Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microscopy
2.3. Roughness
2.4. Contact Angle and Surface Free Energy Evaluation
2.5. Protein Adsorption
2.6. Cell Experiments
2.6.1. Cell Culture
2.6.2. Cell Adhesion and Cell Spreading
2.6.3. Cell Viability
2.7. Statistical Analysis
3. Results
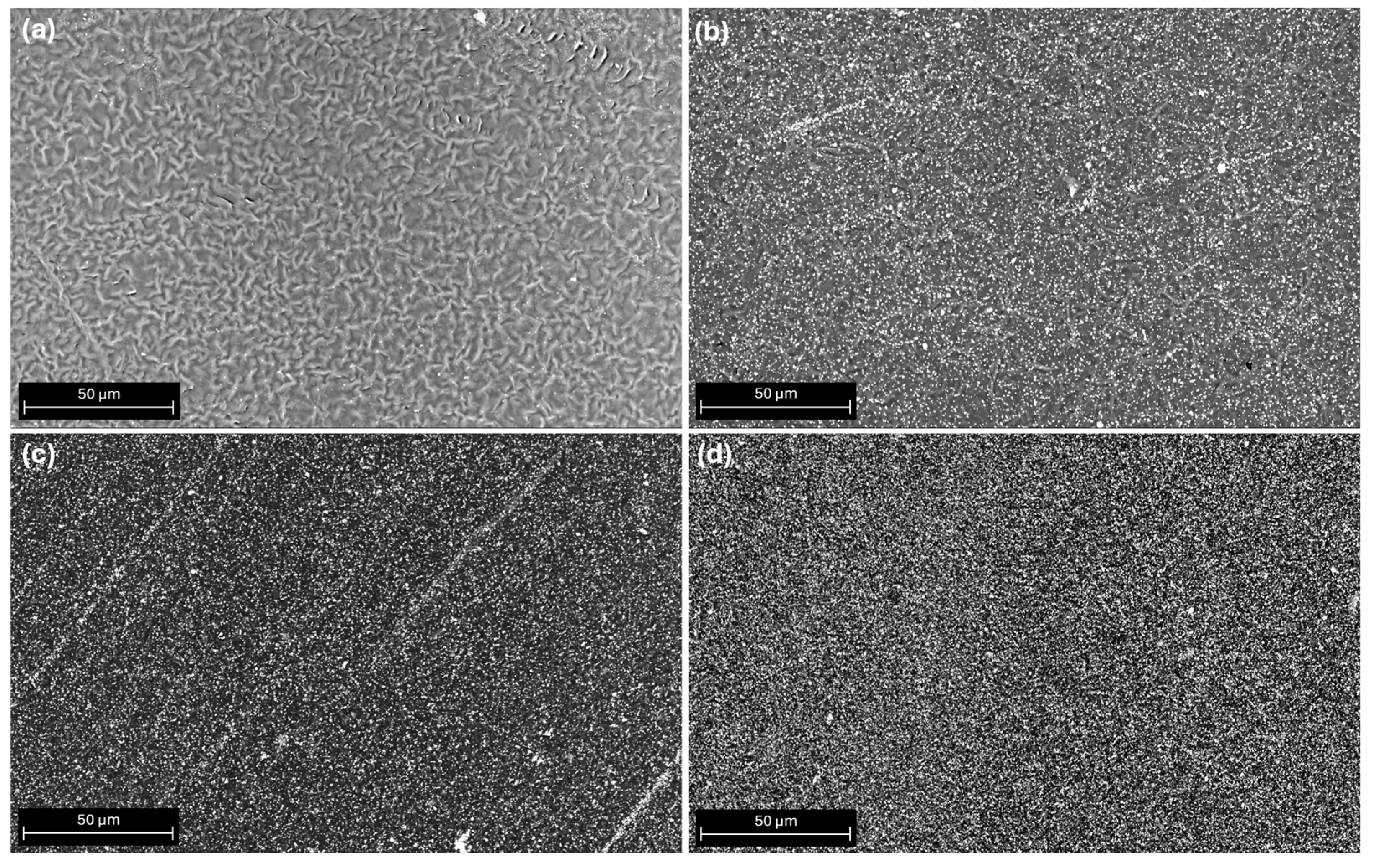
3.1. SEM Morphological Surface Analysis
3.2. Roughness Analysis
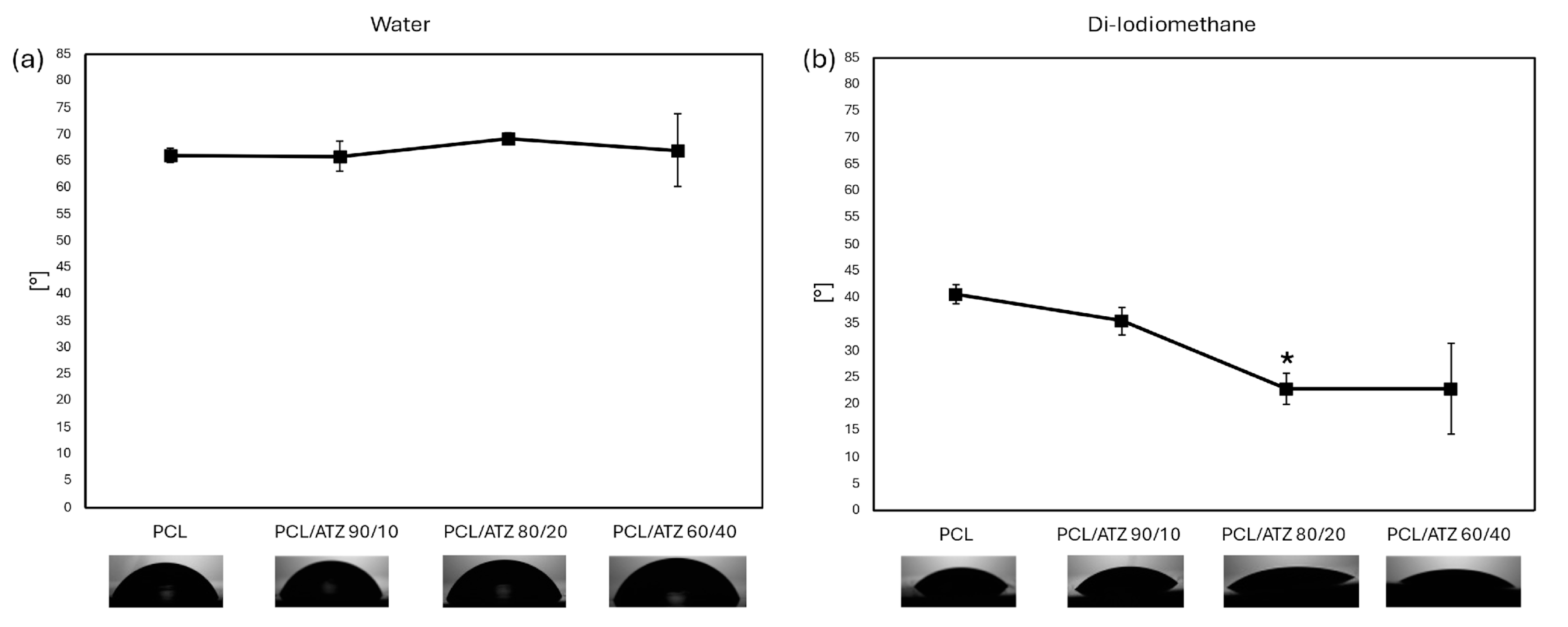
3.3. Wettability and Surface Free Energy Evaluation
3.4. Protein Adsorption
3.5. Cell Experiments
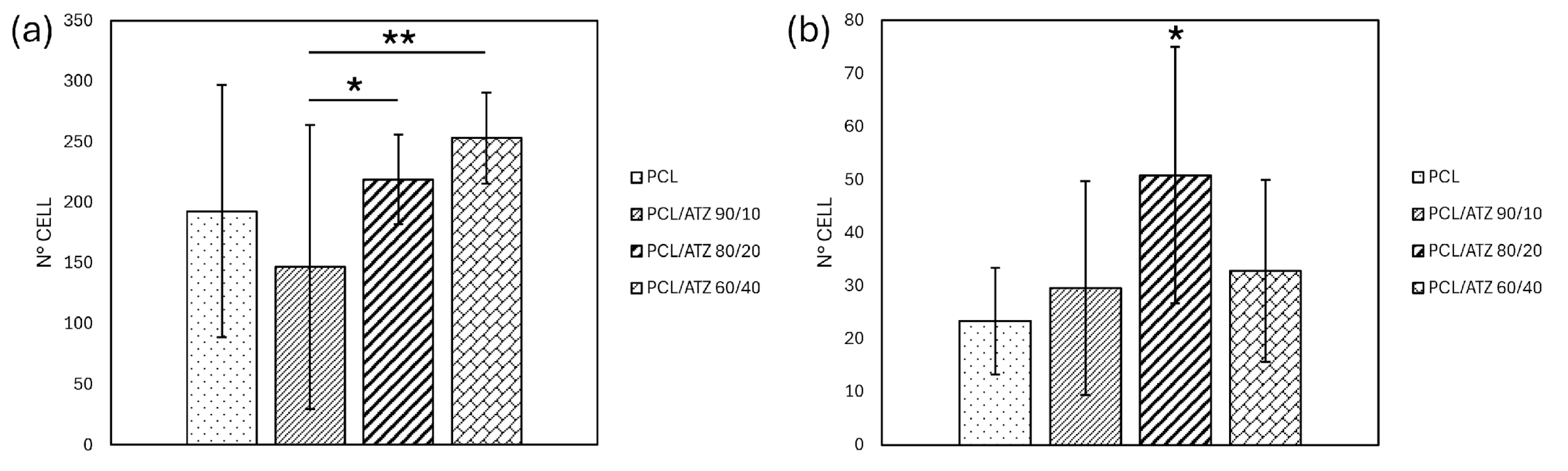
3.5.1. Cell Adhesion and Cell Spreading
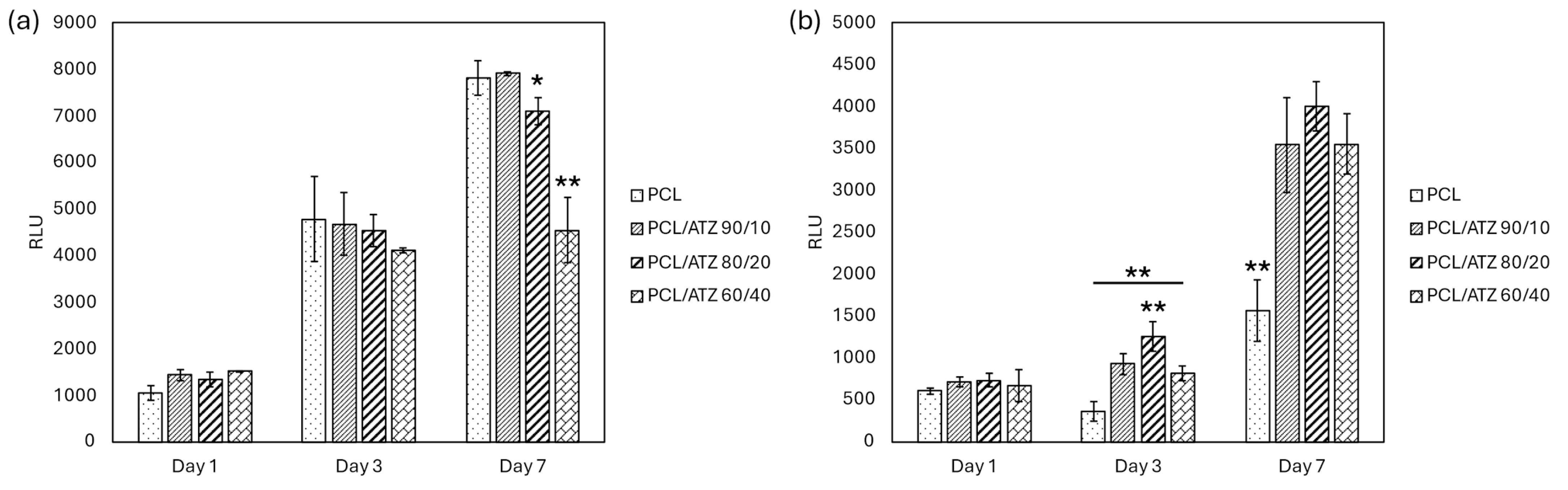
3.5.2. Cell Viability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Sanchez, R.; Dopico, J.; Kalemaj, Z.; Buti, J.; Pardo Zamora, G.; Mardas, N. Comparison of clinical outcomes of immediate versus delayed placement of dental implants: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2022, 33, 231–277. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.A.L.; Mourão, C.F.d.A.B.; Diniz, V.S.; Silva, P.C.; Meirelles, L.; Santos, E.; Schanaider, A. In-vivo bone response to titanium screw implants anodized in sodium sulfate. Acta Cirúrgica Bras. 2014, 29, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Winning, L.; Polyzois, I. What is the influence of implant surface characteristics and/or implant material on the incidence and progression of peri-implantitis? A systematic literature review. Clin. Oral Implant. Res. 2021, 32, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Raes, M.; D’hondt, R.; Teughels, W.; Coucke, W.; Quirynen, M. A 5-year randomized clinical trial comparing minimally with moderately rough implants in patients with severe periodontitis. J. Clin. Periodontol. 2018, 45, 711–720. [Google Scholar] [CrossRef]
