Insulin-Loaded Chitosan–Cellulose-Derivative Hydrogels: In Vitro Permeation of Hormone through Strat-M® Membrane and Rheological and Textural Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogels
2.3. Materials Characterization
2.3.1. In Vitro Pharmaceutical Availability Study
2.3.2. Comparison of Release Profiles
2.3.3. Analysis of Release Kinetics
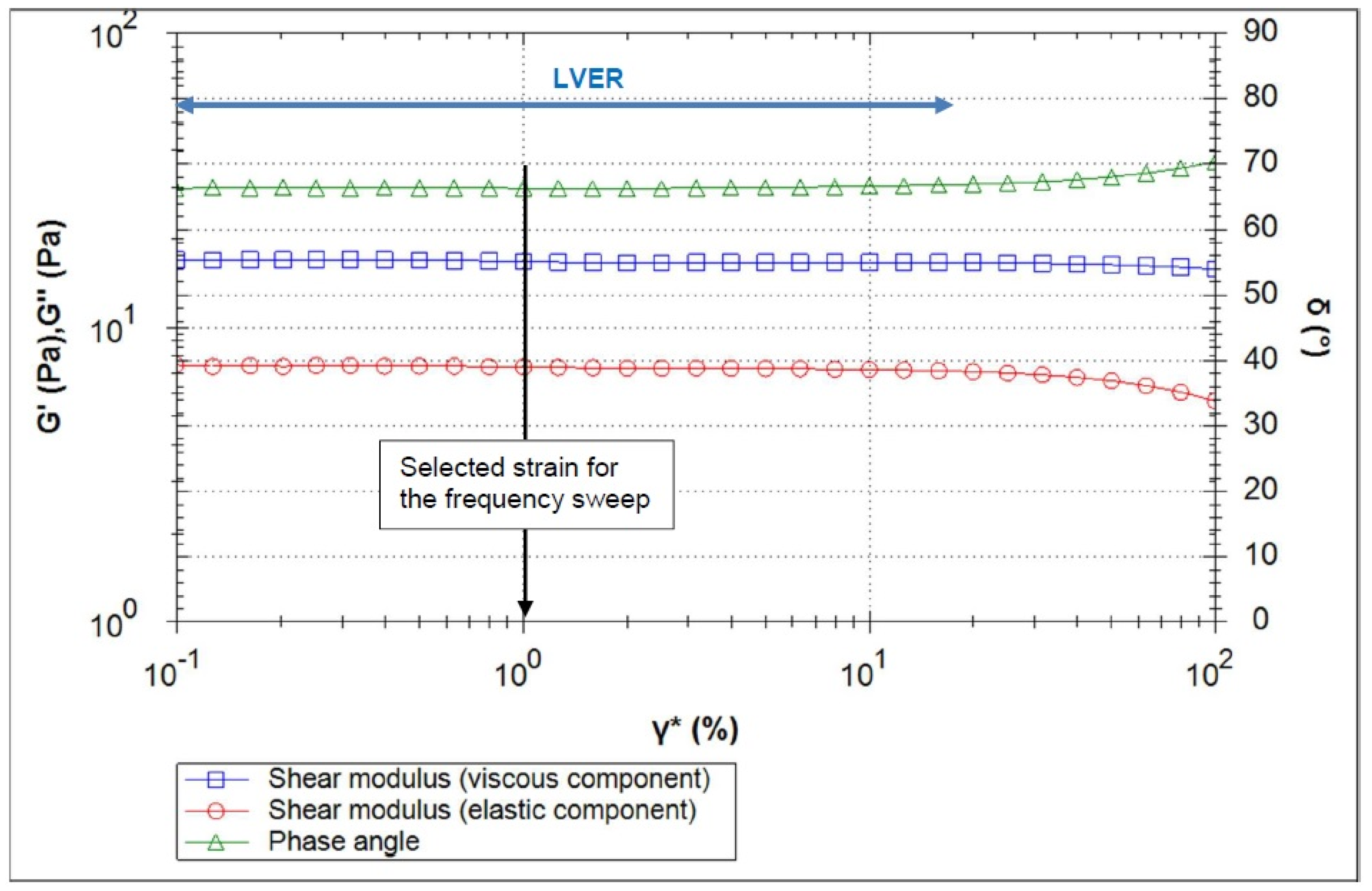
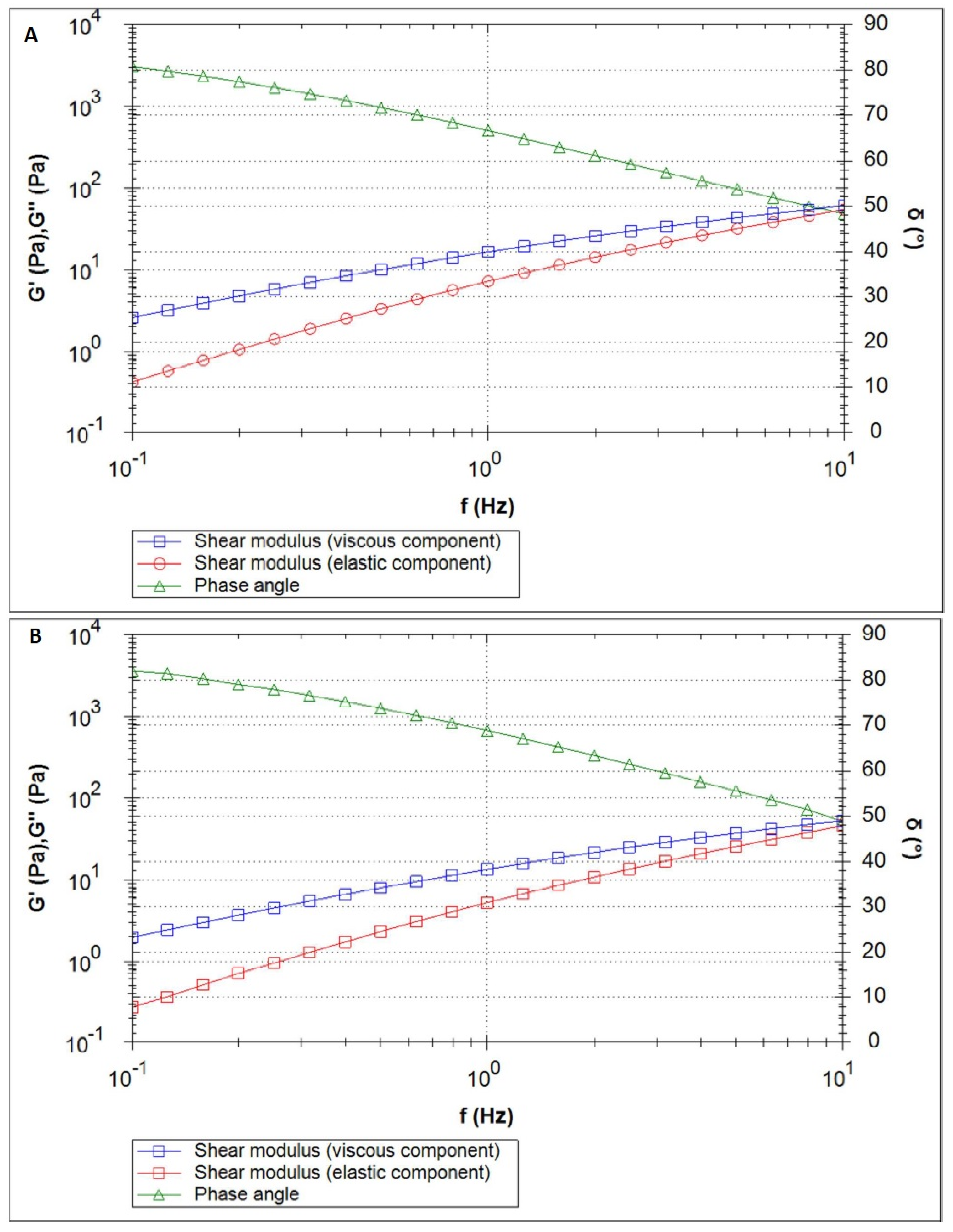
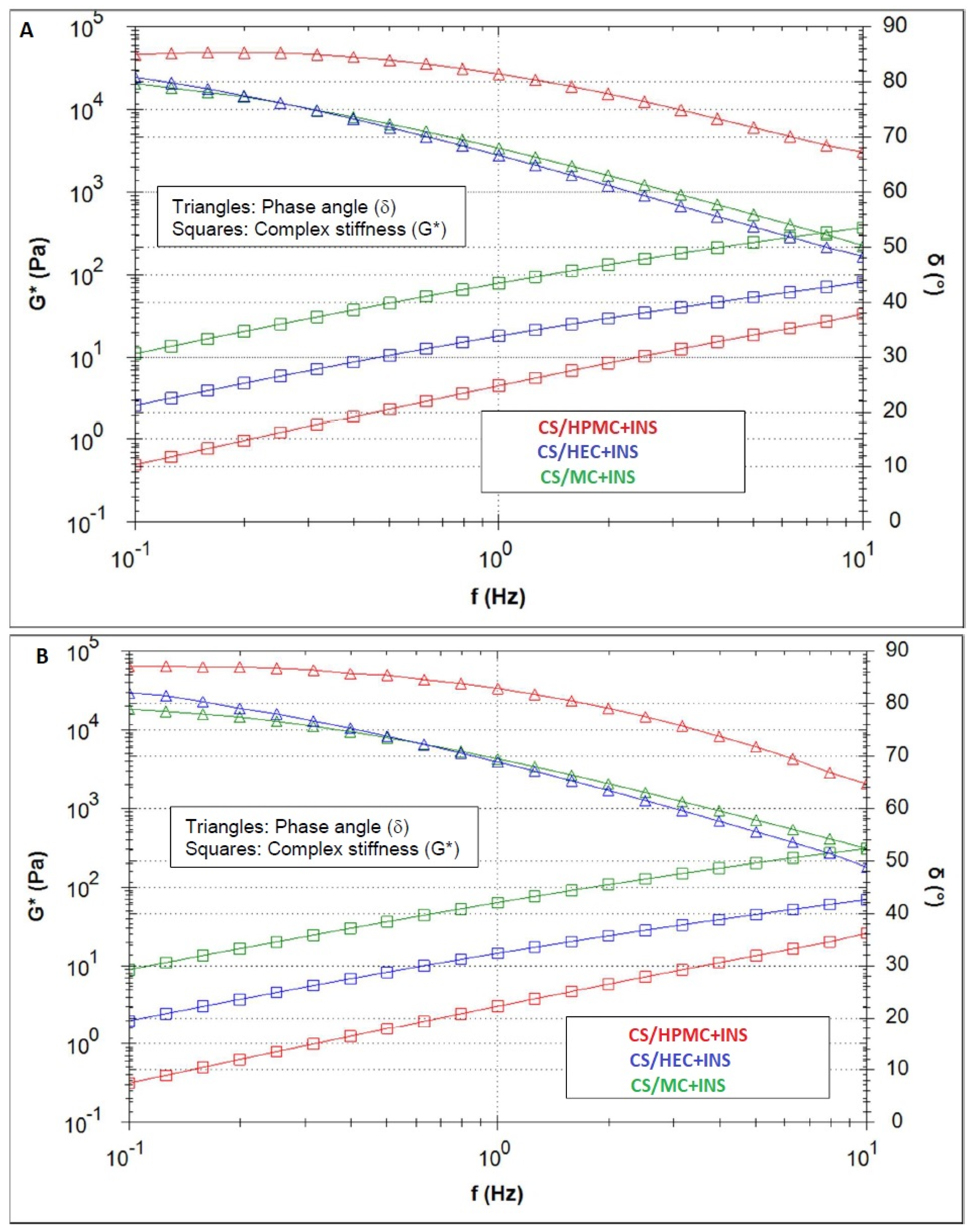
2.3.4. Analysis of Rheological Parameters
Rotational Test
Oscillation Test
2.3.5. Texture Analysis
2.3.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maheshwari, G. Chronic wounds: A rising public health concern. Wounds APAC 2024, 7, 6. [Google Scholar]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Prim. 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low. Extrem. Wounds 2024, 1. [Google Scholar] [CrossRef] [PubMed]
- Wolny, D.; Štěpánek, L.; Horáková, D.; Thomas, J.; Zapletalová, J.; Patel, M.S. Risk Factors for Non-Healing Wounds—A Single-Centre Study. J. Clin. Med. 2024, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62. [Google Scholar] [CrossRef]
- Schul, M.W.; Melin, M.M.; Keaton, T.J. Venous leg ulcers and prevalence of surgically correctable reflux disease in a national registry. J. Vasc. Surg. Venous. Lymphat. Disord. 2023, 11, 511. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, N.; Li, Z.; Xie, X.; Liu, T.; Ouyang, G. The global burden of decubitus ulcers from 1990 to 2019. Sci. Rep. 2021, 11, 21750. [Google Scholar] [CrossRef]
