The Correlations between Microstructures and Color Properties of Nanocrystalline Cellulose: A Concise Review
Abstract
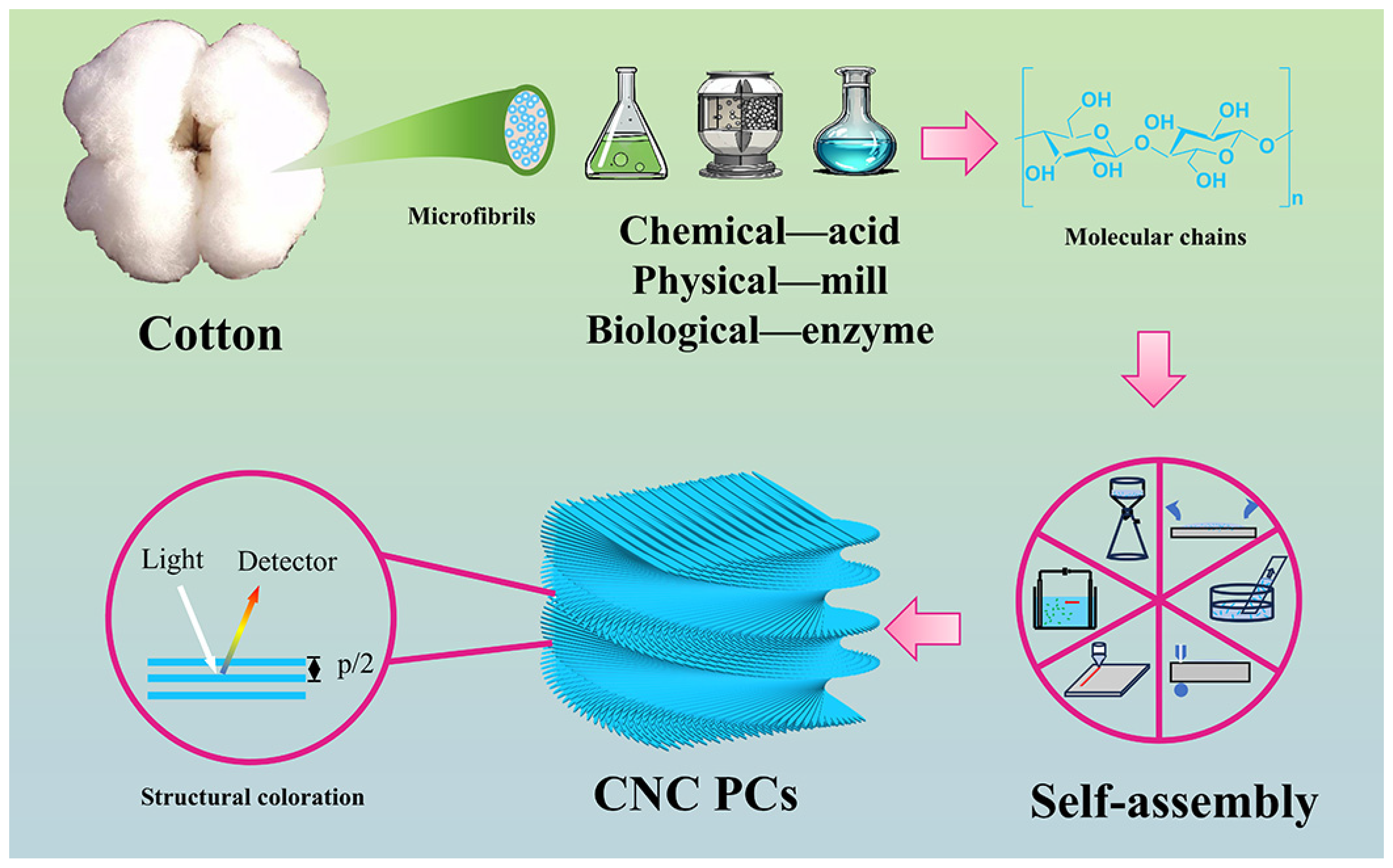
1. Introduction
2. Preparation of Cellulose Nanocrystals
| Method | Raw Materials | Reaction Condition | CNCs Characteristics | Yield/% | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Time | TEMP/°C | L/nm | W/nm | Zeta/mV | ||||
| Inorganic acid hydrolysis | MCC/H2SO4 | 60 min | 45 | 141.3 | 6.5 | −49.4 | 32.7 | [24] |
| MCC/H2SO4 | 10 min | 70 | 200–300 | 5–10 | NA | 25 | [25] | |
| BEKP/FA/FeCl3 | 6 h | 95 | 50–200 | 5–20 | −6.02 | 75.7 | [26] | |
| Organic acid hydrolysis | MCC/ZnCl2/CA | 8 h | 25 | 40–75 | 6–10 | −18.9 | 61.4 | [27] |
| ECF/NaOH/OA | 8 h | 90 | 310 | 16.5 | −34.2 | 59 | [28] | |
| Solid acid hydrolysis | MCC/OA | 4 h | 80 | 350 | 10 | NA | 59 | [29] |
| BEP/FeCl3·6H2O | 10 min | 70 | 322 | 10–27 | −14.1 | 80.1 | [30] | |
| Ionic liquid treatment method | CC/[Bmim][HSO4] | 1.5 h | 100 | 166 | NA | −36.1 | 40.1 | [31] |
| MCC/[DEAPA][Hex] | 3 h | 80 | 410 | 38 | −4.2 | 24 | [32] | |
| MCC/DMSO/ [Bmim][HSO4] | 1.5 h | 90 | 757 | 50 | −20.0 | 60 | [33] | |
| Oxidation method | SBKP/TEMPO/NaBr/NaClO | 10 h | 25 | 185 | 3.5 | −54.0 | 94 | [34] |
| Hardwood | 4.5 h | 170 | 150–250 | 5 | −43.0 | 87 | [35] | |
| MCC/H5IO6/KOH | 14 days | 25 | 116–120 | 3.7–5.3 | −25.5 | 40.1 | [36] | |
| MCC/NaIO4/FeSO4·7H2O | 10 h | 45–60 | 275 | 22.3 | −27.2 | 51.2 | [37] | |
| Wet ball milling | MCC | 0.5–16 h | 25 | 120–400 | 3–10 | NA | 20 | [38] |
| MCC/H3PO4 | 15–60 min | 25 | 230–290 | 8–10 | −23.0 | 76 | [39] | |
| Enzymolysis approach | MCC | 72 h | 50 | 347–350 | 35.5 | NA | 13.1 | [40] |
| BEPF | 21 h | 50 | 600 | 30 | NA | NA | [21] | |
3. Liquid Crystal Phase Self-Assembly of Cellulose Nanocrystals
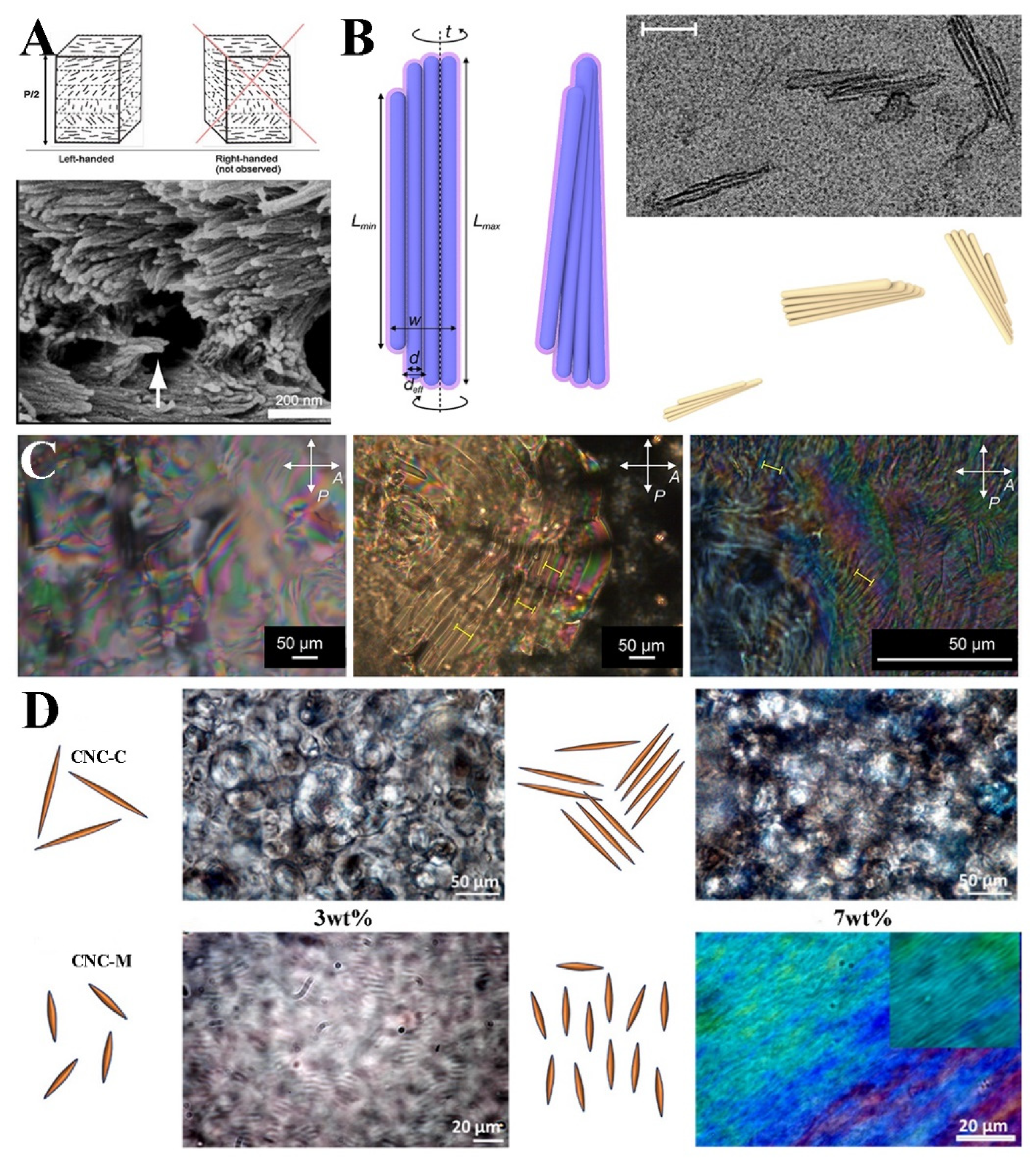
4. Cellulose Nanocrystal Photonic Crystal Film
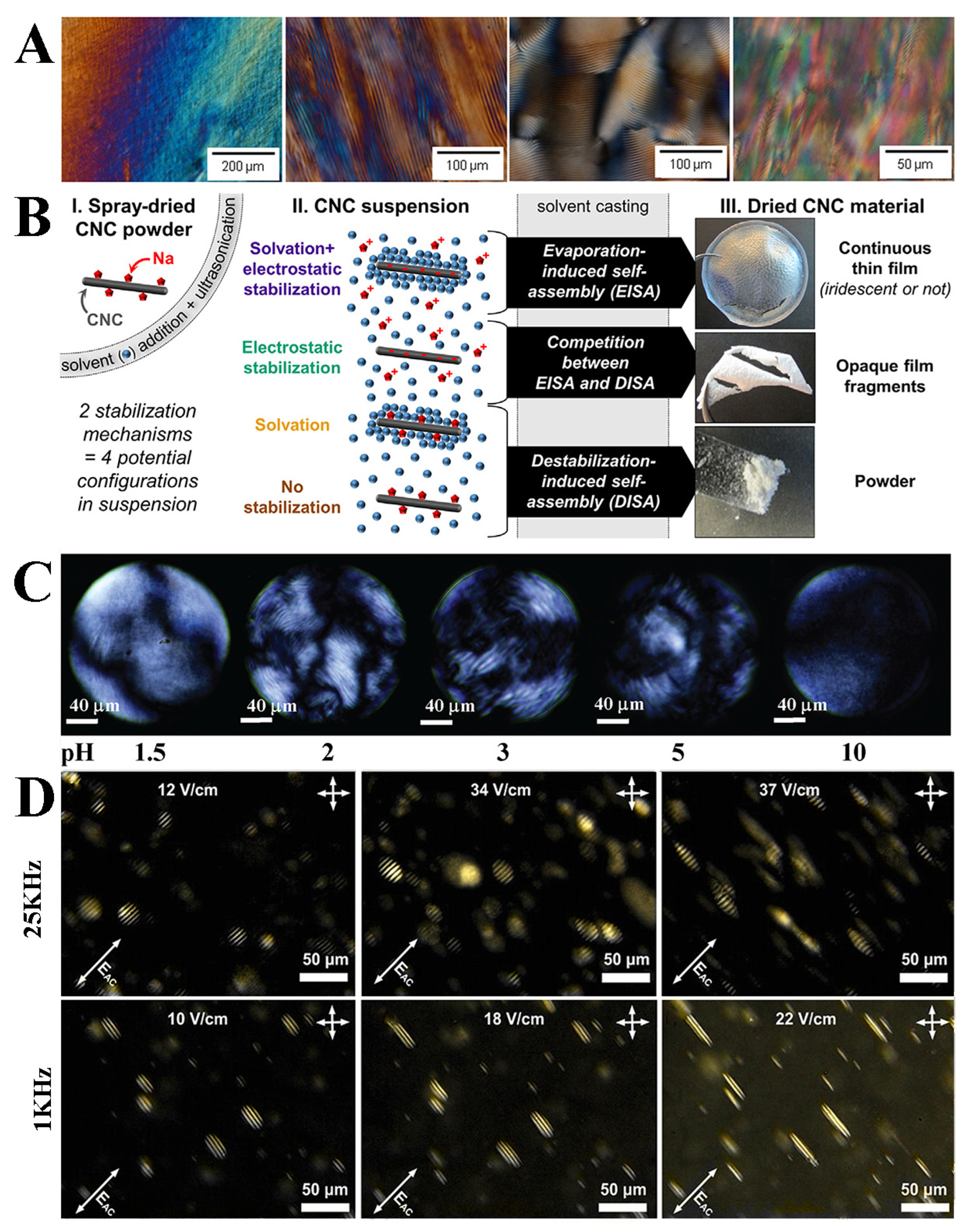
4.1. Preparation of Cellulose Nanocrystalline Photonic Crystal Films
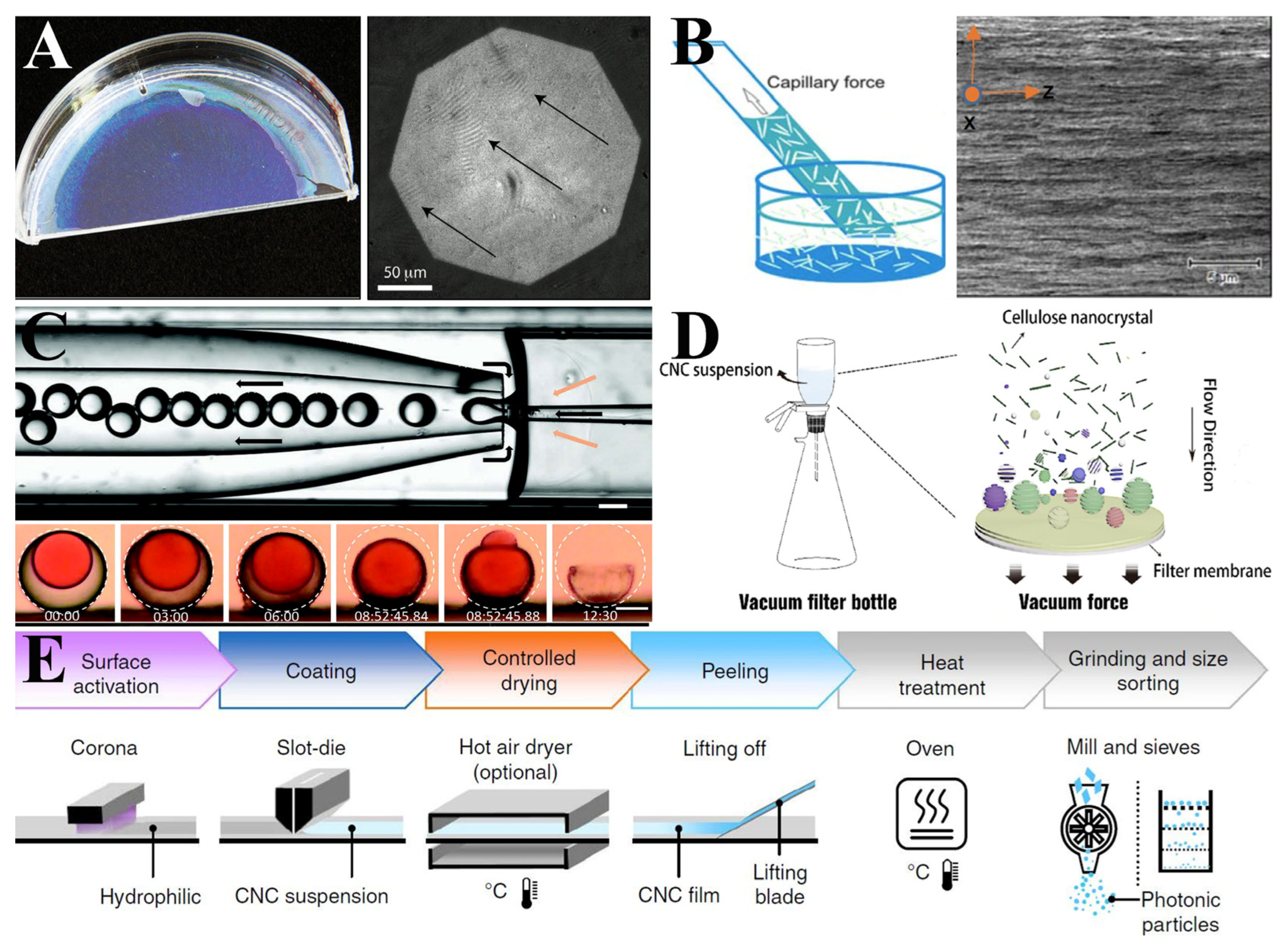
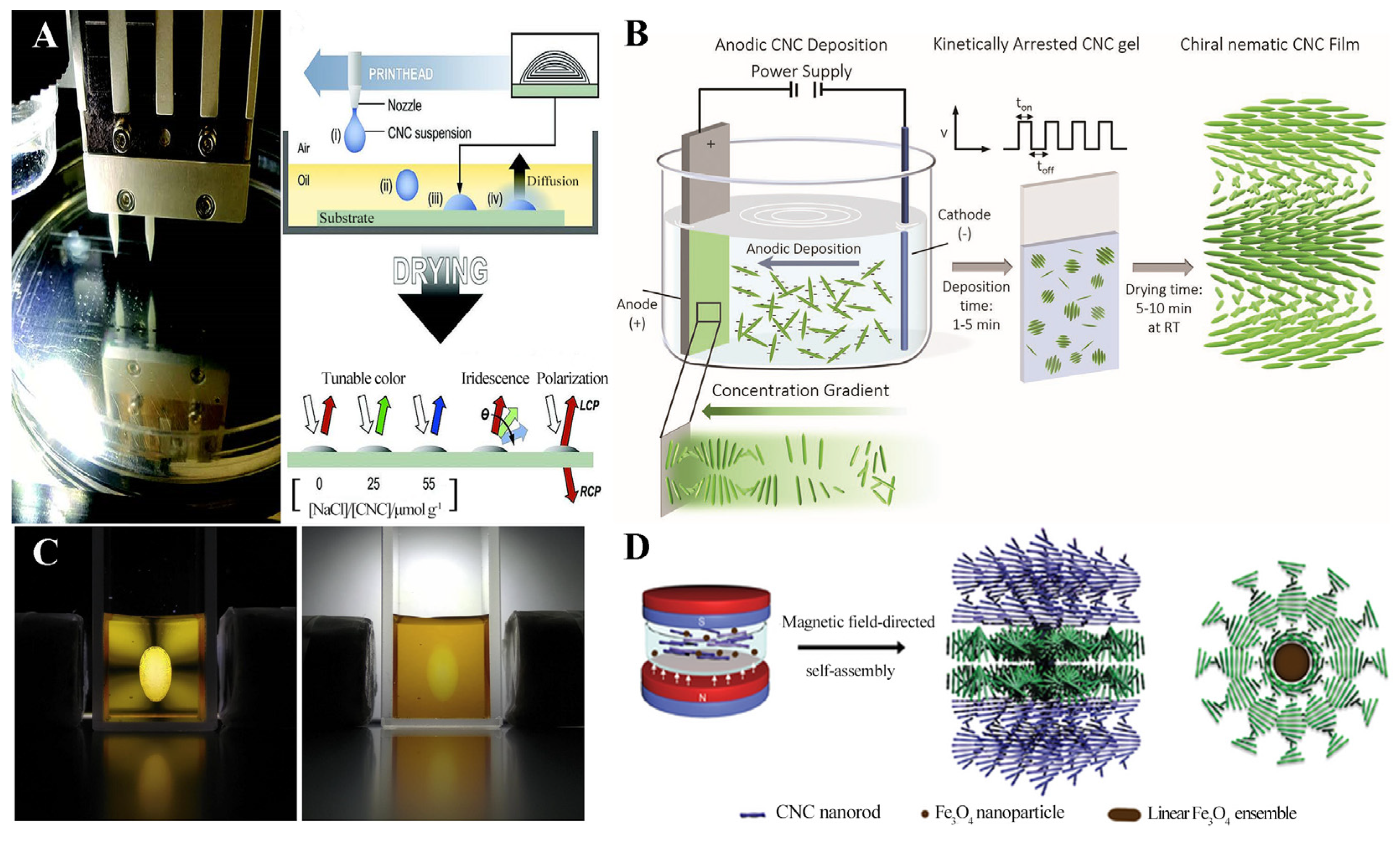
4.2. Structural Color of Cellulose Nanocrystalline Photonic Crystal Films

5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuthill, I.C.; Allen, W.L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M.E.; Hill, G.E.; Jablonski, N.G.; Jiggins, C.D.; Kelber, A. The biology of color. Science 2017, 357, eaan0221. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R. The diversity and implications of animal structural colours. J. Exp. Biol. 1998, 201, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Wang, J.; Jiang, L. Bio-inspired photonic crystals with superwettability. Chem. Soc. Rev. 2016, 45, 6833–6854. [Google Scholar] [CrossRef]
- Azofeifa, D.; Hernández-Jiménez, M.; Libby, E.; Solís, A.; Barboza-Aguilar, C.; Vargas, W. A quantitative assessment approach of feasible optical mechanisms contributing to structural color of golden-like Chrysina aurigans scarab beetles. J. Quant. Spectrosc. Radiat. Transf. 2015, 160, 63–74. [Google Scholar] [CrossRef]
