In Situ Formation of Acidic Comonomer during Thermal Treatment of Copolymers of Acrylonitrile and Its Influence on the Cyclization Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Polymer Synthesis
2.2. Instrumentation
3. Results and Discussion
3.1. Binary Copolymers of Acrylonitrile and Tert-Butyl Acrylate
3.1.1. Polymer Synthesis and Characterization
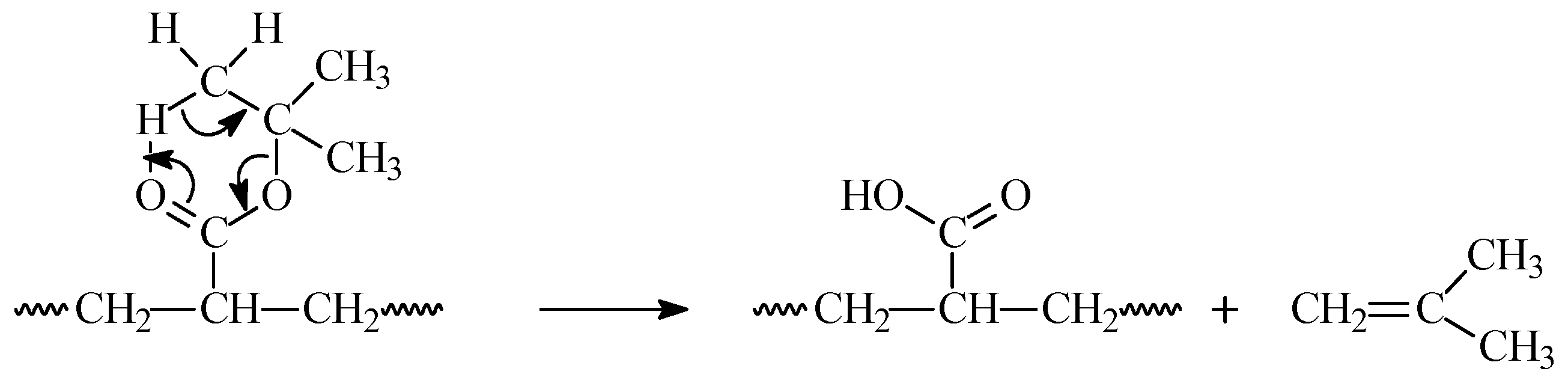
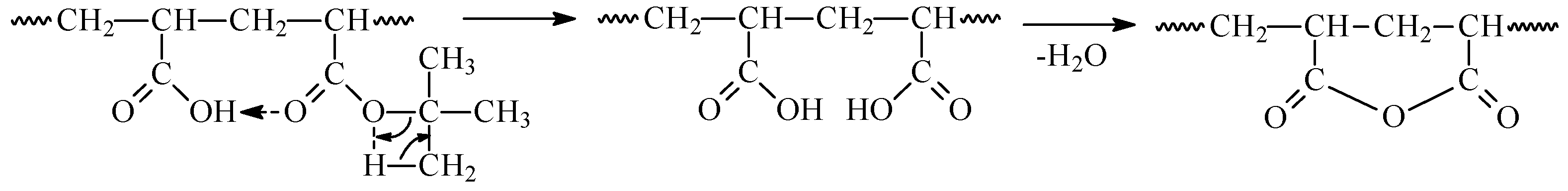
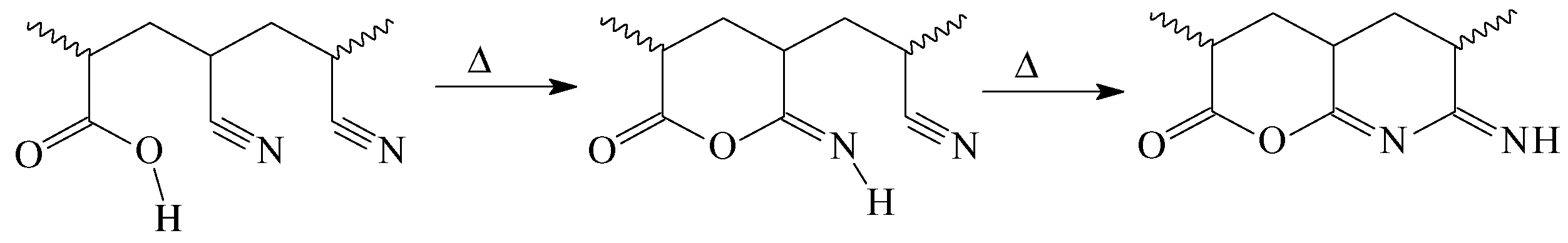
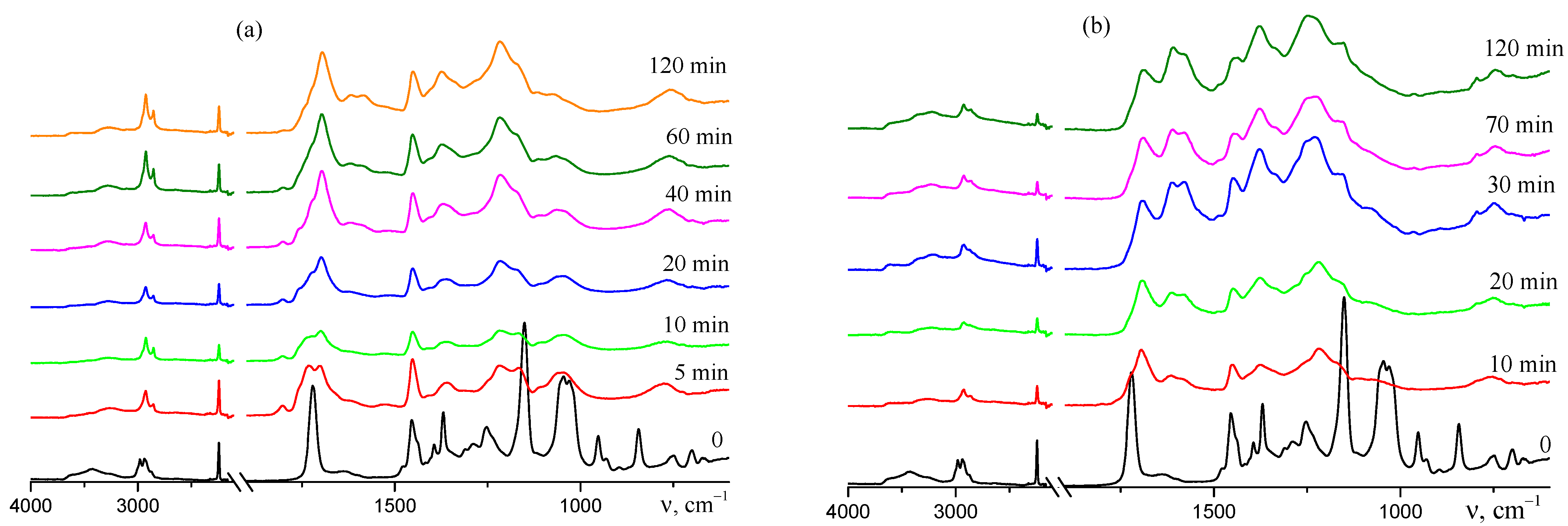
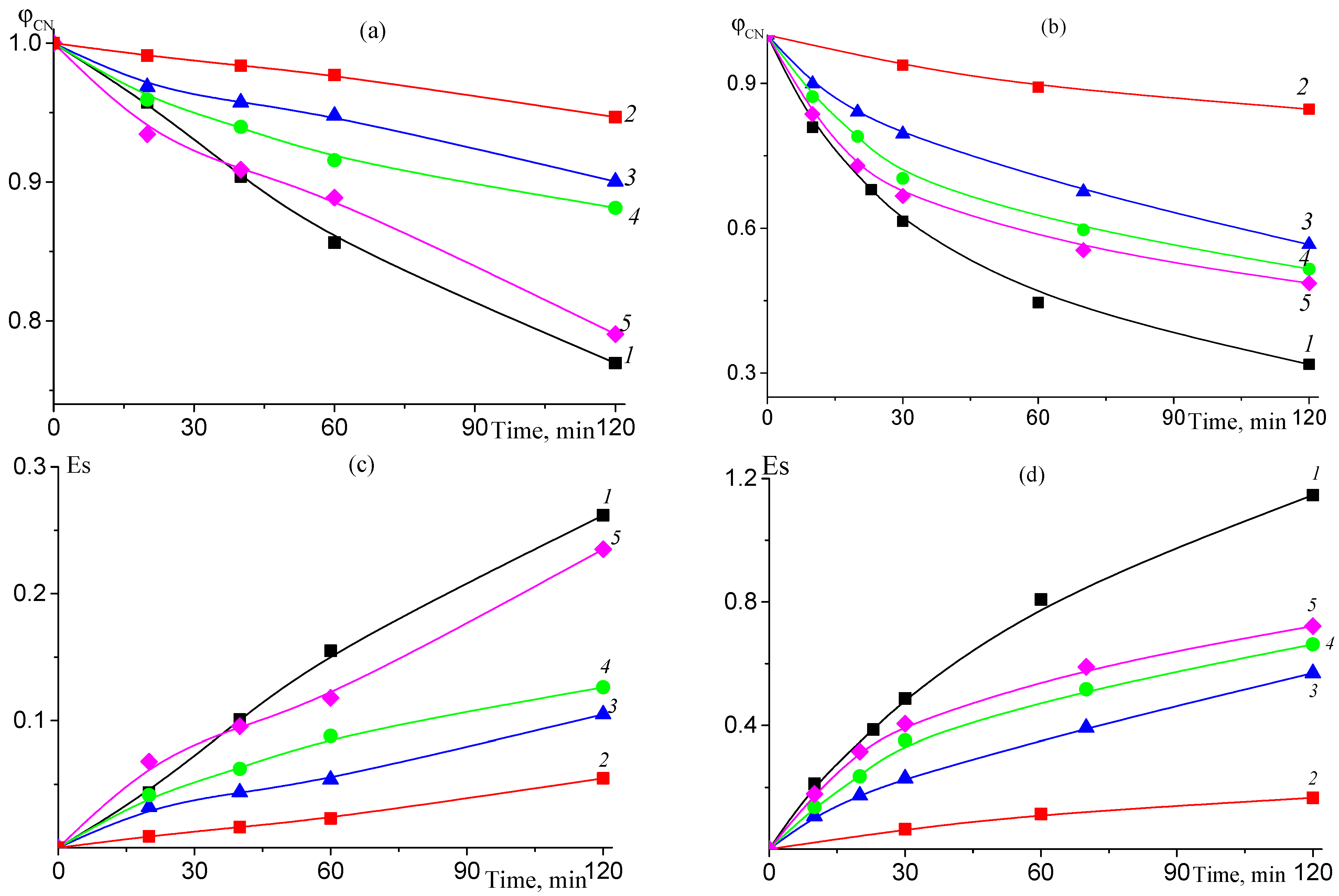
3.1.2. In Situ Formation of Carboxylic Groups during Pyrolysis of AN–TBA Copolymer
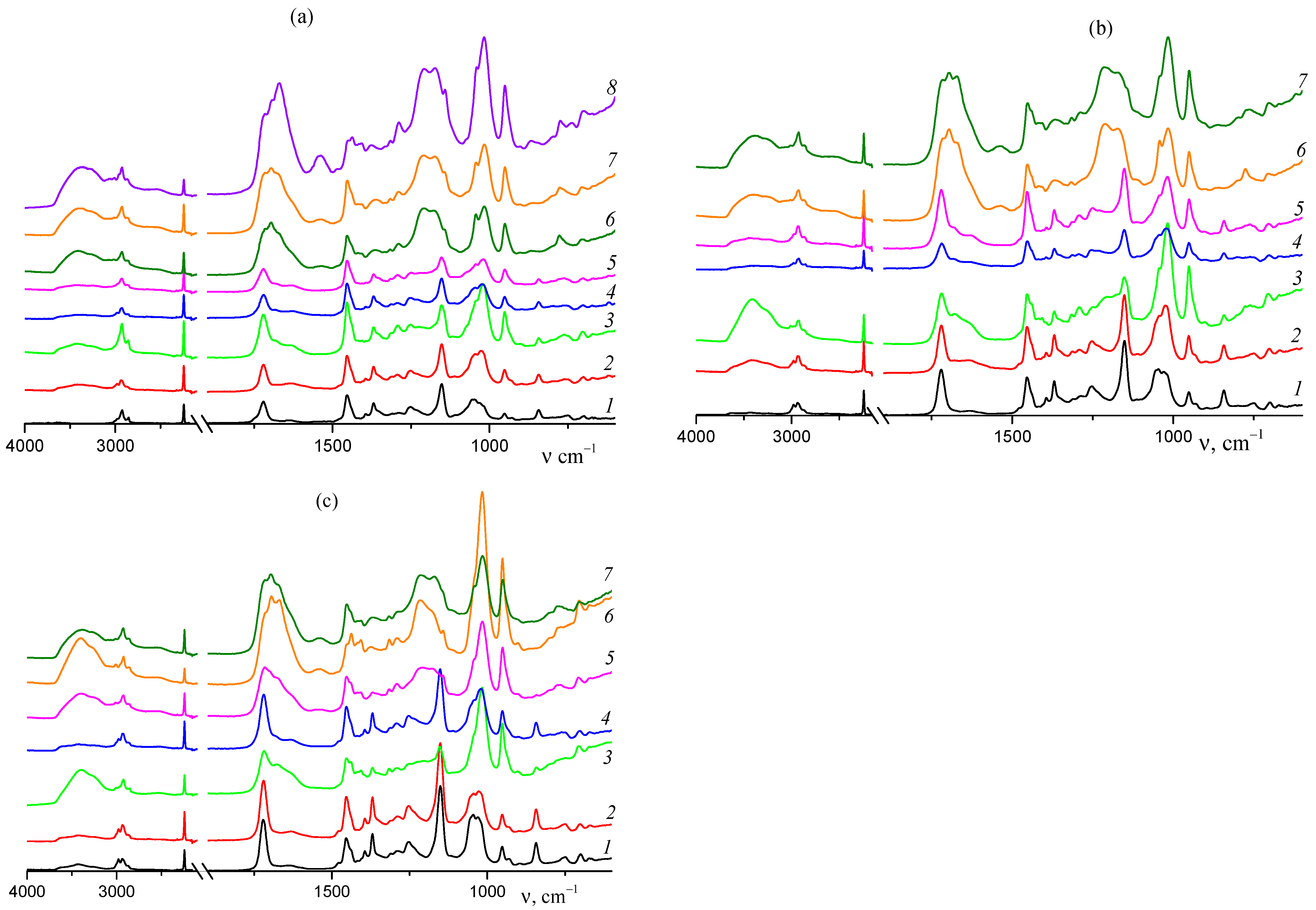
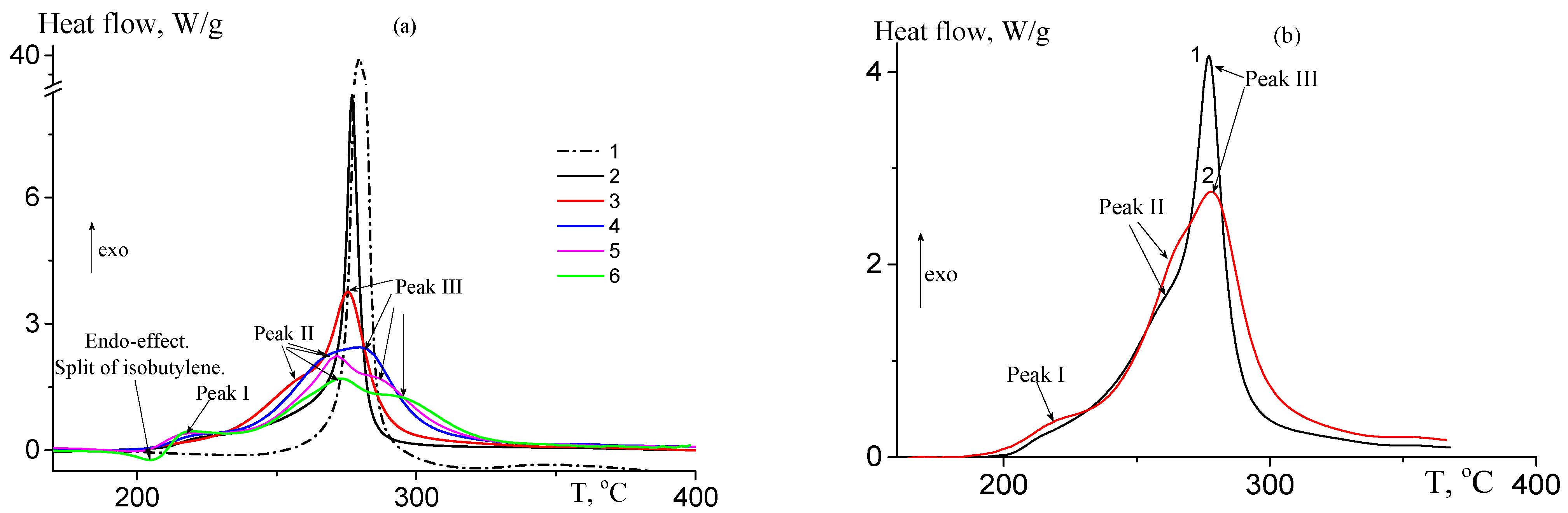
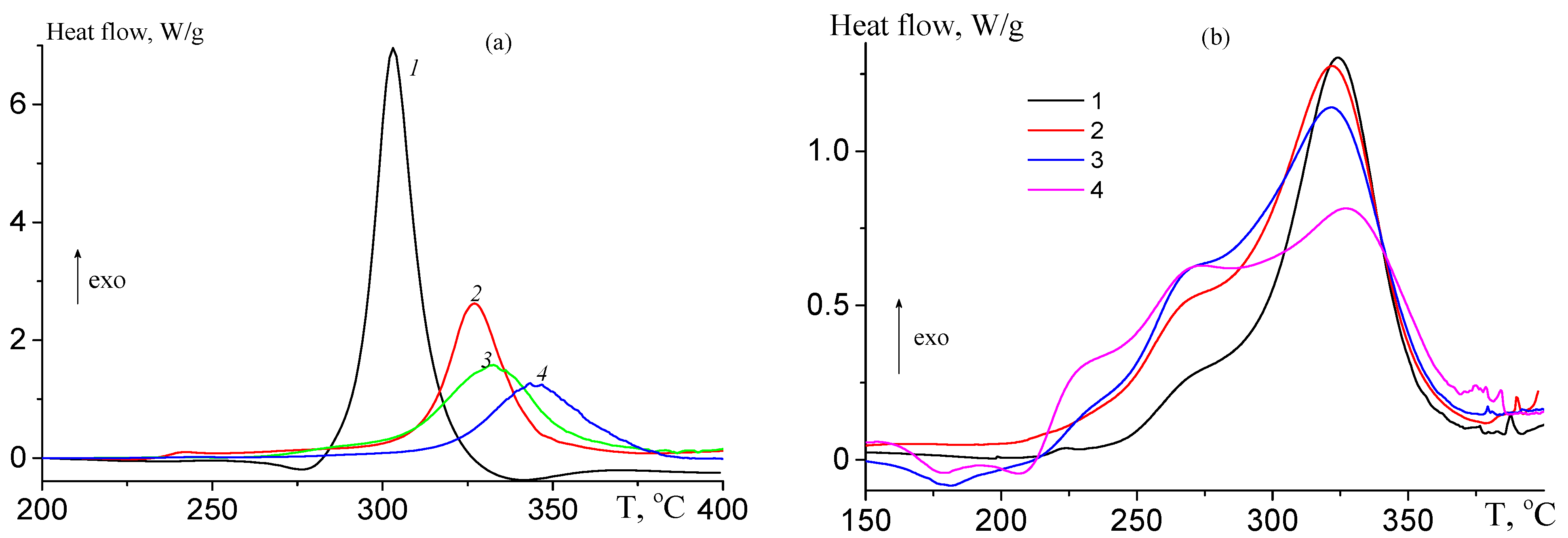
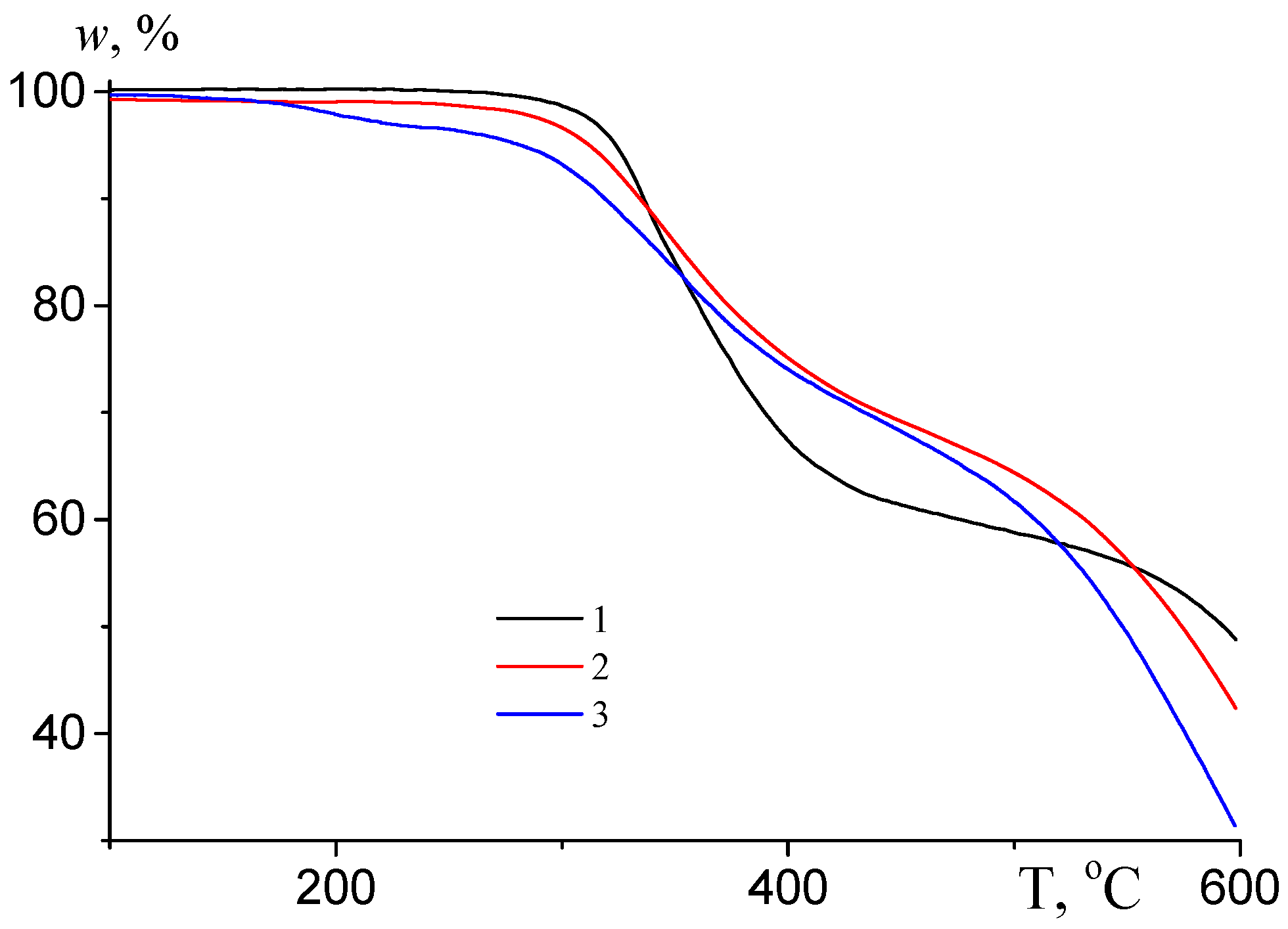
3.1.3. Thermal Behavior under Argon Atmosphere
3.1.4. Thermal Oxidative Stabilization
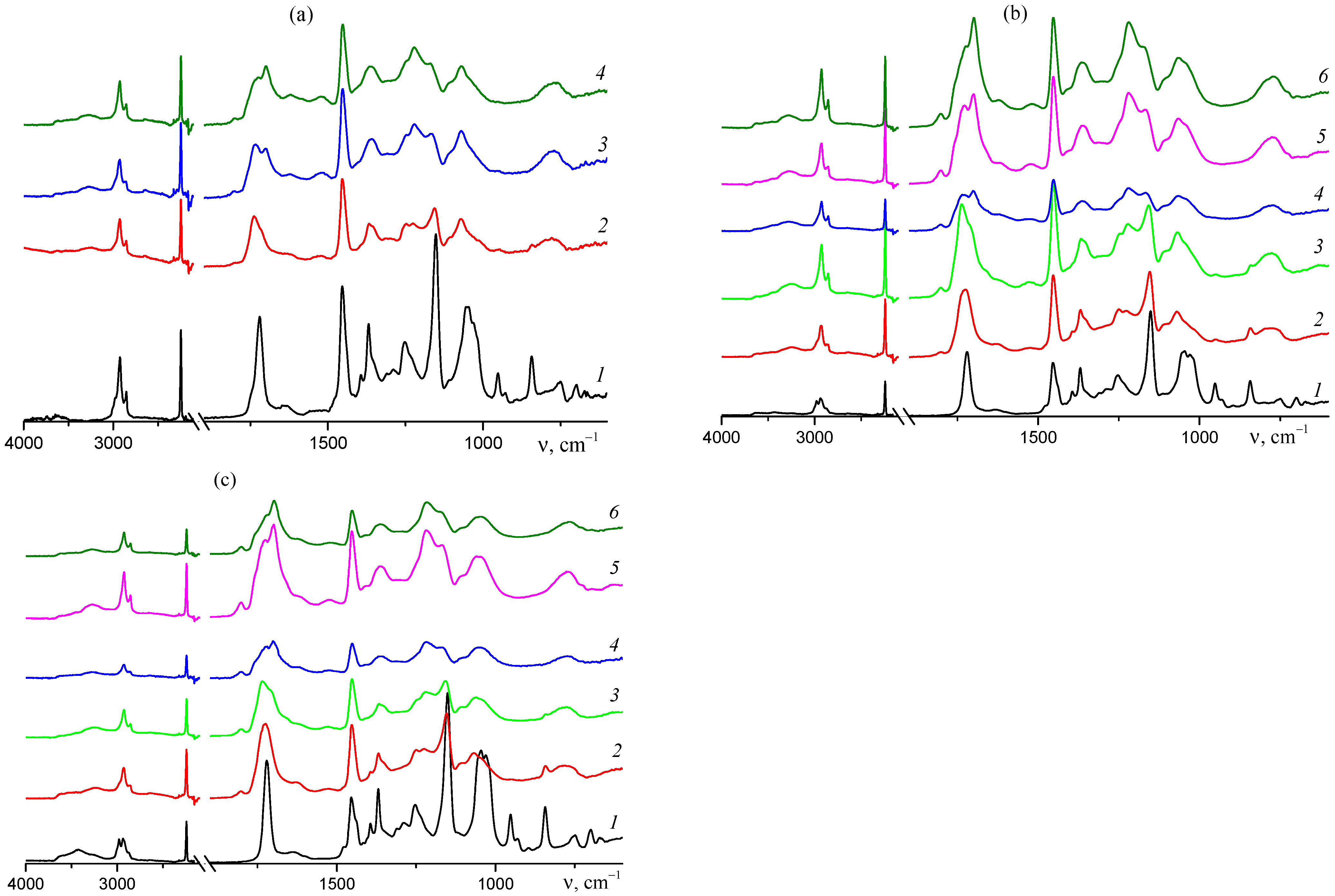
3.2. Ternary Copolymers of Acrylonitrile, Tert-Butyl Acrylate, and n-Butyl Acrylate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers: Precursors, Manufacturing, and Properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, D.-C.J.M.; Buchmeiser, M.R. Carbon Fibers: Precursor Systems, Processing, Structure, and Properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Kaur, J.; Millington, K.; Smith, S. Producing high-quality precursor polymer and fibers to achieve theoretical strength in carbon fibers: A review. J. Appl. Polym. Sci. 2016, 133, 43963. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Recent progress in fabrication, structure and properties of carbon fibers. Polym. Rev. 2012, 52, 234–258. [Google Scholar] [CrossRef]
- Morgan, P. (Ed.) Carbon Fibers and Their Composites; Taylor and Francis: New York, NY, USA, 2005; pp. 185–259. [Google Scholar]
- Standage, A.E.; Matkowsky, R.D. Thermal oxidation of polyacrylonitrile. Eur. Polym. J. 1971, 7, 775–783. [Google Scholar] [CrossRef]