- Fu, J.H.; Wang, H.L. Breaking the wave of peri-implantitis. Periodontology 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Laleman, I.; Lambert, F. Implant connection and abutment selection as a predisposing and/or precipitating factor for peri-implant diseases: A review. Clin. Implant Dent. Relat. Res. 2023, 25, 723–733. [Google Scholar] [CrossRef]
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cells Mater. 2019, 38, 63–78. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, P.; Ge, Y.; Lan, F.; Zhou, X.; He, J.; Wu, Y. Dopamine self-polymerized along with hydroxyapatite onto the preactivated titanium percutaneous implants surface to promote human gingival fibroblast behavior and antimicrobial activity for biological sealing. J. Biomater. Appl. 2018, 32, 1071–1082. [Google Scholar] [CrossRef]
- Genova, T.; Chinigò, G.; Munaron, L.; Rivolo, P.; Luganini, A.; Gribaudo, G.; Cavagnetto, D.; Mandracci, P.; Mussano, F. Bacterial and Cellular Response to Yellow-Shaded Surface Modifications for Dental Implant Abutments. Biomolecules 2022, 12, 1718. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, L.; Kuang, Y.; Zhou, Z.; Li, X. Highly Ordered Nanotube-Like Microstructure on Titanium Dental Implant Surface Fabricated via Anodization Enhanced Cell Adhesion and Migration of Human Gingival Fibroblasts. Int. J. Nanomed. 2024, 19, 2469–2485. [Google Scholar] [CrossRef] [PubMed]