- Steindorfer, M.A.; Schmidt, V.; Belegratis, M.; Stadlober, B.; Krenn, J.R. Detailed simulation of structural color generation inspired by the Morpho butterfly. Opt. Express 2012, 20, 21485–21494. [Google Scholar] [CrossRef]
- Yang, R.; Zaheri, A.; Gao, W.; Hayashi, C.; Espinosa, H.D. AFM identification of beetle exocuticle: Bouligand structure and nanofiber anisotropic elastic properties. Adv. Funct. Mater. 2017, 27, 1603993. [Google Scholar] [CrossRef]
- Kim, H.; Ge, J.; Kim, J.; Choi, S.-e.; Lee, H.; Lee, H.; Park, W.; Yin, Y.; Kwon, S. Structural colour printing using a magnetically tunable and lithographically fixable photonic crystal. Nat. Photonics 2009, 3, 534–540. [Google Scholar] [CrossRef]
- Majoinen, J.; Kontturi, E.; Ikkala, O.; Gray, D.G. SEM imaging of chiral nematic films cast from cellulose nanocrystal suspensions. Cellulose 2012, 19, 1599–1605. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Fu, Y.; Zhou, H. Birefringence- and Optical Distortion-Free Isotropic Polymer Lens Assisted by Photonic Microspheres. ACS Appl. Mater. Interfaces 2020, 12, 44172–44179. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.; Woodcock, J.W.; Krishnamurthy, A.; Obrzut, J.; Gilman, J.W.; Coughlin, E.B. Bouligand nanocomposites: Self-assembly of cellulose nanocrystals with a thermo-responsive polymer. Polymer 2023, 281, 126117. [Google Scholar] [CrossRef]
- Duan, C.; Cheng, Z.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Gao, W.; Chen, K. Chiral photonic liquid crystal films derived from cellulose nanocrystals. Small 2021, 17, 2007306. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Marchi, B.C.; Meng, Z.; Keten, S. Impact resistance of nanocellulose films with bioinspired Bouligand microstructures. Nanoscale Adv. 2019, 1, 1351–1361. [Google Scholar] [CrossRef]
- Chu, G.; Wang, X.; Yin, H.; Shi, Y.; Jiang, H.; Chen, T.; Gao, J.; Qu, D.; Xu, Y.; Ding, D. Free-standing optically switchable chiral plasmonic photonic crystal based on self-assembled cellulose nanorods and gold nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 21797–21806. [Google Scholar] [CrossRef]
- Dumanli, A.G.; van der Kooij, H.M.; Kamita, G.; Reisner, E.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Digital Color in Cellulose Nanocrystal Films. ACS Appl. Mater. Interfaces 2014, 6, 12302–12306. [Google Scholar] [CrossRef]
- Stroobants, A.; Lekkerkerker, H.; Odijk, T. Effect of electrostatic interaction on the liquid crystal phase transition in solutions of rodlike polyelectrolytes. Macromolecules 1986, 19, 2232–2238. [Google Scholar] [CrossRef]
- Chiappini, M.; Dussi, S.; Frka-Petesic, B.; Vignolini, S.; Dijkstra, M. Modeling the cholesteric pitch of apolar cellulose nanocrystal suspensions using a chiral hard-bundle model. J. Chem. Phys. 2022, 156, 014904. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Browne, C.; Batchelor, W.; Garnier, G. Self-assembly of cellulose nanocrystals of different lengths. J. Colloid Interface Sci. 2023, 630, 249–259. [Google Scholar] [CrossRef]