- Trofimenko, E.A.; Bukharkina, T.V.; Verzhichinskaya, S.V. Modification of accelerated thermal stabilization of polyacrylonitrile fibers by creating an oxygen concentration gradient in the production of carbon fiber. Fine Chem. Technol. 2023, 18, 243–253. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Z.; Liu, J.; Liang, J.; Wang, X. Effects of oxygen on the structural evolution of polyacrylonitrile fibers during rapid thermal treatment. RSC Adv. 2020, 10, 6356–6361. [Google Scholar] [CrossRef]
- Bhanu, V.; Rangarajan, P.; Wiles, K.; Bortner, M.; Sankarpandian, M.; Godshall, D.; Wilkes, G. Synthesis and characterization of acrylonitrile methyl acrylate statistical copolymers as melt processable carbon fiber precursors. Polymer 2002, 43, 4841–4850. [Google Scholar] [CrossRef]
- Godshall, D.; Rangarajan, P.; Baird, D.G.; Wilkes, G.L.; Bhanu, V.A.; McGrath, J.E. Incorporation of methyl acrylate in acrylonitrile based copolymers: Effects on melting behavior. Polymer 2003, 44, 4221–4228. [Google Scholar] [CrossRef]
- Devasia, R.; Reghunadhan Nair, C.P.; Sivadasan, P.; Ninan, K.N. High char-yielding poly [acrylonitrile-co-(itaconic ac-id)-co-(methyl acrylate)]: Synthesis and properties. Polym. Int. 2005, 54, 1110–1118. [Google Scholar] [CrossRef]
- Alcalá-Sánchez, D.; Tapia-Picazo, J.C.; Bonilla-Petriciolet, A.; Luna-Bárcenas, G.; López-Romero, J.M.; Álvarez-Castillo, A. Analysis of Terpolymerization systems for the development of carbon fiber precursors of PAN. Int. J. Polym. Sci. 2020, 2020, 8029516. [Google Scholar] [CrossRef]
- Shashidhar, G.V.S.; Satyanarayana, N.; Sundaram, E.V.; Sathaiah, G.; Sirdeshmukh, L. Radiation-Induced Copolymerization of Acrylonitrile with Methyl Acrylate: Synthesis and Characterization. J. Macromol. Sci. Part A Chem. 1990, 27, 1069–1080. [Google Scholar] [CrossRef]
- Han, N.; Zhang, X.-X.; Wang, X.-C. Various Comonomers in Acrylonitrile Based Copolymers: Effects on Thermal Behaviour. Iran. Polym. J. 2010, 19, 243–253. [Google Scholar]