- Liu, Y.; Petreaca, M.; Yao, M.; Martins-Green, M. Cell and molecular mechanisms of keratinocyte function stimulated by insulin during wound healing. BMC Cell Biol. 2009, 10, 1. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Poliks, C.F.; Pilch, P.F.; Smith, B.D.; Fine, A. Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts. Endocrinology 1989, 124, 964–970. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-C.; Lan, C.-C.E. The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 4290. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.A.; Herlitz, K.; Troncoso, F.; Guevara, K.; Acurio, J.; Aguayo, C.; Godoy, A.S.; González, M. Pro-angiogenic role of insulin: From physiology to pathology. Front. Physiol. 2017, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-W.; Hung, L.-C.; Chen, Y.-C.; Wang, W.-H.; Lin, C.-Y.; Tzeng, H.-H.; Suen, J.-L.; Chen, Y.-H. Insulin Reduces Inflammation by Regulating the Activation of the NLRP3 Inflammasome. Front. Immunol. 2021, 11, 587229. [Google Scholar] [CrossRef]
- Goyal, R.; Faizy, A.F.; Siddiqui, S.S.; Singhai, M. Evaluation of TNF-α and IL-6 Levels in Obese and Non-obese Diabetics: Pre- and Postinsulin Effects. N. Am. J. Med. Sci. 2012, 4, 180. [Google Scholar] [CrossRef]
- Kaur, P.; Choudhury, D. Insulin Promotes Wound Healing by Inactivating NFkβP50/P65 and Activating Protein and Lipid Biosynthesis and alternating Pro/Anti-inflammatory Cytokines Dynamics. Biomol. Concepts 2019, 10, 11. [Google Scholar] [CrossRef]
- Almulathanon, A.A.Y.; Mohammad, J.A.; Allwash, T.A. Evaluation the effects of insulin on oxidant/antioxidant status in type 1 diabetic patients. Pharmacia 2021, 68, 699. [Google Scholar] [CrossRef]
- Song, Y.; Ding, W.; Bei, Y.; Xiao, Y.; Tong, H.-D.; Wang, L.-B.; Ai, L.-Y. Insulin is a potential antioxidant for diabetes-associated cognitive decline via regulating Nrf2 dependent antioxidant enzymes. Biomed. Pharmacother. 2018, 104, 474. [Google Scholar] [CrossRef]