- Pawcenis, D.; Leśniak, M.; Szumera, M.; Sitarz, M.; Profic-Paczkowska, J. Effect of hydrolysis time, pH and surfactant type on stability of hydrochloric acid hydrolyzed nanocellulose. Int. J. Biol. Macromol. 2022, 222, 1996–2005. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X.; Jia, M.; Roux, J.C. Preparation and mechanism analysis of morphology-controlled cellulose nanocrystals via compound enzymatic hydrolysis of eucalyptus pulp. J. Appl. Polym. Sci. 2020, 137, 48407. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, M.Z.; Moniruzzaman, M. Engineering Ionic liquids as a sustainable platform for nanocellulose processing from bioresources: Overview and current status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Vafakish, B.; Patel, R.; Falua, K.J.; Dunlop, M.J.; Acharya, B. Cellulose nanocrystals in the development of biodegradable materials: A review on CNC resources, modification, and their hybridization. Int. J. Biol. Macromol. 2023, 258, 128834. [Google Scholar] [CrossRef]
- Ma, T.; Hu, X.; Lu, S.; Cui, R.; Zhao, J.; Hu, X.; Song, Y. Cellulose nanocrystals produced using recyclable sulfuric acid as hydrolysis media and their wetting molecular dynamics simulation. Int. J. Biol. Macromol. 2021, 184, 405–414. [Google Scholar] [CrossRef]
- Calvo, V.; Álvarez Sánchez, M.A.; Guemes, L.; Martinez-Baron, C.; Baúlde, S.; Criado, A.; Gonzalez-Dominguez, J.M.; Maser, W.K.; Benito, A.M. Preparation of cellulose nanocrystals: Controlling the crystalline type by one-pot acid hydrolysis. ACS Macro Lett. 2023, 12, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl 3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Zhou, W.; Zhao, P.; Peng, Z.; Lin, X.; Yang, J. One-step hydrolysis for preparation of carboxylated cellulose nanocrystals with high yield at room temperature and their iridescent phenomenon. Cellulose 2024, 31, 7993–8005. [Google Scholar] [CrossRef]
- Cherian, R.M.; Varghese, R.T.; Antony, T.; Malhotra, A.; Kargarzadeh, H.; Chauhan, S.R.; Chauhan, A.; Chirayil, C.J.; Thomas, S. Non-cytotoxic, highly functionalized cellulose nanocrystals with high crystallinity and thermal stability derived from a novel agromass of Elettaria cardamomum, using a soft and benign mild oxalic acid hydrolysis. Int. J. Biol. Macromol. 2023, 253, 126571. [Google Scholar] [CrossRef]
- Song, K.; Ji, Y.; Wang, L.; Wei, Y.; Yu, Z. A green and environmental benign method to extract cellulose nanocrystal by ball mill assisted solid acid hydrolysis. J. Clean. Prod. 2018, 196, 1169–1175. [Google Scholar] [CrossRef]
- Yang, W.; Mei, Z.; Feng, S.; Li, C.; Guo, J.; Bian, H.; Xiao, H.; Dai, H.; Hu, C.; Han, J. Cellulose nanocrystal preparation via rapid hydrolysis of wood cellulose fibers using recyclable molten ferric chloride hexahydrate. ACS Sustain. Chem. Eng. 2023, 11, 10172–10182. [Google Scholar] [CrossRef]