- Wiles, K.B.; Bhanu, V.A.; Pasquale, A.J.; Long, T.E.; McGrath, J.E. Monomer reactivity ratios for acrylonitrile-methyl acrylate free-radical copolymerization. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2994–3001. [Google Scholar] [CrossRef]
- Peng, W.W.; Han, N.; Tang, X.F.; Liu, H.H.; Zhang, X.X. Preparation and Characterization of Melt-Spun Poly(Acrylonitrile Methylacrylate) Hollow Fiber. Adv. Mater. Res. 2011, 332, 339–342. [Google Scholar] [CrossRef]
- Hao, J.; Liu, Y.; Lu, C. Effect of acrylonitrile sequence distribution on the thermal stabilization reactions and carbon yields of poly(acrylonitrile-co-methyl acrylate). Polym. Degrad. Stab. 2018, 147, 89–96. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kuzin, M.S.; Vashchenko, A.F.; Toms, R.V.; Varfolomeeva, L.A.; Chernikova, E.V.; Shambilova, G.K.; Kulichikhin, V.G. Fiber Spinning of Polyacrylonitrile Terpolymers Containing Acrylic Acid and Alkyl Acrylates. Fibers 2023, 11, 65. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Poteryaeva, Z.A.; Plutalova, A.V. Controlled copolymerization of acrylonitrile in bulk via the reversible addition-fragmentation chain-transfer mechanism. Polym. Sci. Ser. B 2014, 56, 109–117. [Google Scholar] [CrossRef]
- Izumi, Z.; Kitagawa, H. Effect of reaction medium on copolymerization of acrylonitrile and methyl acrylate. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 1967–1975. [Google Scholar] [CrossRef]
- Reddy, G.V.R.; Ranganathan, R.; Sivakumar, S.; Sriram, R. Emulsion copolymerizations of methyl acrylate with methyl methacrylate and with acrylonitrile. Des. Monomers Polym. 2002, 5, 97–114. [Google Scholar] [CrossRef][Green Version]
- Kamide, K.; Miyazaki, Y.; Kobayashi, H. Dilute Solution Properties of Random Acrylonitrile/Methyl Acrylate Copolymer. Polym. J. 1982, 14, 591–602. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Gervald, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Chernikova, E.V. Reversible addition–fragmentation chain transfer based copolymers of acrylonitrile and alkyl acrylates as possible precursors for carbon fibers: Synthesis and thermal behavior during stabilization. Polym. Int. 2022, 71, 646–655. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R. New aspects in the oxidative stabilization of PAN-based carbon fibers. Carbon 1996, 34, 1427–1445. [Google Scholar] [CrossRef]
