Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review
Abstract
1. Introduction
2. Cross-Linking Agents for Ternary Materials
2.1. Glyoxal
2.2. Glutaraldehyde
2.3. Dialdehyde Starch
2.4. Dialdehyde Chitosan
2.5. EDC/NHS
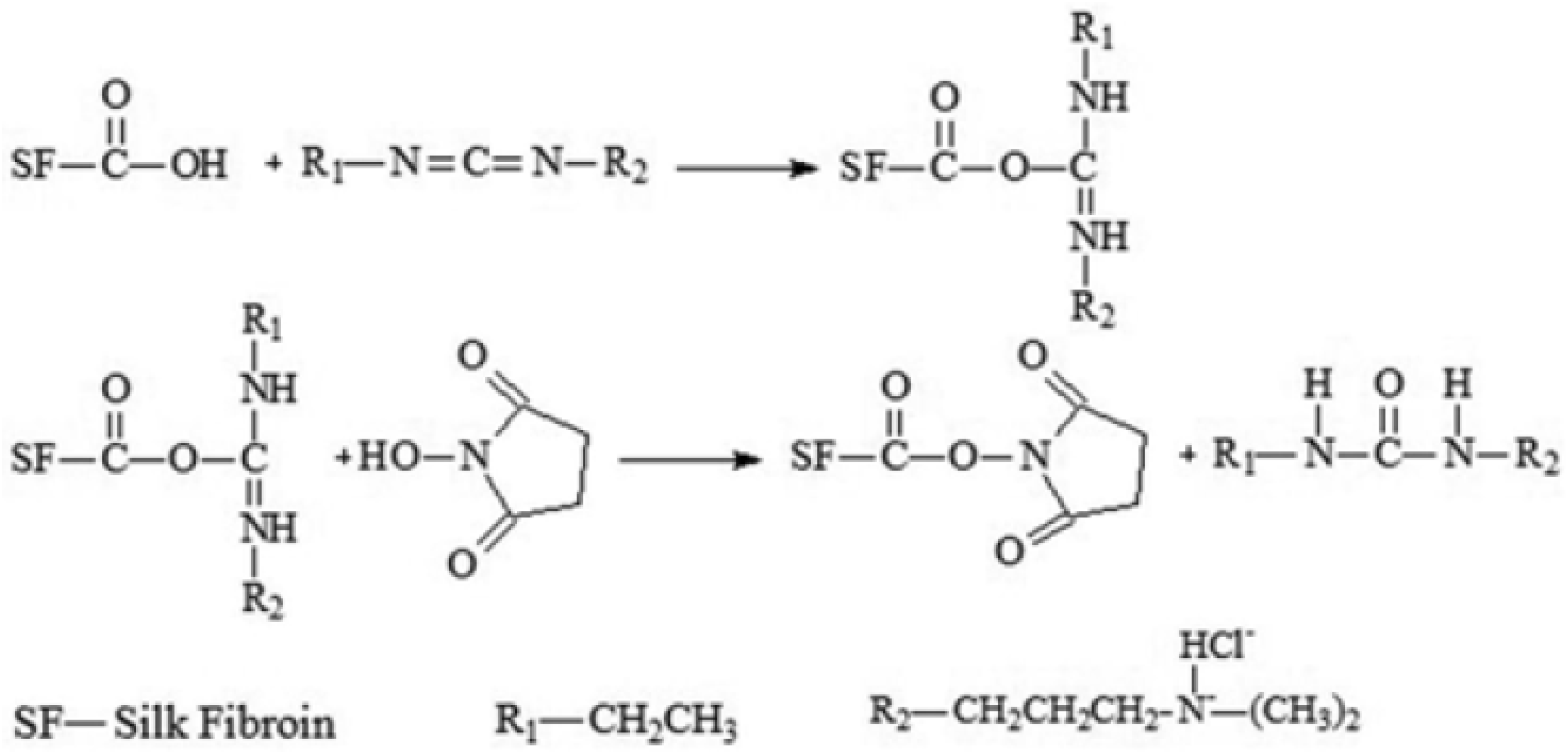
2.6. Other Agents

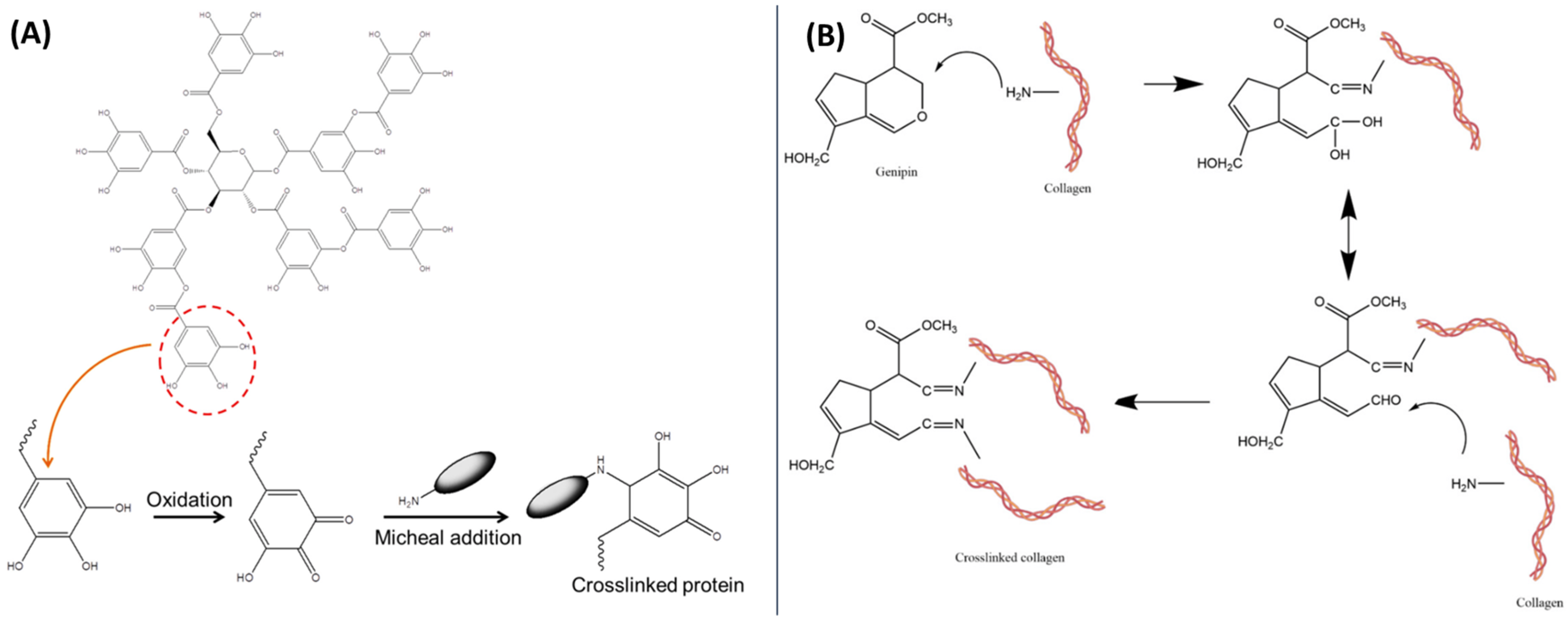
3. Critical Comparison of Cross-Linking Agents Commonly Used for Ternary Materials Cross-Linking
4. Conclusions and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Dongre, R.S.; Sadasivuni, K.K.; Deshmukh, K.; Mehta, A.; Basu, S.; Meshram, J.S.; Al-Maadeed, M.A.A.; Karim, A. Natural Polymer Based Composite Membranes for Water Purification: A Review. Polym.-Plast. Technol. Mater. 2019, 58, 1295–1310. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural Polymer Based Cling Films for Food Packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18. [Google Scholar]
- Kulkarni, V.; Butte, K.; Rathod, S. Natural Polymers—A Comprehensive Review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A. How to Improve Physico-chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-linking of Silk Fibroin—A Review. Materials 2021, 14, 1510. [Google Scholar] [CrossRef]
- Tuwalska, A.; Grabska-Zielińska, S.; Sionkowska, A. Chitosan/Silk Fibroin Materials for Biomedical Applications—A Review. Polymers 2022, 14, 1343. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piatek, J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A. Chitosan/Collagen Blends with Inorganic and Organic Additive—A Review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Geanaliu-Nicolae, R.E.; Andronescu, E. Blended Natural Support Materials—Collagen Based Hydrogels Used in Biomedicine. Materials 2020, 13, 5641. [Google Scholar] [CrossRef]
- Hein, S.; Wang, K.; Stevens, W.F.; Kjems, J. Chitosan Composites for Biomedical Applications: Status, Challenges and Perspectives. Mater. Sci. Technol. 2008, 24, 1053–1061. [Google Scholar] [CrossRef]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-Based Blends for Biomedical Applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef] [PubMed]
- Rogovina, S.Z.; Vikhoreva, G.A. Polysaccharide-Based Polymer Blends: Methods of Their Production. Glycoconj. J. 2006, 23, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B.; Michalska, M. The Miscibility of Collagen/Hyaluronic Acid/Chitosan Blends Investigated in Dilute Solutions and Solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wisniewska, J.; Wisniewski, M. Collagen-Synthetic Polymer Interactions in Solution and in Thin Films. J. Mol. Liq. 2009, 145, 135–138. [Google Scholar] [CrossRef]
- Thakur, G.; Rodrigues, F.C.; Singh, K. Crosslinking Biopolymers for Advanced Drug Delivery and Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1078, 213–231. [Google Scholar]
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Pandolfi, D.; Paradossi, G.; Palocci, C. Biofabrication of Genipin-Crosslinked Peptide Hydrogels and Their Use in the Controlled Delivery of Naproxen. New Biotechnol. 2017, 37, 138–143. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration-Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical Crosslinking of Biopolymeric Scaffolds: Current Knowledge and Future Directions of Crosslinked Engineered Bone Scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. Current Methods of Collagen Cross-Linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Karimi, F.; Lau, K.; Kim, H.N.; Och, Z.; Lim, K.S.; Whitelock, J.; Lord, M.; Rnjak-Kovacina, J. Surface Biofunctionalization of Silk Biomaterials Using Dityrosine Cross-Linking. ACS Appl. Mater. Interfaces 2022, 14, 31551–31566. [Google Scholar] [CrossRef]
- Maziz, A.; Leprette, O.; Boyer, L.; Blatché, C.; Bergaud, C. Tuning the Properties of Silk Fibroin Biomaterial via Chemical Cross-Linking. Biomed. Phys. Eng. Express 2018, 4, 065012. [Google Scholar] [CrossRef]