- Rasri, W.; Thu, V.T.; Corpuz, A.; Nguyen, L.T. Preparation and characterization of cellulose nanocrystals from corncob via ionic liquid [Bmim][HSO4] hydrolysis: Effects of major process conditions on dimensions of the product. RSC Adv. 2023, 13, 19020–19029. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.B.A.; Vieira, S.R.; Pessôa, L.C.; Santana, J.S.; Lemos, P.V.F.; de Souza, C.O.; Cardoso, L.G.; de Jesus Assis, D.; Mussagy, C.U.; Santos-Ebinuma, V.C. Impact of ionic liquid’s cation alkyl chain length and reaction time on cellulose nanocrystals preparation. Carbohydr. Polym. Technol. Appl. 2023, 6, 100390. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, H.B.; Goto, M.; Moniruzzaman, M. Cellulose nanocrystals preparation from microcrystalline cellulose using ionic liquid-DMSO binary mixture as a processing medium. J. Mol. Liq. 2022, 346, 118208. [Google Scholar] [CrossRef]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-free preparation of cellulose nanocrystals by TEMPO oxidation and subsequent cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y. High energy oxidation and organosolv solubilization for high yield isolation of cellulose nanocrystals (CNC) from Eucalyptus hardwood. Sci. Rep. 2018, 8, 16505. [Google Scholar] [CrossRef]
- Liu, P.; Pang, B.; Dechert, S.; Zhang, X.C.; Andreas, L.B.; Fischer, S.; Meyer, F.; Zhang, K. Structure selectivity of alkaline periodate oxidation on lignocellulose for facile isolation of cellulose nanocrystals. Angew. Chem. Int. Ed. 2020, 59, 3218–3225. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.; Duan, C.; Li, J.; Zeng, J.; Xu, J.; Gao, W.; Chen, K. Novel carboxylated cellulose nanocrystals synthesized by co-oxidation of sodium periodate/Fenton as a green solid emulsifier for oil-in-water Pickering emulsion. J. Colloid. Interf. Sci. 2023, 630, 604–617. [Google Scholar] [CrossRef]
- Kang, X.; Kuga, S.; Wang, C.; Zhao, Y.; Wu, M.; Huang, Y. Green preparation of cellulose nanocrystal and its application. ACS Sustain. Chem. Eng. 2018, 6, 2954–2960. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Annamalai, P.K.; Morrow, I.C.; Martin, D. Production of cellulose nanocrystals via a scalable mechanical method. RSC Adv. 2015, 5, 57133–57140. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Guo, Y.; Peng, S.; Liu, G.; Zhao, J. Effect of endoglucanases from different glycoside hydrolase families on enzymatic preparation of cellulose nanocrystal. Ind. Crops Prod. 2020, 155, 112755. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Yao, A.; Tan, L.; Guo, R.; Zhou, M.; Zhang, Y.; Zhu, P. Preparation of cellulose nanocrystals and their application in reinforcing viscose filaments. Cellulose 2020, 27, 10553–10565. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Florencio, C.; Baccarin, G.S.; Farinas, C.S. Cellulose nanostructures obtained using enzymatic cocktails with different compositions. Int. J. Biol. Macromol. 2022, 207, 299–307. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Su, W. Highly efficient preparation of cellulose nanocrystals by mechano-enzymatic hydrolysis: A mechanism study. Catal. Sci. Technol. 2023, 13, 618–623. [Google Scholar] [CrossRef]
- Banvillet, G.; Depres, G.; Belgacem, N.; Bras, J. Alkaline treatment combined with enzymatic hydrolysis for efficient cellulose nanofibrils production. Carbohydr. Polym. 2021, 255, 117383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xu, W.; Wu, H.; Shao, Y.; Han, X.; Zhou, M.; Gu, P.; Li, Z. Preparation methods of cellulose nanocrystal and its application in treatment of environmental pollution: A mini-review. Colloid Interface Sci. Commun. 2023, 53, 100707. [Google Scholar] [CrossRef]