- Parpaiola, A.; Cecchinato, D.; Toia, M.; Bressan, E.; Speroni, S.; Lindhe, J. Dimensions of the healthy gingiva and peri-implant mucosa. Clin. Oral Implant. Res. 2015, 26, 657–662. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17, 55. [Google Scholar] [CrossRef]
- Schierano, G.; Ramieri, G.; Cortese, M.; Aimetti, M.; Preti, G. Organization of the connective tissue barrier around long-term loaded implant abutments in man. Clin. Oral Implant. Res. 2002, 13, 460–464. [Google Scholar] [CrossRef]
- Vallée, A.; Faga, M.; Mussano, F.; Catalano, F.; Tolosano, E.; Carossa, S.; Altruda, F.; Martra, G. Alumina–zirconia composites functionalized with laminin-1 and laminin-5 for dentistry: Effect of protein adsorption on cellular response. Colloids Surf. B: Biointerfaces 2014, 114, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Mussano, F.; Faga, M.G.; Menicucci, G.; Manzella, C.; Sabione, C.; Genova, T.; Degerfeld, M.M.v.; Peirone, B.; Cassenti, A. An alumina toughened zirconia composite for dental implant application: In vivo animal results. BioMed Res. Int. 2015, 2015, 157360. [Google Scholar] [CrossRef]
- Maji, A.; Choubey, G. Microstructure and mechanical properties of alumina toughened zirconia (ATZ). Mater. Today: Proc. 2018, 5, 7457–7465. [Google Scholar] [CrossRef]
- Duraccio, D.; Strongone, V.; Faga, M.; Auriemma, F.; Mussano, F.; Genova, T.; Malucelli, G. The role of different dry-mixing techniques on the mechanical and biological behavior of UHMWPE/alumina-zirconia composites for biomedical applications. Eur. Polym. J. 2019, 120, 109274. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, P.; Qin, Q.; Jiang, K.; Luo, Y.; Xiang, C.; He, J.; Chen, L.; Jiang, D.; Cui, W. 3D-printed bone regeneration scaffolds modulate bone metabolic homeostasis through vascularization for osteoporotic bone defects. Biomaterials 2024, 311, 122699. [Google Scholar] [CrossRef]
- Antunovic, F.; Tolosa, F.; Klein, C.; Ocaranza, R. Polycaprolactone-based scaffolds for guided tissue regeneration in periodontal therapy: A systematic review. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231211416. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Zheng, K.; Li, X.; Li, L.; Xu, Y. 3D polycaprolactone/gelatin-oriented electrospun scaffolds promote periodontal regeneration. ACS Appl. Mater. Interfaces 2022, 14, 46145–46160. [Google Scholar] [CrossRef]
- Khor, H.L.; Ng, K.W.; Schantz, J.-T.; Phan, T.-T.; Lim, T.C.; Teoh, S.-H.; Hutmacher, D. Poly (ε-caprolactone) films as a potential substrate for tissue engineering an epidermal equivalent. Mater. Sci. Eng. C 2002, 20, 71–75. [Google Scholar] [CrossRef]
- Chunyan, Z.; Lan, C.; Jiajia, L.; Dongwei, S.; Jun, Z.; Huinan, L. In vitro evaluation of degradation, cytocompatibility and antibacterial property of polycaprolactone/hydroxyapatite composite coating on bioresorbable magnesium alloy. J. Magnes. Alloys 2022, 10, 2252–2265. [Google Scholar] [CrossRef]