- Partlow, B.P.; Applegate, M.B.; Omenetto, F.G.; Kaplan, D.L. Dityrosine Cross-Linking in Designing Biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. [Google Scholar] [CrossRef]
- Dunn, R.M. Cross-Linking in Biomaterials. Plast. Reconstr. Surg. 2012, 130, 18S–26S. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkilä, M.I.; Willför, S.M.; Tenkanen, M. Films from Glyoxal-Crosslinked Spruce Galactoglucomannans Plasticized with Sorbitol. Int. J. Polym. Sci. 2012, 2012, 482810. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk Fibroin/Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials toward Bone Tissue Regeneration. Materials 2020, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Mazur, O.; Michalska-Sionkowska, M.; Łukowicz, K.; Osyczka, A.M. The Preparation and Characterization of Chitosan-Based Hydrogels Cross-Linked by Glyoxal. Materials 2021, 14, 2449. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Rau, R.; Glomb, M.A. Modification and Cross-Linking of Proteins by Glycolaldehyde and Glyoxal: A Model System. J. Agric. Food Chem. 2018, 66, 10835–10843. [Google Scholar] [CrossRef]
- Yang, Q.; Dou, F.; Liang, B.; Shen, Q. Studies of Cross-Linking Reaction on Chitosan Fiber with Glyoxal. Carbohydr. Polym. 2005, 59, 205–210. [Google Scholar] [CrossRef]
- Jawad, A.H.; Norrahma, S.S.A.; Hameed, B.H.; Ismail, K. Chitosan-Glyoxal Film as a Superior Adsorbent for Two Structurally Different Reactive and Acid Dyes: Adsorption and Mechanism Study. Int. J. Biol. Macromol. 2019, 135, 569–581. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Pin, J.M.; Zasada, L.; Sonne, M.M.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Kleszczyński, K. Assessment of Melatonin-Cultured Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials for Wound Healing. Antioxidants 2022, 11, 570. [Google Scholar] [CrossRef]
- Alfindee, M.N.; Sweah, Z.J.; Saki, T.A. Preparation and Characterization of Polymer Blends Based on Carboxymethyl Cellulose, Polyvinyl Alcohol, and Polyvinylpyrrolidone. Egypt. J. Chem. 2021, 64, 2679–2684. [Google Scholar] [CrossRef]
- Mugnaini, G.; Gelli, R.; Mori, L.; Bonini, M. How to Cross-Link Gelatin: The Effect of Glutaraldehyde and Glyceraldehyde on the Hydrogel Properties. ACS Appl. Polym. Mater. 2023, 5, 9192–9202. [Google Scholar] [CrossRef]
- Doustdar, F.; Olad, A.; Ghorbani, M. Effect of Glutaraldehyde and Calcium Chloride as Different Crosslinking Agents on the Characteristics of Chitosan/Cellulose Nanocrystals Scaffold. Int. J. Biol. Macromol. 2022, 208, 912–924. [Google Scholar] [CrossRef]
- Steitz, M.; Zouhair, S.; Khan, M.B.; Breitenstein-Attach, A.; Fritsch, K.; Tuladhar, S.R.; Wulsten, D.; Wolkers, W.F.; Sun, X.; Hao, Y.; et al. A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application. Bioengineering 2023, 10, 1247. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, C.; Duan, L.; Li, G. Physicochemical Properties of Collagen Solutions Cross-Linked by Glutaraldehyde. Connect. Tissue Res. 2014, 55, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, Y.; Lu, W.W.; Leong, J.C.; Zhang, W.; Zhang, J.; Zhang, M.; Yao, K. Preparation and Histological Evaluation of Biomimetic Three-Dimensional Hydroxyapatite/Chitosan-Gelatin Network Composite Scaffolds. Biomaterials 2002, 23, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, Y.; Cai, J.; Ma, S.; Wang, X. Coagulation Property of Hyaluronic Acid-Collagen/Chitosan Complex Film. J. Mater. Sci. Mater. Med. 2008, 19, 3621–3629. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun Chitosan/Polycaprolactone-Hyaluronic Acid Bilayered Scaffold for Potential Wound Healing Applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Sangeetha, V.; Sudha, P.N.; Gomathi, T. Experimental Analysis of Binary and Ternary Polymer Blends of Nanochitosan. Mater. Today Proc. 2016, 3, 2169–2177. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Y.; Ting, K.; Li, Q.; Gao, Q. Preparation and Emulsification Properties of Dialdehyde Starch Nanoparticles. Food Chem. 2019, 286, 467–474. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Para, A. Structural and Molecular Properties of Dialdehyde Starch. Carbohydr. Polym. 2006, 63, 360–366. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, W.; Xiao, J.; Zhao, X.; Zhu, Y.; Wu, Y. Preparation and Characterization of Dialdehyde Starch by One-Step Acid Hydrolysis and Oxidation. Int. J. Biol. Macromol. 2017, 103, 1257–1264. [Google Scholar] [CrossRef]