- Marchessault, R.; Morehead, F.; Walter, N. Liquid crystal systems from fibrillar polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure–properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y. Electron microdiffraction reveals the nanoscale twist geometry of cellulose nanocrystals. Nanoscale 2019, 11, 21767–21774. [Google Scholar] [CrossRef]
- Lim, J.H.; Jing, Y.; Park, S.; Nishiyama, Y.; Veron, M.; Rauch, E.; Ogawa, Y. Structural Anisotropy Governs the Kink Formation in Cellulose Nanocrystals. J. Phys. Chem. Lett. 2023, 14, 3961–3969. [Google Scholar] [CrossRef]
- Gonçalves, D.P.; Hegmann, T. Chirality Transfer from an Innately Chiral Nanocrystal Core to a Nematic Liquid Crystal: Surface-Modified Cellulose Nanocrystals. Angew. Chem. Int. Ed. Engl. 2021, 60, 17344–17349. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.P.; Ogolla, T.; Hegmann, T. Chirality Transfer from an Innately Chiral Nanocrystal Core to a Nematic Liquid Crystal 2: Lyotropic Chromonic Liquid Crystals. ChemPhysChem 2023, 24, e202200685. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Kam, D.; Levi-Kalisman, Y.; Gray, D.G.; Shoseyov, O. Surface charge influence on the phase separation and viscosity of cellulose nanocrystals. Langmuir 2018, 34, 3925–3933. [Google Scholar] [CrossRef]
- Bruckner, J.R.; Kuhnhold, A.; Honorato-Rios, C.; Schilling, T.; Lagerwall, J.P. Enhancing self-assembly in cellulose nanocrystal suspensions using high-permittivity solvents. Langmuir 2016, 32, 9854–9862. [Google Scholar] [CrossRef]
- Bruel, C.; Davies, T.S.; Carreau, P.J.; Tavares, J.R.; Heuzey, M.-C. Self-assembly behaviors of colloidal cellulose nanocrystals: A tale of stabilization mechanisms. J. Colloid. Interf. Sci. 2020, 574, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Evans, J.; Wang, N.; Guo, T.; He, S. pH dependence of the chirality of nematic cellulose nanocrystals. Sci. Rep. 2019, 9, 11290. [Google Scholar] [CrossRef]
- Mao, Y.; Bleuel, M.; Lyu, Y.; Zhang, X.; Henderson, D.; Wang, H.; Briber, R.M. Phase separation and stack alignment in aqueous cellulose nanocrystal suspension under weak magnetic field. Langmuir 2018, 34, 8042–8051. [Google Scholar] [CrossRef]
- Qu, D.; Zussman, E. Electric Field-Driven Control of Cholesteric Cellulose Nanocrystal Orientation and Morphology. Adv. Opt. Mater. 2022, 10, 2101659. [Google Scholar] [CrossRef]
- Parton, T.G.; Parker, R.M.; van de Kerkhof, G.T.; Narkevicius, A.; Haataja, J.S.; Frka-Petesic, B.; Vignolini, S. Chiral self-assembly of cellulose nanocrystals is driven by crystallite bundles. Nat. Commun. 2022, 13, 2657. [Google Scholar] [CrossRef]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.; Gray, D. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Roman, M.; Gray, D.G. Parabolic focal conics in self-assembled solid films of cellulose nanocrystals. Langmuir 2005, 21, 5555–5561. [Google Scholar] [CrossRef] [PubMed]