- Dang, W.; Liu, J.; Wang, X.; Yan, K.; Zhang, A.; Yang, J.; Liang, J. Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere. Polymers 2020, 12, 63. [Google Scholar] [CrossRef]
- Bajaj, P.; Roopanwal, A. Thermal stabilization of acrylic precursors for the production of carbon fibers: An overview. J. Macromol. Sci. Part C 1997, 37, 97–147. [Google Scholar] [CrossRef]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Miller, G.C.; Yu, J.; Joseph, R.M.; Choudhury, S.R.; Mecham, S.J.; Baird, D.G.; Riffle, J.S. Melt-spinnable polyacrylonitrile copolymer precursors for carbon fibers. Polymer 2017, 126, 87–95. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paliwal, D.K.; Bajaj, P. Melting behavior of acrylonitrile polymers. J. Appl. Polym. Sci. 1998, 70, 2703–2709. [Google Scholar] [CrossRef]
- Cai, J.Y.; McDonnell, J.; Brackley, C.; O’Brien, L.; Church, J.S.; Millington, K.; Phair-Sorensen, N. Polyacrylonitrile-based precursors and carbon fibers derived from advanced RAFT technology and conventional methods—The 1st comparative study. Mater. Today Commun. 2016, 9, 22–29. [Google Scholar] [CrossRef]
- Chae, H.G.; Newcomb, B.A.; Gulgunje, P.V.; Liu, Y.; Gupta, K.K.; Kamath, M.G.; Lyons, K.M.; Ghoshal, S.; Pramanik, C.; Giannuzzi, L.; et al. High strength and high modulus carbon fibers. Carbon 2015, 93, 81–87. [Google Scholar] [CrossRef]
- Morris, E.A.; Weisenberger, M.C.; Bradley, S.B.; Abdallah, M.G.; Mecham, S.J.; Pisipati, P.; McGrath, J.E. Synthesis, spinning, and properties of very high molecular weight poly(acrylonitrile-co-methyl acrylate) for high performance precursors for carbon fiber. Polymer 2014, 55, 6471–6482. [Google Scholar] [CrossRef]
- Li, W.; Long, D.; Miyawaki, J.; Qiao, W.; Ling, L.; Mochida, I.; Yoon, S.-H. Structural features of polyacrylonitrile-based carbon fibers. J. Mater. Sci. 2011, 47, 919–928. [Google Scholar] [CrossRef]