- Lin, W.; Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G. Collagen Cryogel Cross-Linked by Dialdehyde Starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is Dialdehyde Starch a Valuable Cross-Linking Agent for Collagen/Elastin Based Materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Du, Y.; Fan, L. Dialdehyde Starch-Crosslinked Chitosan Films and Their Antimicrobial Effects. J. Polym. Sci. B Polym. Phys. 2003, 41, 993–997. [Google Scholar] [CrossRef]
- Kamoun, E.A. N-Succinyl Chitosan-Dialdehyde Starch Hybrid Hydrogels for Biomedical Applications. J. Adv. Res. 2016, 7, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Einipour, S.K.; Sadrjahani, M.; Rezapour, A. Preparation and Evaluation of Antibacterial Wound Dressing Based on Vancomycin-Loaded Silk/Dialdehyde Starch Nanoparticles. Drug Deliv. Transl. Res. 2022, 12, 2778–2792. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Sun, Y.; Wu, Y.; Wang, J.; Ding, Y.; Cheng, J.; Guo, M. Mechanical, Microstructural, and Rheological Characterization of Gelatin-Dialdehyde Starch Hydrogels Constructed by Dual Dynamic Crosslinking. LWT 2022, 161, 113374. [Google Scholar] [CrossRef]
- Gennadios, A.; Handa, A.; Froning, G.W.; Weller, C.L.; Hanna, M.A. Physical Properties of Egg White-Dialdehyde Starch Films. J. Agric. Food Chem. 1998, 46, 1297–1302. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and Characterization of Collagen/Hyaluronic Acid/Chitosan Film Crosslinked with Dialdehyde Starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The Application of Chitosan/Collagen/Hyaluronic Acid Sponge Cross-Linked by Dialdehyde Starch Addition as a Matrix for Calcium Phosphate in Situ Precipitation. Int. J. Biol. Macromol. 2018, 107, 470–477. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The Comparison of Physic-Chemical Properties of Chitosan/Collagen/Hyaluronic Acid Composites with Nano-Hydroxyapatite Cross-Linked by Dialdehyde Starch and Tannic Acid. Polym. Test. 2017, 62, 171–176. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska, S. Incorporation of Magnetite Particles in 3D Matrices Made from the Blends of Collagen, Chitosan, and Hyaluronic Acid. Adv. Polym. Technol. 2018, 37, 2905–2914. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Olewnik-Kruszkowska, E.; Reczyńska, K.; Pamuła, E. Is Dialdehyde Chitosan a Good Substance to Modify Physicochemical Properties of Biopolymeric Materials? Int. J. Mol. Sci. 2021, 22, 3391. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-Chemical Characterization and Biological Tests of Collagen/Silk Fibroin/Chitosan Scaffolds Cross-Linked by Dialdehyde Starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Kirui, G.K.; Madivoli, E.S.; Nzilu, D.M.; Kareru, P.G.; Waudo, W. Antifungal Activity of Dialdehyde Chitosan against Aspergillus Brasiliensis and Candida Albicans. Biomass Convers. Biorefin 2024. [Google Scholar] [CrossRef]
- R, M.D.; Usharani, N.; Saravanan, N.; Kanth, S.V. In Vitro and in Silico Approach towards Antimicrobial and Antioxidant Behaviour of Water-Soluble Chitosan Dialdehyde Biopolymers. Carbohydr. Res. 2024, 542, 109192. [Google Scholar] [CrossRef]
- Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- Bam, P.; Bhatta, A.; Krishnamoorthy, G. Design of Biostable Scaffold Based on Collagen Crosslinked by Dialdehyde Chitosan with Presence of Gallic Acid. Int. J. Biol. Macromol. 2019, 130, 836–844. [Google Scholar] [CrossRef]