- Dumanli, A.G.; Kamita, G.; Landman, J.; van der Kooij, H.; Glover, B.J.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Controlled, bio-inspired self-assembly of cellulose-based chiral reflectors. Adv. Opt. Mater. 2014, 2, 646–650. [Google Scholar] [CrossRef]
- Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Tactoid annealing improves order in self-assembled cellulose nanocrystal films with chiral nematic structures. Langmuir 2018, 34, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lin, T.; Lu, L.; Yu, M.; Yin, X. Enhanced homogeneity and flexibility in a humidity sensor using cellulose nanocrystal-based composite film with circular shear flow. Int. J. Biol. Macromol. 2024, 263, 130293. [Google Scholar] [CrossRef]
- Klockars, K.W.; Yau, N.E.; Tardy, B.L.; Majoinen, J.; Kämäräinen, T.; Miettunen, K.; Boutonnet, E.; Borghei, M.; Beidler, J.; Rojas, O. Asymmetrical coffee rings from cellulose nanocrystals and prospects in art and design. Cellulose 2019, 26, 491–506. [Google Scholar] [CrossRef]
- Cherpak, V.; Korolovych, V.; Geryak, R.; Turiv, T.; Nepal, D.; Kelly, J.; Bunning, T.; Lavrentovich, O.; Heller, W.; Tsukruk, V. Robust chiral organization of cellulose nanocrystals in capillary confinement. Nano Lett. 2018, 18, 6770–6777. [Google Scholar] [CrossRef]
- Ackroyd, A.J.; De Paolis, A.; Xu, Y.-T.; Momeni, A.; Hamad, W.Y.; MacLachlan, M.J. Self-assembly of cellulose nanocrystals confined to square capillaries. Nanoscale 2023, 15, 14388–14398. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Greca, L.G.; Rojas, O.J.; Tardy, B.L. Controlling superstructure formation and macro-scale adhesion via confined evaporation of cellulose nanocrystals. Cellulose 2023, 30, 741–751. [Google Scholar] [CrossRef]
- Geng, Y.; Honorato-Rios, C.; Noh, J.; Lagerwall, J.P. Cholesteric Spherical Reflectors with Tunable Color from Single-Domain Cellulose Nanocrystal Microshells. Adv. Mater. 2024, 36, 2305251. [Google Scholar] [CrossRef]
- Lv, J.; Ding, D.; Yang, X.; Hou, K.; Miao, X.; Wang, D.; Kou, B.; Huang, L.; Tang, Z. Biomimetic chiral photonic crystals. Angew. Chem. Int. Ed. 2019, 58, 7783–7787. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Ye, C.; Xiong, R. Structural, Optical, and Mechanical Insights into Cellulose Nanocrystal Chiral Nematic Film Engineering by Two Assembly Techniques. Biomacromolecules 2024, 25, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, Y.; Hu, J.; Yang, J.; Feng, F.; Yu, Y.; Liu, P.; Men, Y.; Zhang, J. Origin of vacuum-assisted chiral self-assembly of cellulose nanocrystals. Carbohydr. Polym. 2020, 245, 116459. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, J.; Shi, L.; Xing, T.; Gao, X.; Xu, Y. Chiral Pearlescent Cellulose Nanocrystals Films with Broad-Range Tunable Optical Properties for Anti-Counterfeiting Applications. Small 2024, 20, 2306810. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Zhu, D.; Zheng, Z.; Wang, X. New Finding of Inversed Right-Handed Helix in Dynamically Rotational Evaporation-Induced Iridescent CNC Film. Adv. Opt. Mater. 2024, 12, 2400927. [Google Scholar] [CrossRef]