- Rangarajan, P.; Yang, J.; Bhanu, V.; Godshall, D.; McGrath, J.; Wilkes, G.; Baird, D. Effect of comonomers on melt processability of polyacrylonitrile. J. Appl. Polym. Sci. 2002, 85, 69–83. [Google Scholar] [CrossRef]
- Chang, S.-H. Thermal analysis of acrylonitrile copolymers containing methyl acrylate. J. Appl. Polym. Sci. 1994, 54, 405–407. [Google Scholar] [CrossRef]
- Maksimov, N.M.; Toms, R.V.; Balashov, M.S.; Gerval’D, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Kuzin, M.S.; Skvortsov, I.Y.; Kulichikhin, V.G.; Chernikova, E.V. Novel Potential Precursor of Carbon Fiber Based on Copolymers of Acrylonitrile, Acrylamide, and Alkyl Acrylates. Polym. Sci. Ser. B 2022, 64, 670–687. [Google Scholar] [CrossRef]
- König, S.; Kreis, P.; Herbert, C.; Wego, A.; Steinmann, M.; Wang, D.; Frank, E.; Buchmeiser, M.R. Melt-Spinning of an Intrinsically Flame-Retardant Polyacrylonitrile Copolymer. Materials 2020, 13, 4826. [Google Scholar] [CrossRef]
- Hall, M.E.; Zhang, J.; Horrocks, A.R. The flammability of polyacrylonitrile and its copolymers III. Effect of flame retardants. Fire Mater. 1994, 18, 231–241. [Google Scholar] [CrossRef]
- Deng, W.; Lobovsky, A.; Iacono, S.T.; Wu, T.; Tomar, N.; Budy, S.M.; Long, T.; Hoffman, W.P.; Dennis, D.W.S., Jr. Poly (acrylonitrile–co-1-vinylimidazole): A new melt processable carbon fiber precursor. Polymer 2011, 52, 622–628. [Google Scholar] [CrossRef]
- Mahmood, S.F.; Batchelor, B.; Jung, M.; Park, K.; Voit, W.E.; Novak, B.M.; Yang, D. Study of a melt processable polymer precursor for carbon fiber. Carbon Lett. 2019, 29, 605–612. [Google Scholar] [CrossRef]
- Han, N.; Li, H.; Li, C.; Zhang, X.; Li, W.; Wang, D. Synthesis and Properties of Melt Processable Acrylonitrile-N-vinylimidazole Copolymers and Fiber. Chem. J. Chin. Univ. 2015, 36, 2073–2080. Available online: http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20150309 (accessed on 25 August 2024).