- Liu, X.; Dan, N.; Dan, W.; Gong, J. Feasibility Study of the Natural Derived Chitosan Dialdehyde for Chemical Modification of Collagen. Int. J. Biol. Macromol. 2016, 82, 989–997. [Google Scholar] [CrossRef]
- Wissink, M.J.B.; Beernink, R.; Pieper, J.S.; Poot, A.A.; Engbers, G.H.M.; Beugeling, T.; Van Aken, W.G.; Feijen, J. Immobilization of Heparin to EDC/NHS-Crosslinked Collagen. Characterization and in Vitro Evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New Composite Materials Prepared by Calcium Phosphate Precipitation in Chitosan/Collagen/Hyaluronic Acid Sponge Cross-Linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef]
- Liu, R.; Ming, J.; Zhang, H.; Zuo, B. EDC/NHS Crosslinked Electrospun Regenerated Tussah Silk Fibroin Nanofiber Mats. Fibers Polym. 2012, 13, 613–617. [Google Scholar] [CrossRef]
- Mao, J.S.; Liu, H.F.; Yin, Y.J.; Yao, K. De The Properties of Chitosan-Gelatin Membranes and Scaffolds Modified with Hyaluronic Acid by Different Methods. Biomaterials 2003, 24, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, Y.; Yao, K. Construction of Chitosan-Gelatin-Hyaluronic Acid Artificial Skin in Vitro. J. Biomater. Appl. 2007, 21, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mao, J.; Yao, K.; Yang, G.; Cui, L.; Cao, Y. A Study on a Chitosan-Gelatin-Hyaluronic Acid Scaffold as Artificial Skin in Vitro and Its Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2004, 15, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic Mineralized Hierarchical Hybrid Scaffolds Based on in Situ Synthesis of Nano-Hydroxyapatite/Chitosan/Chondroitin Sulfate/Hyaluronic Acid for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef]
- Mathews, S.; Bhonde, R.; Gupta, P.K.; Totey, S. Novel Biomimetic Tripolymer Scaffolds Consisting of Chitosan, Collagen Type 1, and Hyaluronic Acid for Bone Marrow-Derived Human Mesenchymal Stem Cells-Based Bone Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1825–1834. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Thanyacharoen, T.; Thongchai, K.; Techasakul, S.; Ummartyotin, S. Preparation of Chitosan/Hydrolyzed Collagen/Hyaluronic Acid Based Hydrogel Composite with Caffeic Acid Addition. Int. J. Biol. Macromol. 2020, 162, 1937–1943. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pavasant, P.; Supaphol, P. Preparation and Characterization of Caffeic Acid-Grafted Electrospun Poly(l-Lactic Acid) Fiber Mats for Biomedical Applications. ACS Appl. Mater. Interfaces 2012, 4, 3031–3040. [Google Scholar] [CrossRef]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus Aureus Clinical Strains. Biomed. Res. Int. 2018, 2018, 413504. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the Use of Natural Antioxidants in Active Food Packaging: A Review. Food Addit. Contam.-Part A 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, H.; Wang, Y.; Qiu, Z.; Wu, J.; Cao, J.; Xu, K.; Zhang, Y.; Jiang, Y.; Wang, M. Feasibility of Caffeic Acid as a Crosslinking Agent in Modifying Acellular Extracellular Matrices. Biochem. Biophys. Res. Commun. 2023, 677, 182–189. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. Scaffolds Based on Chitosan and Collagen with Glycosaminoglycans Cross-Linked by Tannic Acid. Polym. Test. 2018, 65, 163–168. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-Crosslinked Chitosan Hydrogels as Biomedical and Pharmaceutical Aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- MacAya, D.; Ng, K.K.; Spector, M. Injectable Collagen-Genipin Gel for the Treatment of Spinal Cord Injury: In Vitro Studies. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid—Based Injectable Hydrogels for Tissue Engineering Applications—Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Guzdek-Zając, K.; Karewicz, A.; Horak, W.; Lach, R.; Wójcik, K.; Nowakowska, M. Bioactive yet Antimicrobial Structurally Stable Collagen/Chitosan/Lysine Functionalized Hyaluronic Acid—Based Injectable Hydrogels for Potential Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 155, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Łańcucka, J.; Gilarska, A.; Buła, A.; Horak, W.; Łatkiewicz, A.; Nowakowska, M. Genipin Crosslinked Bioactive Collagen/Chitosan/Hyaluronic Acid Injectable Hydrogels Structurally Amended via Covalent Attachment of Surface-Modified Silica Particles. Int. J. Biol. Macromol. 2019, 136, 1196–1208. [Google Scholar] [CrossRef]