- Di Maro, M.; Pedraza, R.; Mosca Balma, A.; Gomez d’Ayala, G.; Poggetto, G.D.; Malucelli, G.; Roato, I.; Duraccio, D.; Mussano, F.; Faga, M.G. Influence of Dry-Mixing and Solvent Casting Blending Techniques on the Mechanical and Biological Behavior of Novel Biocompatible Poly (ε-caprolactone)/Alumina-Toughened Zirconia Scaffolds Obtained by 3D Printing. J. Compos. Sci. 2024, 8, 194. [Google Scholar] [CrossRef]
- Waldner, C.; Hirn, U. Modeling liquid penetration into porous materials based on substrate and liquid surface energies. J. Colloid Interface Sci. 2023, 640, 445–455. [Google Scholar] [CrossRef]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.-W.; Venkatesan, T. Surface energy and wettability of van der Waals structures. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef]
- Roato, I.; Genova, T.; Duraccio, D.; Ruffinatti, F.A.; Zanin Venturini, D.; Di Maro, M.; Mosca Balma, A.; Pedraza, R.; Petrillo, S.; Chinigò, G. mechanical and biological characterization of PMMA/Al2O3 composites for dental implant abutments. Polymers 2023, 15, 3186. [Google Scholar] [CrossRef]
- Berta, G.N.; Di Scipio, F.; Yang, Z.; Oberto, A.; Abbadessa, G.; Romano, F.; Carere, M.E.; Ceccarelli, A.; Hirsch, E.; Mognetti, B. Chemical Oral Cancerogenesis Is Impaired in PI3Kγ Knockout and Kinase-Dead Mice. Cancers 2021, 13, 4211. [Google Scholar] [CrossRef]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]
- Stringer, C.; Pachitariu, M. Cellpose3: One-click image restoration for improved cellular segmentation. bioRxiv 2024. bioRxiv:2010.579780. [Google Scholar]
- JCGM 100:2008; Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement, JCGM 100: 2008 GUM 1995 with Minor Corrections. Joint Committee for Guides in Metrology: Sèvres, France, 2008; p. 98.