- Hidekazu, Y.; Manabu, K.; Keiichi, F.; Masahiro, N.; Yoritsuna, Y.; Hiromi, M.; Koji, H.; Satoshi, O.; Keiichi, I.; Daizoh, S.; et al. Insulin Treatment Directly Restores Neutrophil Phagocytosis and Bactericidal Activity in Diabetic Mice and Thereby Improves Surgical Site Staphylococcus Aureus Infection. Infect. Immun. 2012, 80, 4409. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A. The Potential of Pharmaceutical Hydrogels in the Formulation of Topical Administration Hormone Drugs. Polymers 2022, 14, 3307. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Chen, J.; Shen, T.; Jin, T.; Zeng, B.; Li, L.; Yang, C.; Mu, Z.; Deng, H.; et al. An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds. Acta Biomater. 2022, 146, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Wilczyński, S.; Dolińska, B. Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane. Polymers 2023, 15, 3639. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Maciążek-Jurczyk, M.; Pożycka, J.; Dolińska, B. Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, D.; Liu, D.; Su, J.; Jin, Y.; Wang, D.; Han, B.; Jiang, Z.; Liu, B. Applications of Chitosan and its Derivatives in Skin and Soft Tissue Diseases. Front. Bioeng. Biotechnol. 2022, 10, 894667. [Google Scholar] [CrossRef]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Dhlamini, K.S.; Selepe, C.T.; Ramalapa, B.; Tshweu, L.; Ray, S.S. Reimagining Chitosan-Based Antimicrobial Biomaterials to Mitigate Antibiotic Resistance and Alleviate Antibiotic Overuse: A Review. Macromol. Mater. Eng. 2024, 309, 2400018. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Yan, T.; Kong, S.; Ouyang, Q.; Li, C.; Hou, T.; Chen, Y.; Li, S. Chitosan-Gentamicin Conjugate Hydrogel Promoting Skin Scald Repair. Mar. Drugs 2020, 18, 233. [Google Scholar] [CrossRef]