- Jayachandran, B.; Parvin, T.N.; Alam, M.M.; Chanda, K.; MM, B. Insights on Chemical Crosslinking Strategies for Proteins. Molecules 2022, 27, 8124. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Miłek, O.; Michalska-Sionkowska, M.; Osyczka, A.M. Bio-Studies of Scaffolds Based on Chitosan/Tannic Acid Cross-Linked by Glyoxal. Mater. Lett. 2021, 292, 129667. [Google Scholar] [CrossRef]
- Wang, L.; Stegemann, J.P. Glyoxal Crosslinking of Cell-Seeded Chitosan/Collagen Hydrogels for Bone Regeneration. Acta Biomater. 2011, 7, 2410–2417. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, K.; Liu, W.; Shen, L.; Li, G. Two-Dimensional Infrared Spectroscopic Study on the Thermally Induced Structural Changes of Glutaraldehyde-Crosslinked Collagen. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 356–363. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Pin, J.M.; Kaczmarek-Szczepańska, B.; Olewnik-Kruszkowska, E.; Sionkowska, A.; Monteiro, F.J.; Steinbrink, K.; Kleszczyński, K. Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment. Polymers 2022, 14, 1818. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sosik, A.; Małkowska, A.; Olewnik-Kruszkowska, E.; Steinbrink, K.; Kleszczyński, K.; Kaczmarek-Szczepańska, B. The Characterization of Scaffolds Based on Dialdehyde Chitosan/Hyaluronic Acid. Materials 2021, 14, 4993. [Google Scholar] [CrossRef] [PubMed]
- Wickramathilaka, M.P.; Tao, B.Y. Characterization of Covalent Crosslinking Strategies for Synthesizing DNA-Based Bioconjugates. J. Biol. Eng. 2019, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Madison, S.A.; Carnali, J.O. PH Optimization of Amidation via Carbodiimides. Ind. Eng. Chem. Res. 2013, 52, 13547–13555. [Google Scholar] [CrossRef]


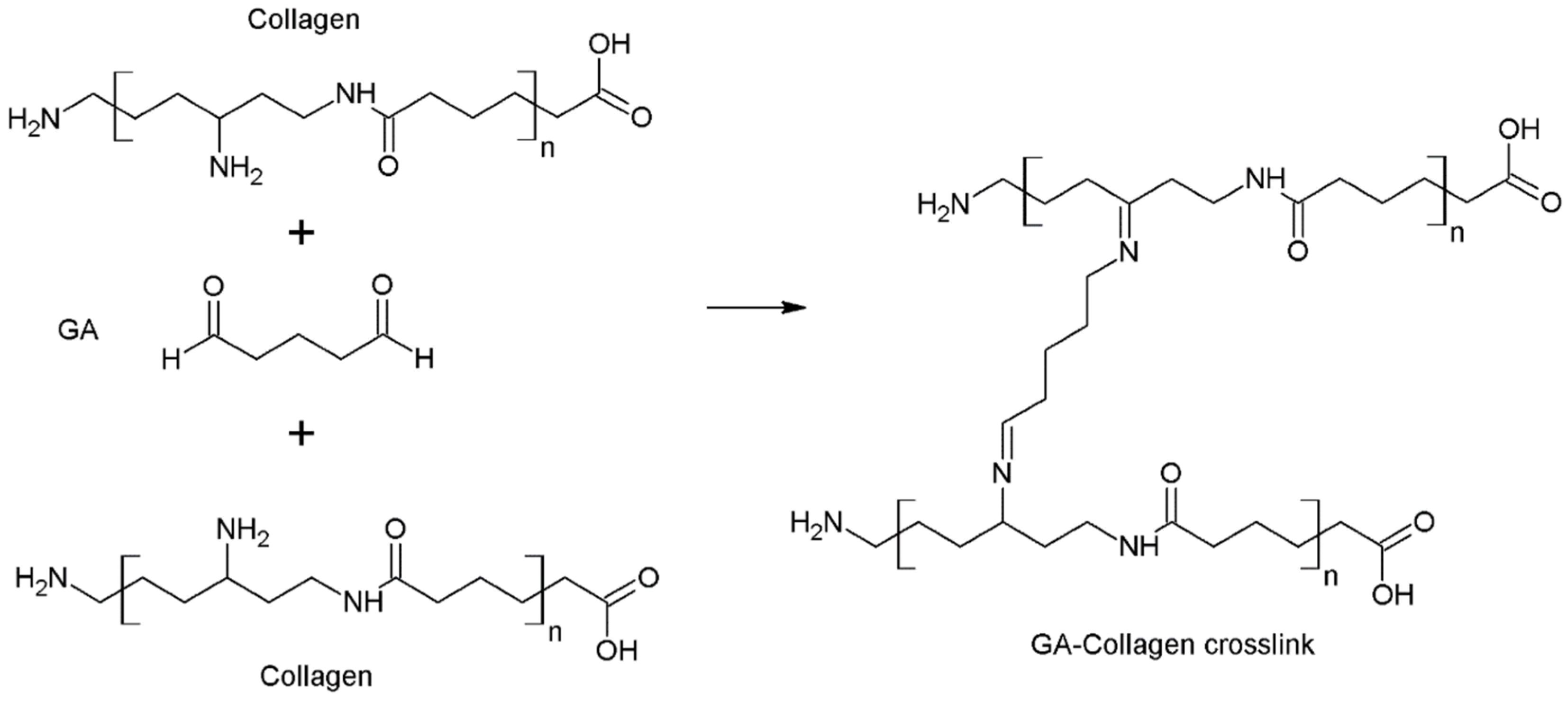


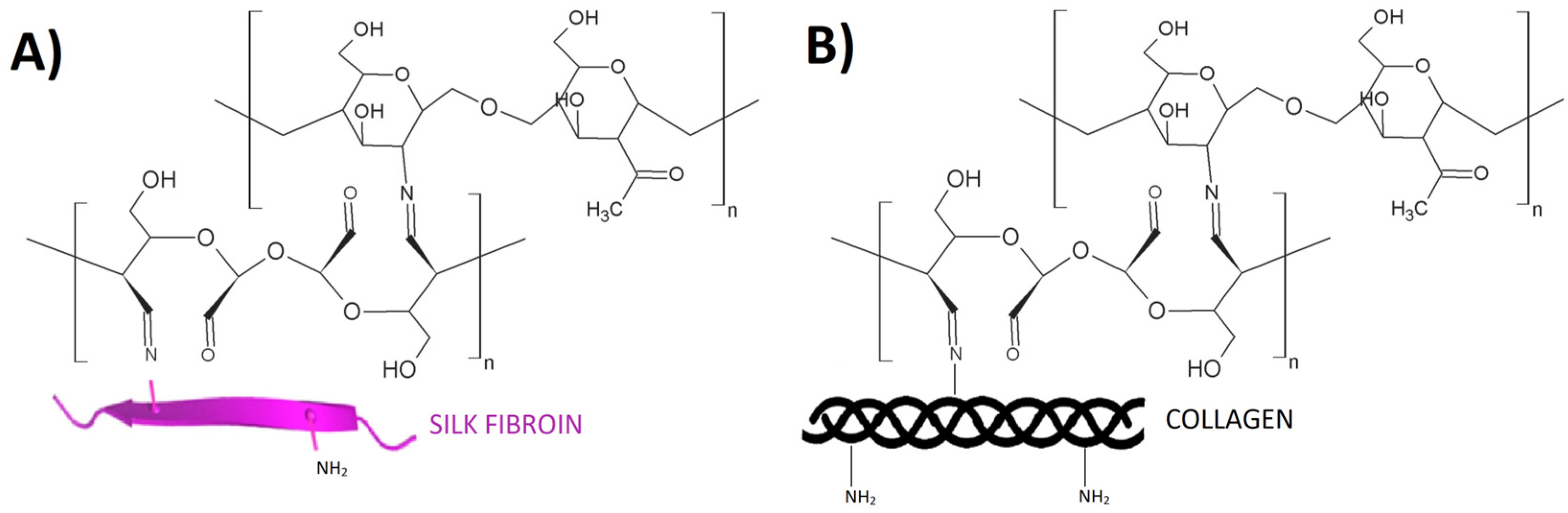


| Cross-Linking Agent | Characteristics | Applications | Limitations | References |
|---|---|---|---|---|
| Glyoxal | the smallest existing dialdehyde; the presence of two adjacent carbonyl groups in structure is responsible for its high reactivity | cross-linking of materials based on polysaccharides, peptides, and their mixtures in different forms; stabilization of polymeric structure; improving the physicochemical properties of materials | (1) toxicity: GLY can exhibit cytotoxicity in higher concentrations, which limits its use in biomedical applications (drug delivery, tissue engineering, or medical devices); residual GLY must be thoroughly removed to avoid toxic effects on cells and tissues; (2) pH sensitivity: an acidic environment may suit for GLY to polysaccharides reaction occurrence because the Schiff base reaction takes place in the presence of acid catalysis; (3) concentration: the metabolic activity of hBMSC (human bone marrow stem cells) was not affected by GLY at low concentrations, but there was a relatively clear cut-off point above which cellular activity decreased; (4) exposure time: at a 1 h incubation time, cell metabolic activity was unaffected up, though at increasing exposure time this value was reduced; (5) low efficiency: the steric structure of GLY can be a disadvantage due to a small molecular size and a possibility to forms shorter and potentially less stable cross-links than other aldehydes (glutaraldehyde) | [5,36,89,90] |