- Dietrich, C.F. Uncertainty, Calibration and Probability: The Statistics of Scientific and Industrial Measurement; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Babich, H.; Sedletcaia, A.; Kenigsberg, B. In vitro cytotoxicity of protocatechuic acid to cultured human cells from oral tissue: Involvement in oxidative stress. Pharmacol. Toxicol. 2002, 91, 245–253. [Google Scholar] [CrossRef]
- Wong, P.-C.; Song, S.-M.; Tsai, P.-H.; Nien, Y.-Y.; Jang, J.S.-C.; Cheng, C.-K.; Chen, C.-H. Relationship between the surface roughness of biodegradable Mg-based bulk metallic glass and the osteogenetic ability of MG63 osteoblast-like cells. Materials 2020, 13, 1188. [Google Scholar] [CrossRef]
- De Avila, E.D.; De Molon, R.S.; Palomari Spolidorio, D.M.; de Assis Mollo Jr, F. Implications of surface and bulk properties of abutment implants and their degradation in the health of periodontal tissue. Materials 2013, 6, 5951–5966. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Poddar, D. Effect of Bulk and Surface Properties of Polymeric Scaffolds on Its Applications in Tissue Engineering. In Tailored Functional Materials for Clean and Sustainable Development; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 59–85. [Google Scholar]
- Greiner, R.; Schwarzl, F. Thermal contraction and volume relaxation of amorphous polymers. Rheol. Acta 1984, 23, 378–395. [Google Scholar] [CrossRef]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Bellini, A. Fused Deposition of Ceramics: A Comprehensive Experimental, Analytical and Computational Study of Material Behavior, Fabrication Process and Equipment Design; Drexel University: Philadelphia, PA, USA, 2002. [Google Scholar]
- Shofner, M.L.; Lozano, K.; Rodríguez-Macías, F.J.; Barrera, E.V. Nanofiber-reinforced polymers prepared by fused deposition modeling. J. Appl. Polym. Sci. 2003, 89, 3081–3090. [Google Scholar] [CrossRef]
- Puszka, A.; Sikora, J.W. New Segmented Poly (Thiourethane-Urethane) s Based on Poly (ε-Caprolactone) Diol Soft Segment: Synthesis and Characterization. Materials 2022, 15, 4940. [Google Scholar] [CrossRef]
- Carvalho, A.; Grenho, L.; Fernandes, M.H.; Daskalova, A.; Trifonov, A.; Buchvarov, I.; Monteiro, F.J. Femtosecond laser microstructuring of alumina toughened zirconia for surface functionalization of dental implants. Ceram. Int. 2020, 46, 1383–1389. [Google Scholar]
- Yang, Y.; Knust, S.; Schwiderek, S.; Qin, Q.; Yun, Q.; Grundmeier, G.; Keller, A. Protein adsorption at nanorough titanium oxide surfaces: The importance of surface statistical parameters beyond surface roughness. Nanomaterials 2021, 11, 357. [Google Scholar] [CrossRef]
- Martinez, M.A.F.; Balderrama, Í.d.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface roughness of titanium disks influences the adhesion, proliferation and differentiation of osteogenic properties derived from human. Int. J. Implant Dent. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Hyzy, S.L.; Berg, M.E.; Schneider, J.M.; Hotchkiss, K.; Schwartz, Z.; Boyan, B.D. Osteoblast lineage cells can discriminate microscale topographic features on titanium–aluminum–vanadium surfaces. Ann. Biomed. Eng. 2014, 42, 2551–2561. [Google Scholar] [CrossRef]