- Droguet, B.E.; Liang, H.-L.; Frka-Petesic, B.; Parker, R.M.; De Volder, M.F.; Baumberg, J.J.; Vignolini, S. Large-scale fabrication of structurally coloured cellulose nanocrystal films and effect pigments. Nat. Mater. 2022, 21, 352–358. [Google Scholar] [CrossRef]
- Williams, C.A.; Parker, R.M.; Kyriacou, A.; Murace, M.; Vignolini, S. Inkjet printed photonic cellulose nanocrystal patterns. Adv. Mater. 2024, 36, 2307563. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Saito, T.; Koga, H.; Nogi, M. One-pot hierarchical structuring of nanocellulose by electrophoretic deposition. ACS Nano 2022, 16, 18390–18397. [Google Scholar] [CrossRef]
- Atifi, S.; Mirvakili, M.N.; Williams, C.A.; Bay, M.M.; Vignolini, S.; Hamad, W.Y. Fast Self-Assembly of Scalable Photonic Cellulose Nanocrystals and Hybrid Films via Electrophoresis. Adv. Mater. 2022, 34, 2109170. [Google Scholar] [CrossRef]
- Mhatre, S.; Niu, X.; Bautista, G.F.; Sumanasinghe, S.; Rojas, O.J. Electric field-modulated evaporative thin film deposition of bio-particles for piezoelectric applications. Nanoscale 2024, 16, 12611–12623. [Google Scholar] [CrossRef]
- Cranston, E.; Gray, D. Formation of cellulose-based electrostatic layer-by-layer films in a magnetic field. Sci. Technol. Adv. Mater. 2006, 7, 319. [Google Scholar] [CrossRef]
- Wang, P.-X.; Hamad, W.Y.; MacLachlan, M. Liquid crystalline tactoidal microphases in ferrofluids: Spatial positioning and orientation by magnetic field gradients. J. Chem. 2019, 5, 681–692. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Jeong, K.J.; Yu, X.; Yu, X.; Xu, Y. Homeotropic concentric helix orientations in chiral nematic cellulose nanocrystal films by local magnetic fields. Adv. Opt. Mater. 2022, 10, 2102616. [Google Scholar] [CrossRef]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M.J.L. Phase separation behavior in aqueous suspensions of bacterial cellulose nanocrystals prepared by sulfuric acid treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef]
- Mu, X.; Gray, D.G. Formation of chiral nematic films from cellulose nanocrystal suspensions is a two-stage process. Langmuir 2014, 30, 9256–9260. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Vasilyev, G.; Qu, D.; Deng, S.; Bai, L.; Rojas, O.J.; Zussman, E. Structural arrest and phase transition in glassy nanocellulose colloids. Langmuir 2020, 36, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-A.; Facchine, E.G.; Khan, S.A.; Rojas, O.J.; Spontak, R. Mesophase characteristics of cellulose nanocrystal films prepared from electrolyte suspensions. J. Colloid. Interf. Sci. 2021, 599, 207–218. [Google Scholar] [CrossRef]
- Lin, M.; Raghuwanshi, V.S.; Browne, C.; Simon, G.P.; Garnier, G. Modulating the chiral nanoarchitecture of cellulose nanocrystals through interaction with salts and polymer. J. Colloid. Interf. Sci. 2022, 613, 207–217. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Ma, Z.; Tian, D.; Gu, M.; Lin, F. Structure–color mechanism of iridescent cellulose nanocrystal films. RSC Adv. 2014, 4, 39322–39331. [Google Scholar] [CrossRef]