- Jamil, S.N.A.M.; Daik, R.; Ahmad, I. Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber. Materials 2014, 7, 6207–6223. [Google Scholar] [CrossRef] [PubMed]
- Rwei, S.-P.; Way, T.-F.; Chiang, W.-Y.; Tseng, J.-C. Thermal analysis and melt spinnability of poly(acrylonitrile-co-methyl acrylate) and poly(acrylonitrile-co-dimethyl itaconate) copolymers. Text. Res. J. 2017, 88, 1479–1490. [Google Scholar] [CrossRef]
- Rangarajan, P.; Bhanu, V.; Godshall, D.; Wilkes, G.; McGrath, J.; Baird, D. Dynamic oscillatory shear properties of potentially melt processable high acrylonitrile terpolymers. Polymer 2002, 43, 2699–2709. [Google Scholar] [CrossRef]
- Schaefgen, J.R.; Sarasohn, I.M. Observations on the thermolytic decomposition of poly(tert-butyl acrylate). J. Polym. Sci. 1962, 58, 1049–1061. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Collins, G.L.; Thomas, N.W.; Williams, G.E. Kinetic relationships between heat generation and nitrile consumption in the reaction of poly(acrylonitrile) in air at 265 °C. Carbon 1988, 26, 671–679. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Terpugova, P.S.; Trifilov, M.Y. Controlled synthesis of acrylic homo- and copolymers in the presence of trithiocarbonates as reversible addition-fragmentation chain transfer agents. Polym. Sci. Ser. A 2009, 51, 658–666. [Google Scholar] [CrossRef]
- Grant, D.H.; Grassie, N. The thermal decomposition of poly(t-butyl methacrylate). Polymer 1960, 1, 445–455. [Google Scholar] [CrossRef]
- Maurer, J.J.; Eustace, D.J.; Ratcliffe, C.T. Thermal characterization of poly(acrylic acid). Macromolecules 1987, 20, 196–202. [Google Scholar] [CrossRef]
- Özlem, S.; Hacaloglu, J. Thermal degradation of poly(n-butyl methacrylate), poly(n-butyl acrylate) and poly(t-butyl acrylate). J. Anal. Appl. Pyrolysis 2013, 104, 161–169. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Shaova, A.A.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Chernikova, E.V. Copolymers of Acrylonitrile and Acrylic Acid: Effect of Composition and Distribution of Chain Units on the Thermal Behavior of Copolymers. Polym. Sci. Ser. B 2020, 62, 102–115. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Gervald, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Berkovich, A.K.; Chernikova, E.V. Influence of synthesis method on the properties of carbon fiber precursors based on acrylonitrile and acrylic acid copolymers. Polym. Sci. Ser. B 2020, 62, 660–670. [Google Scholar] [CrossRef]