- Bellon, B.; Pippenger, B.; Stähli, A.; Degen, M.; Parisi, L. Cementum and enamel surface mimicry influences soft tissue cell behavior. J. Periodontal Res. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saberian, E.; Jenča, A.; Seyfaddini, R.; Jenča, A.; Zare-Zardini, H.; Petrášová, A.; Jenčová, J. Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study. Biomolecules 2024, 14, 719. [Google Scholar] [CrossRef]
- Nazarov, D.; Ezhov, I.; Yudintceva, N.; Shevtsov, M.; Rudakova, A.; Kalganov, V.; Tolmachev, V.; Zharova, Y.; Lutakov, O.; Kraeva, L. Antibacterial and osteogenic properties of Ag nanoparticles and Ag/TiO2 nanostructures prepared by atomic layer deposition. J. Funct. Biomater. 2022, 13, 62. [Google Scholar] [CrossRef]
- Riivari, S.; Närvä, E.; Kangasniemi, I.; Willberg, J.; Närhi, T. Epithelial cell attachment and adhesion protein expression on novel in sol TiO2 coated zirconia and titanium alloy surfaces. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2022, 110, 2533–2541. [Google Scholar] [CrossRef]



| Liquid | γ [mN/m] | γP [mN/m] | γD [mN/m] |
|---|---|---|---|
| Water | 72.8 | 43.7 | 29.1 |
| Di-iodomethane | 50 | 2.6 | 47.4 |
| Sample | Ra [μm] | Rq [μm] | Rsk | Rku | Rz [μm] |
|---|---|---|---|---|---|
| PCL | 0.103 ± 0.019 | 0.173 ± 0.035 | 3.273 ± 0.525 | 40.284 ± 15.079 | 0.613 ± 0.107 |
| PCL/ATZ 90/10 | 0.122 ± 0.016 | 0.234 ± 0.102 | −1.597 ± 2.772 | 85.616 ± 43.422 | 0.711 ± 0.063 |
| PCL/ATZ 80/20 | 0.106 ± 0.002 | 0.143 ± 0.007 | −0.644 ± 0.594 | 10.712 ± 9.004 | 0.641 ± 0.010 |
| PCL/ATZ 60/40 | 0.207 ± 0.001 | 0.306 ± 0.002 | −1.003 ± 0.252 | 11.909 ± 2.799 | 1.296 ± 0.015 |
| Sample | Sa [μm] | Sq [μm] | Ssk | Sku | Sz [μm] |
|---|---|---|---|---|---|
| PCL | 0.122 ± 0.021 | 0.238 ± 0.066 | 1.825 ± 2.553 | 27.887 ± 22.966 | 4.233 ± 2.141 |
| PCL/ATZ 90/10 | 0.135 ± 0.007 | 0.227 ± 0.016 | −1.049 ± 1.461 | 91.515 ± 91.120 | 13.005 ± 6.984 |
| PCL/ATZ 80/20 | 0.062 ± 0.004 | 0.102 ± 0.017 | −0.123 ± 12.575 | 1400.300 ± 1223.800 | 13.861 ± 5.640 |
| PCL/ATZ 60/40 | 0.155 ± 0.035 | 0.248 ± 0.057 | 1.515 ± 5.255 | 163.070 ± 217.170 | 14.631 ± 4.138 |
| Sample | Surface Energy: Total [mN/m] | Surface Energy: Polar [mN/m] | Surface Energy: Dispersive [mN/m] |
|---|---|---|---|
| PCL | 4.15 | 1.77 | 2.38 |
| PCL/ATZ 90/10 | 4.14 | 1.72 | 2.43 |
| PCL/ATZ 80/20 | 4.02 | 1.48 | 2.54 |
| PCL/ATZ 60/40 | 4.09 | 1.56 | 2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedraza, R.; Mosca Balma, A.; Roato, I.; Orrico, C.; Genova, T.; Baima, G.; Berta, G.N.; Giura, A.; Ribotta, L.; Duraccio, D.; et al. Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application. Polymers 2024, 16, 2521. https://doi.org/10.3390/polym16172521
Pedraza R, Mosca Balma A, Roato I, Orrico C, Genova T, Baima G, Berta GN, Giura A, Ribotta L, Duraccio D, et al. Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application. Polymers. 2024; 16(17):2521. https://doi.org/10.3390/polym16172521
Chicago/Turabian StylePedraza, Riccardo, Alessandro Mosca Balma, Ilaria Roato, Clarissa Orrico, Tullio Genova, Giacomo Baima, Giovanni Nicolao Berta, Andrea Giura, Luigi Ribotta, Donatella Duraccio, and et al. 2024. "Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application" Polymers 16, no. 17: 2521. https://doi.org/10.3390/polym16172521
APA StylePedraza, R., Mosca Balma, A., Roato, I., Orrico, C., Genova, T., Baima, G., Berta, G. N., Giura, A., Ribotta, L., Duraccio, D., Faga, M. G., & Mussano, F. (2024). Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application. Polymers, 16(17), 2521. https://doi.org/10.3390/polym16172521