- Peng, Y.; Via, B. The effect of cellulose nanocrystal suspension treatment on suspension viscosity and casted film property. Polymers 2021, 13, 2168. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhou, T.; Hou, A.; Liang, J.; Ma, T.; Xie, K.; Gao, A. A Tunable Hydrophilic–Hydrophobic, Stimulus Responsive, and Robust Iridescent Structural Color Bionic Film with Chiral Photonic Crystal Nanointerface. Small 2024, 20, 2311283. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Q.; Meng, X.; Li, Y.; Peng, H.; Whittaker, A.K.; Zhu, S. Ultrasensitive magnetic tuning of optical properties of films of cholesteric cellulose nanocrystals. ACS Nano 2020, 14, 9440–9448. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, S.; Sun, J.; Song, J.; Li, H.; Guo, J. Polyethylene glycol regulates the pitch and liquid crystal behavior of cellulose nanocrystal-based photonic crystals. Int. J. Biol. Macromol. 2024, 260, 129544. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gui, X.; Wan, Z.; Qi, Y.; Wang, S.; Zhang, H.; Niu, M.; Guo, Y. Efficient preparation of cellulose nanocrystals and regulation of cholesteric liquid crystals for advanced anti-counterfeiting. J. Appl. Polym. Sci. 2024, 141, e56043. [Google Scholar] [CrossRef]
- Meng, Y.; Zong, G.; Sun, Y.; He, Z.; Dong, C.; Long, Z. Flexible multiple-stimulus-responsive photonic films based on layer-by-layer assembly of cellulose nanocrystals and deep eutectic solvents. Chem. Eng. J. 2024, 488, 150713. [Google Scholar] [CrossRef]
- Jia, S.; Du, J.; Xie, Y.; Yang, B.; Tao, T.; Yu, L.; Zhang, Y.; Zhang, J.; Tang, W.; Gong, J. cellulose-based photonic crystal film with multiple stimulus-responsive performances. Chem. Mater. 2024, 36, 7967–7975. [Google Scholar] [CrossRef]
- Dong, X.; Li, D.; Wu, J.; Zhang, Z.; Wang, Z.; Song, F.; Wang, X.; Wang, Y. Non-iridescent and wide-color-range structural coloration enabled by cellulose nanocrystals with a controlled long-range photonic structure and helical pitch. ACS Sustain. Chem. Eng. 2022, 10, 10641–10648. [Google Scholar] [CrossRef]
- Boott, C.E.; Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Cellulose nanocrystal elastomers with reversible visible color. Angew. Chem. 2020, 132, 232–237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Zhou, X.; Wang, D.; Li, D.; Lei, W.; Fang, C.; Hu, J.; Luo, R. The Correlations between Microstructures and Color Properties of Nanocrystalline Cellulose: A Concise Review. Polymers 2024, 16, 2774. https://doi.org/10.3390/polym16192774
Zhu K, Zhou X, Wang D, Li D, Lei W, Fang C, Hu J, Luo R. The Correlations between Microstructures and Color Properties of Nanocrystalline Cellulose: A Concise Review. Polymers. 2024; 16(19):2774. https://doi.org/10.3390/polym16192774
Chicago/Turabian StyleZhu, Keming, Xing Zhou, Dong Wang, Dexiang Li, Wanqing Lei, Changqing Fang, Jingbo Hu, and Rubai Luo. 2024. "The Correlations between Microstructures and Color Properties of Nanocrystalline Cellulose: A Concise Review" Polymers 16, no. 19: 2774. https://doi.org/10.3390/polym16192774
APA StyleZhu, K., Zhou, X., Wang, D., Li, D., Lei, W., Fang, C., Hu, J., & Luo, R. (2024). The Correlations between Microstructures and Color Properties of Nanocrystalline Cellulose: A Concise Review. Polymers, 16(19), 2774. https://doi.org/10.3390/polym16192774







