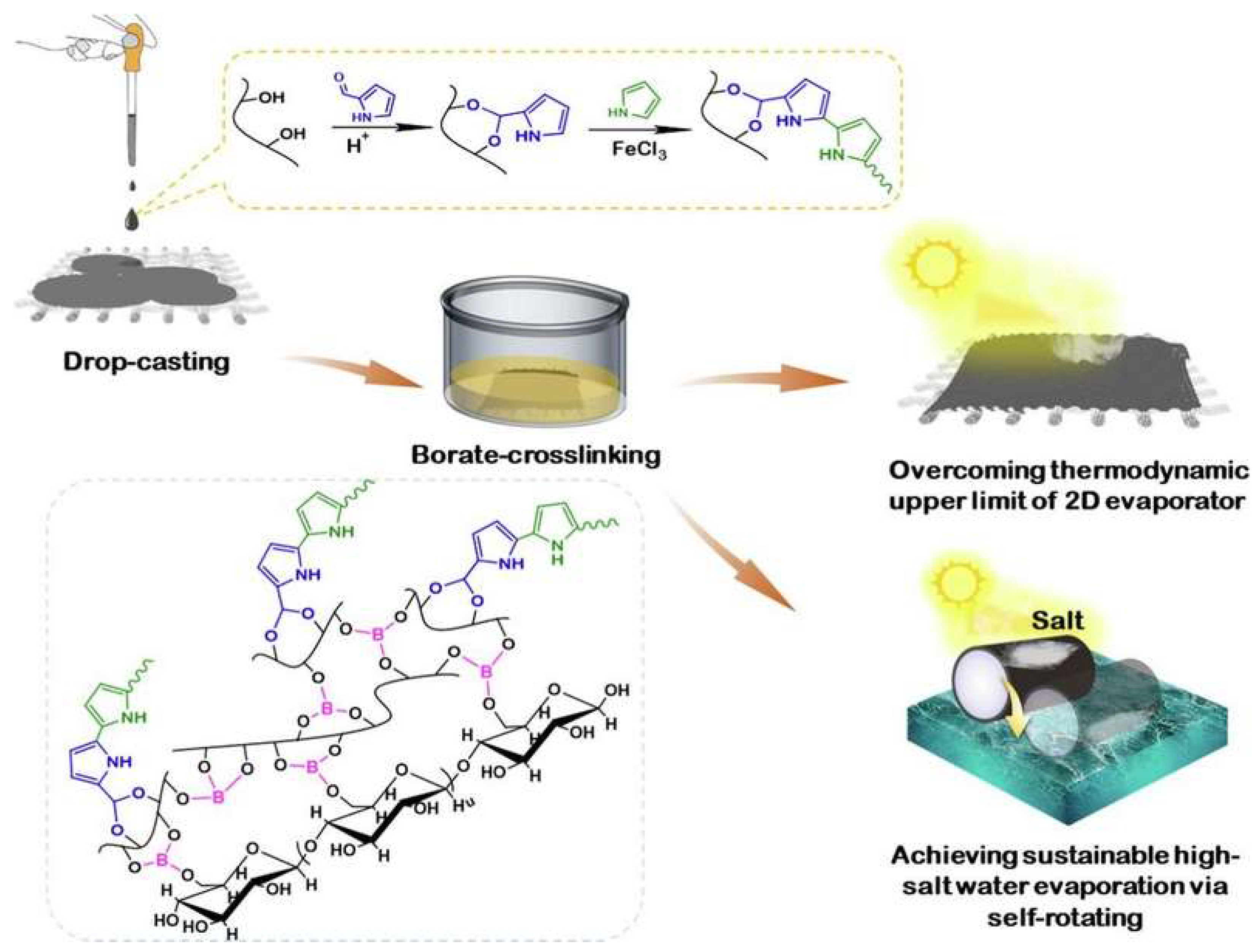

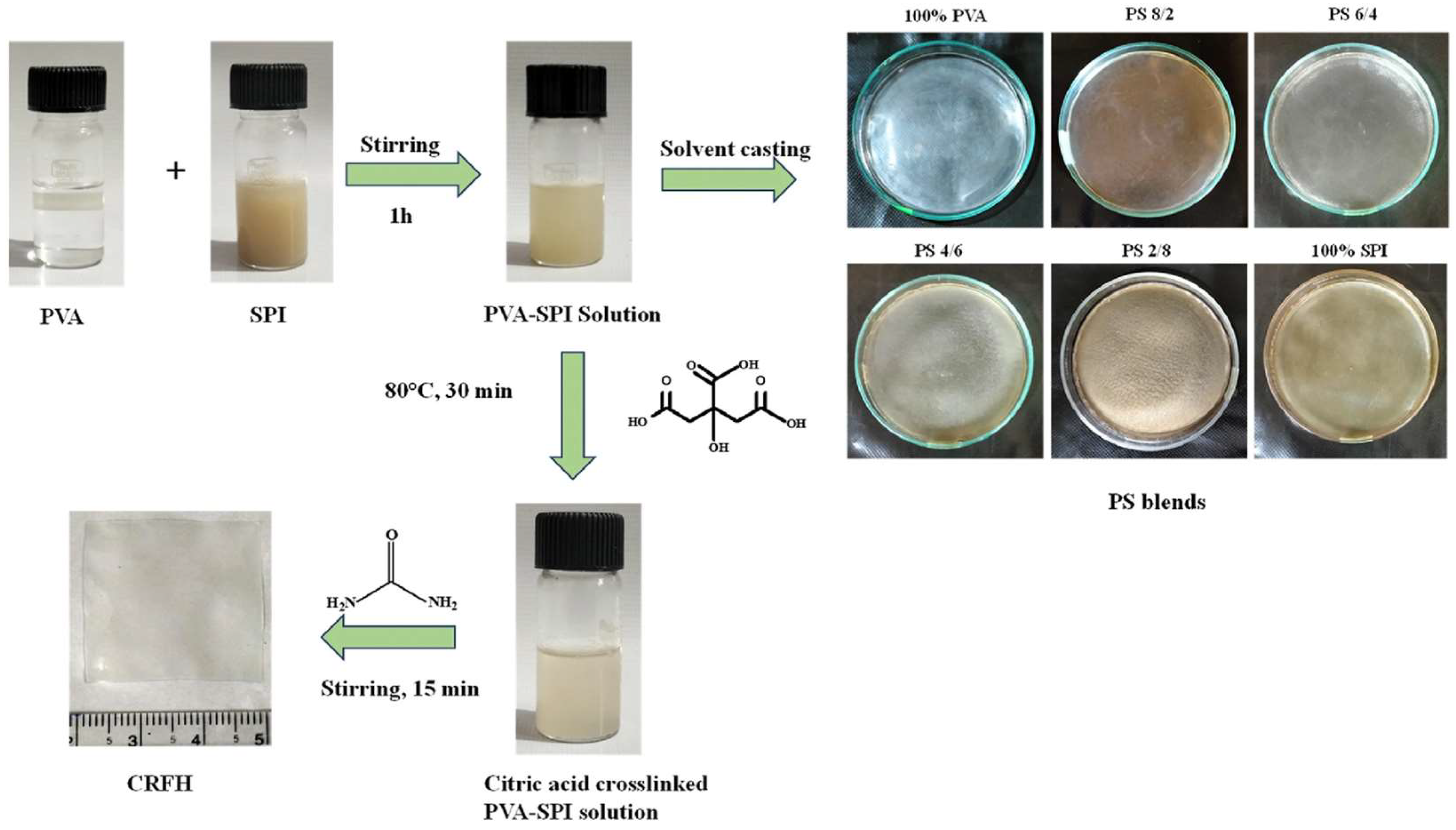
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. https://doi.org/10.3390/polym16192755
Liang X, Zhong H-J, Ding H, Yu B, Ma X, Liu X, Chong C-M, He J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers. 2024; 16(19):2755. https://doi.org/10.3390/polym16192755
Chicago/Turabian StyleLiang, Xiaoxu, Hai-Jing Zhong, Hongyao Ding, Biao Yu, Xiao Ma, Xingyu Liu, Cheong-Meng Chong, and Jingwei He. 2024. "Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications" Polymers 16, no. 19: 2755. https://doi.org/10.3390/polym16192755
APA StyleLiang, X., Zhong, H.-J., Ding, H., Yu, B., Ma, X., Liu, X., Chong, C.-M., & He, J. (2024). Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers, 16(19), 2755. https://doi.org/10.3390/polym16192755