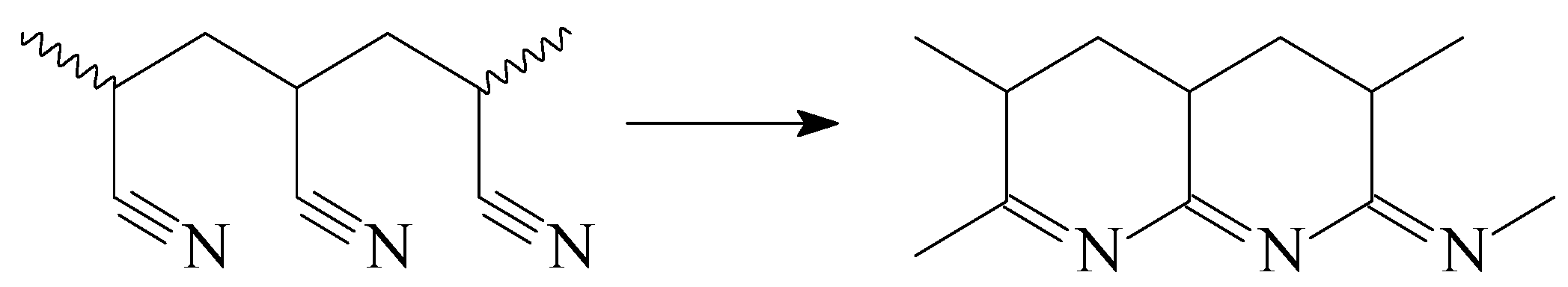
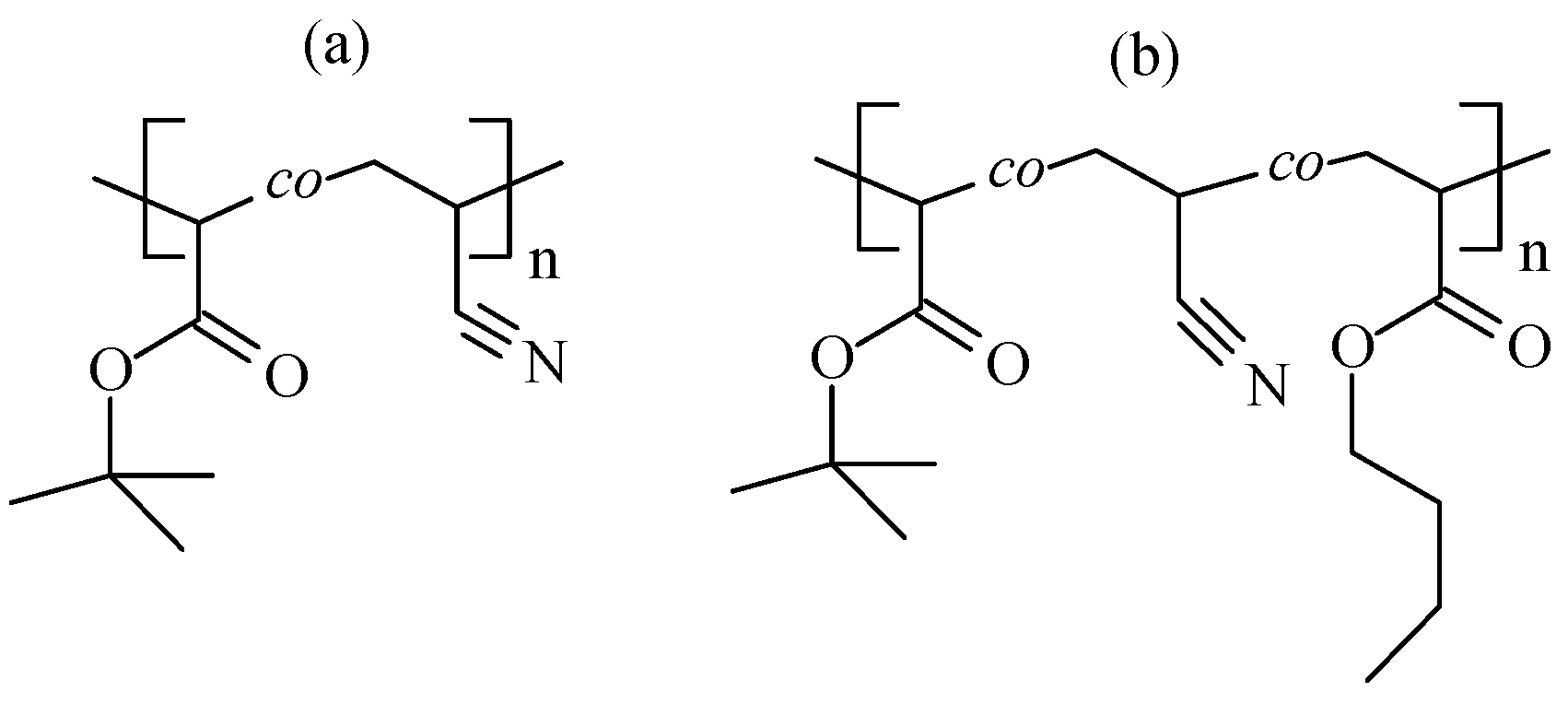
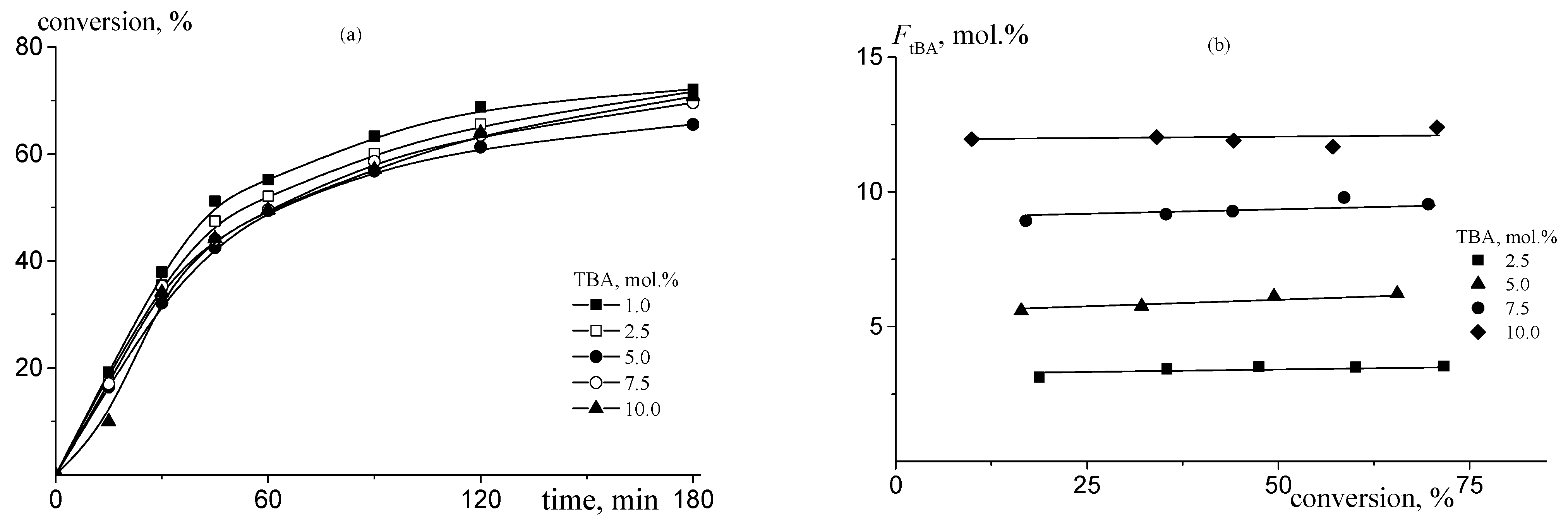
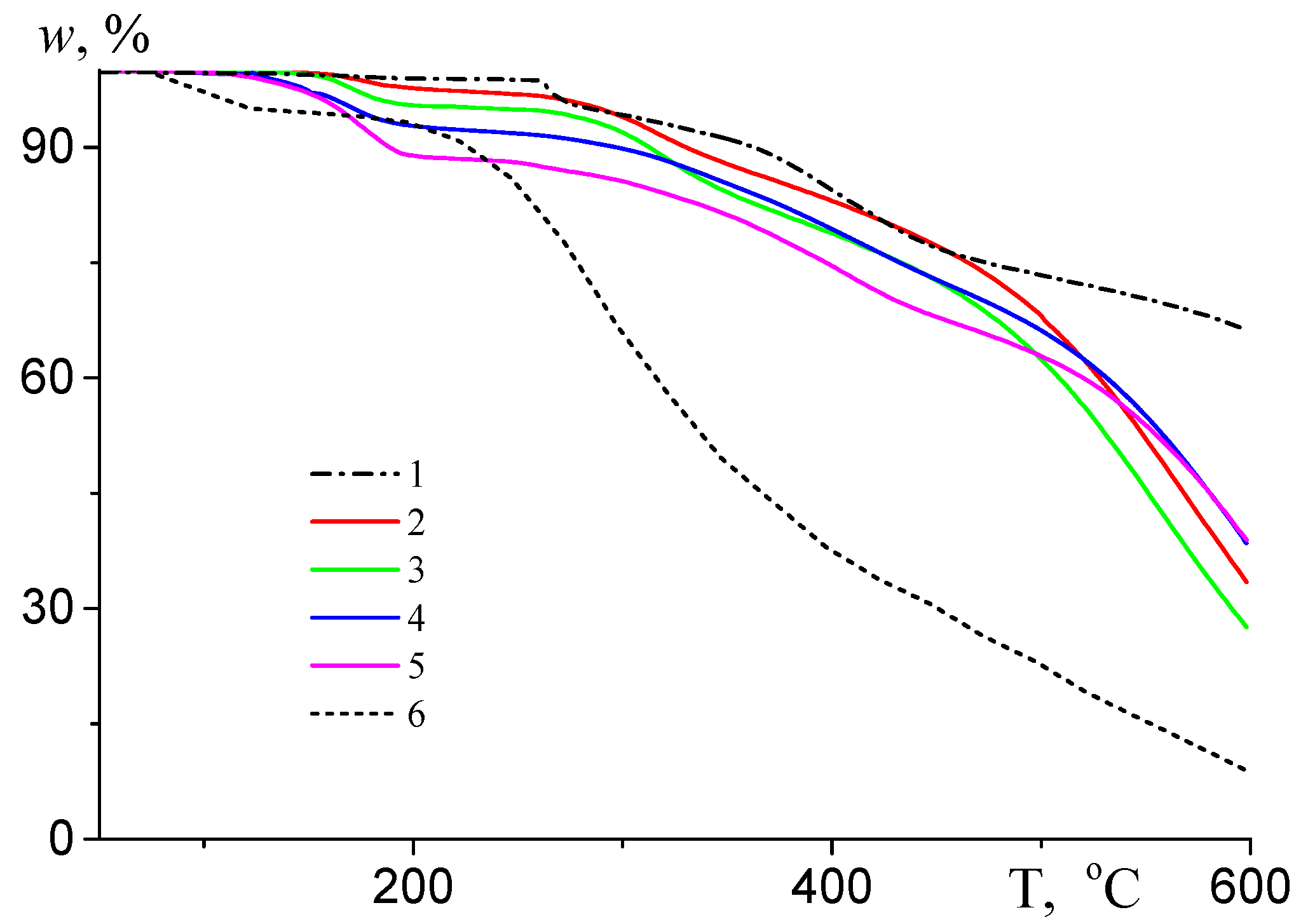








| Monomer | f, mol.% (a) | ME, mol.% (b) | CPSK, wt.% (c) | CDMSO, wt. % | Facrylate, mol% (d) | Mn, kg·mole−1 | Đ |
|---|---|---|---|---|---|---|---|
| TBA | 1.0 | 0.05 | 2 | 80 | 1.7 | 62.5 | 2.1 |
| 2.5 | 0.05 0.3 | 2 | 80 | 3.5 | 61.8 22.4 | 2.1 2.8 | |
| 5.0 | 0.05 0.3 | 2 | 80 | 6.2 | 57.4 27.7 | 1.9 2.2 | |
| 7.5 | 0.05 | 2 | 80 | 9.5 | 50.4 | 2.0 | |
| 10.0 | 0.05 | 2 | 80 | 12.1 | 58.6 | 1.7 | |
| BA | 10.0 15.0 20.0 | 0.3 | 2 | 80 | 11.2 15.6 20.5 | 27.2 25.8 24.7 | 1.7 1.8 1.9 |
| BA/TBA | 15.0/1.0 15.0/2.5 15.0/5.0 15.0/10.0 | 0.3 | 2 | 80 | 15.4 (e) 16.4 (e) 18.2 (e) 22.5 (e) | 25.3 24.8 19.3 18.6 | 1.8 2.0 2.3 2.3 |
| TBA, Mole % | FAN-AN-AN | FAN-AN-TBA | FAN-TBA-AN | <NAN>n | <NTBA>n |
|---|---|---|---|---|---|
| 1.0 | 0.980 | 0.020 | 0 | 99 | 1 |
| 2.5 | 0.950 | 0.049 | 0.001 | 40 | 1 |
| 5.0 | 0.902 | 0.096 | 0.002 | 20 | 1 |
| 7.5 | 0.854 | 0.140 | 0.006 | 13 | 1 |
| 10.0 | 0.808 | 0.181 | 0.011 | 10 | 1 |
| fTBA, mol.% | TBA Content in Copolymer | Isobutylene, wt % | ||
|---|---|---|---|---|
| mol.% | wt % | Theoretical | Experiment | |
| 1.0 | 1.7 | 4.0 | 1.7 | 0.8 |
| 2.5 | 3.5 | 8.0 | 3.5 | 2.5 |
| 5.0 | 6.2 | 13.8 | 6.0 | 5.1 |
| 7.5 | 9.5 | 20.2 | 8.8 | 7.8 |
| 10.0 | 12.1 | 24.9 | 10.9 | 11.5 |
| fTBA | ME, mol.% | Mn (kDa)/Đ | Tp, °C (a) | Heat flow at Tp, W/g (b) | −ΔH, J/g | Ea, kJ/mole |
|---|---|---|---|---|---|---|
| 1.0 | 0.05 | 220/277 | 8.4 | 547 | 187 ± 12 (III) | |
| 2.5 | 0.05 0.3 | 61.9/2.1 22.4/2.9 | 218/258/275 217/261/277 | 3.9 4.1 | 687 667 | 98 ± 1 (II)/161 ± 18 (III) – |
| 5.0 | 0.05 0.3 | 57.4/1.9 27.7/2.2 | 220/265/280 220/266/278 | 2.4 2.8 | 680 701 | 118 ± 4 (II)/217 ± 16 (III) – |
| 7.5 (c) | 0.05 | 50.4/2.0 | 218/272/285 | 2.2 | 575 | 114 ± 2 (II)/146 ± 7 (III) |