| Glutaraldehyde | the most commonly employed cross-linking agent with high reactivity and efficiency; excellent soluble in water and organic solvents; easy available; low cost | improving the mechanical properties of collagen-based biomaterials; reagent to stabilize a polymer structure against thermal or enzymatic degradation; cross-linking agent for the preparation of heart valves, vascular grafts, elastic cartilages, tendon xerographs, or artificial skin | (1) Coll concentration in material: if the concentration of Coll is too low, the improvement in any important properties (e.g., thermal stability and mechanical strength) is not observed; if the concentration of Coll is too high, the inhomogeneous reaction may occur; (2) toxicity: GLU can exhibit cytotoxicity in higher concentrations, which limits its use in biomedical applications (in vivo medical implants or drug delivery systems); residual GLU must be thoroughly removed to avoid toxic effects on cells and tissues; (3) concentration of cross-linking agent: the limit for GLU concentration is 8% w/v, which allows for the elimination of the toxic effect; (4) impossibility to cell incorporation: cells cannot be incorporated into materials during fabrication because of the possible cytotoxicity of GLU, and therefore, cells must be seeded onto the exterior of scaffolds post-fabrication; (5) pH sensitivity: the reactivity of GLU is highly dependent on pH; at acidic pH, the aldehyde groups are more reactive, while at neutral or alkaline pH, they are less reactive; it requires careful control of reaction conditions; (6) irritation: GLU is a strong irritant to the skin, eyes, and respiratory system, posing safety concerns during handling and manufacturing processes | [5,24,33,38,89,90,91] |
| Dialdehyde starch | natural agent; derivative of starch; can be obtained by an oxidation process; exhibits excellent chemical, physical, and biochemical properties due to the presence of active aldehyde groups | cross-linking agent for proteins, polysaccharides, and their mixtures; component of materials used in biomedical field and packaging industry | (1) too low degree of starch oxidation: it results in reduced cross-linking efficiency and may limit its application where high reactivity is required; (2) stability of aldehyde groups: the aldehyde groups in DAS can undergo side reactions, such as hydration or oxidation into carboxylic acids, which can reduce their reactivity over time; this instability can affect the consistency and efficiency of the cross-linking process; (3) limited solubility: DAS is less soluble in water compared to native starch due to the loss of hydroxyl groups during oxidation, which can be problematic in applications where high solubility is required; (4) cytotoxicity: free aldehyde groups can exhibit cytotoxicity, which makes DAS potentially harmful in biomedical applications unless the unreacted aldehyde groups are properly quenched, neutralized or DAS residues will be thoroughly removed | [52,53,54,55] |
| Dialdehyde chitosan | derivative of chitosan; can be synthesized from chitosan by periodate oxidation; it demonstrates antifungal and antibacterial activity; it exhibits antioxidant activity | cross-linking agent for proteins and polysaccharides and their mixtures; component of materials used in the biomedical field and packaging industry | (1) too low degree of starch oxidation: it results in reduced cross-linking efficiency and may limit its application where high reactivity is required; (2) stability of aldehyde groups: the aldehyde groups in DAC can undergo side reactions, such as hydration or oxidation into carboxylic acids, which can reduce their reactivity over time; this instability can affect the consistency and efficiency of the cross-linking process; (3) poor solubility: DAC has reduced solubility in comparison to native chitosan due to the loss of hydroxyl groups during oxidation, which can be problematic in applications where high solubility is required; (4) cytotoxicity: free aldehyde groups can exhibit cytotoxicity, which makes DAS potentially harmful in biomedical applications unless the unreacted aldehyde groups are properly quenched, neutralized, or DAC residues will be thoroughly removed; (5) synthesis challenges: the oxidation process used to obtain DAC requires the precise control of conditions to achieve the desired level of oxidation; over-oxidation or under-oxidation can lead to inconsistent aldehyde content, affecting the cross-linking performance and the properties of the final material | [62,64,65,66,67,68,92,93] |