| 10.0 (c) | 0.05 | 58.6/1.7 | 219/273/292 | 1.7 | 522 | 126 ± 3 (II)/163 ± 6 |
| fTBA | Tp, °C | Heat Flow at Tp, W/g |
|---|---|---|
| 0 | 303 | 38.3 |
| 1.0 | 269/283/313 | 9.3/9.8/8.7 |
| 2.5 | 278/315 | 13.7/14.0 |
| 5.0 | 272/318 | 11.8/14.4 |
| 7.5 | 274/325 | 10.8/13.9 |
| 10.0 | 267/348 | 5.7/5.2 |
| fTBA | Tp, °C | Heat flow at Tp, W/g | −ΔH, J/g |
|---|---|---|---|
| 1.0 | 226/270/324 | 0.03/0.3/1.3 | 340 |
| 2.5 | 230/269/322 | 0.1/0.5/1.2 | 425 |
| 5.0 (a) | 231/270/322 | 0.2/0.6/1.1 | 460 |
| 10.0 (a) | 230/270/327 | 0.3/0.6/0.8 | 430 |
| Temperature of Weight Loss | PAN | P(AN/BA), 15 mol.% BA | P(AN/BA/TBA), 15 mol.% BA, 1.0 mol.% TBA | P(AN/BA/TBA), 15 mol.% BA, 2.5 mol. % TBA |
|---|---|---|---|---|
| T0 | 261.3 | 274.2 | 245.3 | 317.8 |
| T5% | 248.8 | 323.5 | 312.0 | 281.5 |
| T30% | 554.1 | 476.0 | 531.3 | 509.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toms, R.V.; Ismaylov, D.A.; Gervald, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Chernikova, E.V. In Situ Formation of Acidic Comonomer during Thermal Treatment of Copolymers of Acrylonitrile and Its Influence on the Cyclization Reaction. Polymers 2024, 16, 2833. https://doi.org/10.3390/polym16192833
Toms RV, Ismaylov DA, Gervald AY, Prokopov NI, Plutalova AV, Chernikova EV. In Situ Formation of Acidic Comonomer during Thermal Treatment of Copolymers of Acrylonitrile and Its Influence on the Cyclization Reaction. Polymers. 2024; 16(19):2833. https://doi.org/10.3390/polym16192833
Chicago/Turabian StyleToms, Roman V., Daniil A. Ismaylov, Alexander Yu. Gervald, Nickolay I. Prokopov, Anna V. Plutalova, and Elena V. Chernikova. 2024. "In Situ Formation of Acidic Comonomer during Thermal Treatment of Copolymers of Acrylonitrile and Its Influence on the Cyclization Reaction" Polymers 16, no. 19: 2833. https://doi.org/10.3390/polym16192833
APA StyleToms, R. V., Ismaylov, D. A., Gervald, A. Y., Prokopov, N. I., Plutalova, A. V., & Chernikova, E. V. (2024). In Situ Formation of Acidic Comonomer during Thermal Treatment of Copolymers of Acrylonitrile and Its Influence on the Cyclization Reaction. Polymers, 16(19), 2833. https://doi.org/10.3390/polym16192833