| EDC/NHS | “zero length” cross-linking agent which chemically activates a molecule | cross-linking agent to polysaccharides, proteins and their mixtures; improving physicochemical properties of collagen-based materials | (1) instability of EDC: EDC is water-soluble, hydrolytically unstable, and has a relatively short half-life in water; in water conditions, it tends to rapidly hydrolyze into inactive by-products, which can reduce the reaction efficiency; it needs to be used immediately after preparation; (2) pH sensitivity: the reactivity of EDC/NHS is highly dependent on pH; EDC/NHS has an optimal pH range usually between 4.5 and 7.5; it requires careful control of reaction conditions; (3) cross-linking of Coll with EDC/NHS affects cell adhesion and leads to calcification; (4) cytotoxicity: by-products of EDC (urea derivatives) may be toxic or interfere with biological applications (drug delivery and tissue engineering); residues have to be thoroughly removed | [24,33,88,94,95] |
| Cross-Linking Agent | Mixture | Form of Biomaterial | Studies | Reference |
|---|---|---|---|---|
| Glyoxal | Silk fibroin/collagen/chitosan | 3D scaffold | FTIR; density; porosity; moisture content; swelling behaviour; SEM; mechanical properties; in vitro cytotoxicity assay with MG-63 cells | [32] |
| Carboxymethyl cellulose/polyvinyl alcohol/polyvinylpyrrolidone | Hydrogel films | Mechanical properties; SEM; swelling behaviour | [39] | |
| Glutaraldehyde | Hydroxyapatite/chitosan/gelatin | 3D scaffolds | Calvarial osteoblast isolation, seeding, and culture; SEM; immunohistological observation of osteoblasts/scaffold constructs; mineralization study | [44] |
| Hyaluronic acid/type I collagen/chitosan | Films | 3T3 fibroblast culture; MTT assay; MIC assay; AFM | [45] | |
| Chitosan/polycaprolactone/hyaluronic acid | 3D scaffolds | SEM; FTIR-ATR; mechanical properties; buffer uptake ability; WVTR; wettability; porosity; degradation degree; evaluation of antimicrobial property | [46] | |
| Nanochitosan/polyvinylpyrollidone/silk fibroin | Films | FTIR; XRD, TGA, DSC | [47] | |
| Carboxymethyl cellulose/polyvinyl alcohol/polyvinylpyrrolidone | Hydrogel films | Mechanical properties; SEM; swelling behaviour | [39] | |
| Dialdehyde starch | Collagen/hyaluronic acid/chitosan | Films | FTIR; mechanical properties; AFM; contact angle measurements | [58] |
| Chitosan/collagen/hyaluronic acid (with nHAp) | 3D scaffolds | SEM; porosity; density; liquid uptake; mechanical tests; attachment and proliferation of human osteosarcoma SaOS-2 cells | [60] | |
| Chitosan/collagen/hyaluronic acid (with calcium phosphate) | 3D scaffolds | SEM; EDX; FTIR; porosity; density; mechanical properties; attachment and proliferation of human osteosarcoma SaOS-2 cells | [59] | |
| Collagen/chitosan/hyaluronic acid (with magnetic particles) | 3D scaffolds | ATR-FTIR; density; porosity; SEM; swelling ability; mechanical properties | [61] | |
| Chitosan/collagen/silk fibroin | 3D scaffolds | FTIR-ATR; swelling behaviour; water content; porosity; density; SEM; mechanical properties; thermal properties; cytotoxicity test with MG-63 cells | [62] | |
| Collagen/silk fibroin/chitosan | 3D scaffolds | FTIR-ATR; swelling behaviour; water content; porosity; density; SEM; mechanical properties; thermal properties; cytotoxicity test with MG-63 cells | [62] | |
| Silk fibroin/chitosan/collagen | 3D scaffolds | FTIR-ATR; swelling behaviour; water content; porosity; density; SEM; mechanical properties; thermal properties; cytotoxicity test with MG-63 cells | [62] | |
| Dialdehyde chitosan | Chitosan/collagen/silk fibroin | 3D scaffolds | FTIR-ATR; swelling behaviour; water content; porosity; density; SEM; mechanical properties; thermal properties; cytotoxicity test with MG-63 cells | [62] |
| Collagen/silk fibroin/chitosan | 3D scaffolds | FTIR-ATR; swelling behaviour; water content; porosity; density; SEM; mechanical properties; thermal properties; cytotoxicity test with MG-63 cells | [62] | |
| Silk fibroin/chitosan/collagen | 3D scaffolds | FTIR-ATR; swelling behaviour; water content; porosity; density; SEM; mechanical properties; thermal properties; cytotoxicity test with MG-63 cells | [62] | |
| EDC/NHS | Collagen/chitosan/silk fibroin | 3D scaffolds | FTIR; porosity; density; swelling behaviour; moisture content; liquid uptake; SEM; mechanical properties; in vitro cytocompatibility assay with MG-63 cells | [21] |
| Chitosan/collagen/hyaluronic acid | 3D scaffolds | FTIR; SEM; porosity; density; mechanical tests; EDX; adhesion and proliferation of human osteosarcoma SaOS-2 cells studies | [59] | |
| Chitosan/gelatin/hyaluronic acid | Membranes | SEM; XPS; PBS solution adsorption; mechanical testing; in vitro degradation; cytocompatibility with human dermal fibroblasts | [72] | |
| Chitosan/gelatin/hyaluronic acid | 3D scaffolds | SEM; XPS; PBS solution adsorption; mechanical testing; in vitro degradation; cytocompatibility with human dermal fibroblasts | [72] | |
| Chitosan/gelatin/hyaluronic acid | 3D scaffolds | SEM; water uptake ability; mechanical properties; FTIR; in vitro biodegradation; construction of artificial dermis in vitro | [73] | |
| Chitosan/gelatin/hyaluronic acid | 3D scaffold | Water uptake and retention abilities; SEM; culture of fibroblast in scaffolds; attachment and proliferation assay; co-culture of keratinocytes and fibroblasts in scaffolds | [74] | |
| MES and EDC | Chitosan/collagen type I/hyaluronic acid | 3D scaffolds | SEM; swelling ratio; hMSCs culture on the scaffolds; calcein-propidium iodide live-dead assayed; osteoblast differentiation studies; alkaline phosphatase assay; immunofluorescent staining for fibronectin and osteocalcin | [76] |
| Caffeic acid | Chitosan/hydrolyzed collagen/hyaluronic acid | Hydrogels | X-ray diffraction; DSC; thermogravimetric analysis; SEM; swelling behaviour; release of caffeic acid; antioxidant activity; total phenolic content; in vitro degradation behaviour; water solubility; WVTR | [77] |
| Tannic acid | Chitosan/collagen/glycosaminoglycans (GAGs, including HA and CS) | 3D scaffold | FTIR-ATR; SEM; porosity; density; mechanical tests; attachment and proliferation of human osteosarcoma SaOS-2 cells | [82] |
| Genipin | Collagen/chitosan/hyaluronic acid | Hydrogels | Swelling properties; wettability measurements; SEM; mechanical testing; enzymatic degradation studies; Alamar Blue cell viability test; cell morphology analysis | [85] |
| Chitosan/collagen/modified hyaluronic acid | Hydrogels | Swelling ability; contact angle measurements; mechanical properties; enzymatic degradation evaluation; SEM; density; porosity; in vitro osteoblasts culture; antibacterial activity assessment | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabska-Zielińska, S. Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review. Polymers 2024, 16, 2679. https://doi.org/10.3390/polym16182679
Grabska-Zielińska S. Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review. Polymers. 2024; 16(18):2679. https://doi.org/10.3390/polym16182679
Chicago/Turabian StyleGrabska-Zielińska, Sylwia. 2024. "Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review" Polymers 16, no. 18: 2679. https://doi.org/10.3390/polym16182679
APA StyleGrabska-Zielińska, S. (2024). Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review. Polymers, 16(18), 2679. https://doi.org/10.3390/polym16182679