- Ueno, H.; Nakamura, F.; Murakami, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Evaluation effects of chitosan for the extracellular matrix production by fibroblasts and the growth factors production by macrophages. Biomaterials 2001, 22, 2125–2130. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-J.; Cen, J.-K.; Ren, Y.; Li, M.-X. Evaluation of the Anti-Inflammatory Pain Effect of Ginsenoside-Conjugated O-Carboxymethyl Chitosan Particles. Polymers 2023, 15, 4011. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cai, B.; Li, X.; Wang, X. Preparation and Application of Biomass-based Sprayable Hydrogels. Paper Biomater. 2023, 8, 1–19. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, Z.; Ding, M.; Feng, Y.; Yao, J. Advances in cellulose-metal organic framework composites: Preparation and applications. J. Mater. Chem. A 2021, 9, 23353–23363. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H. Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Xu, L.; Lu, H.; Chen, Y.; Wu, C.; Hu, P. A Self-Healing Hydrogel Based on Crosslinked Hyaluronic Acid and Chitosan to Facilitate Diabetic Wound Healing. Int. J. Biol. Macromol. 2022, 220, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wang, T.; Han, X.; Yang, S.; Lai, C.; Yuan, T.; Feng, Z.; He, N. In situ wound sprayable double-network hydrogel: Preparation and characterization. Chin. Chem. Lett. 2022, 33, 1963–1969. [Google Scholar] [CrossRef]
- Mumuni, A.M.; Calister, E.U.; Aminu, N.; Franklin, C.K.; Musiliu Oluseun, A.; Usman, M.; Abdulmumuni, B.; James, Y.O.; Ofokansi, C.K.; Anthony, A.A.; et al. Mucin-Grafted Polyethylene Glycol Microparticles Enable Oral Insulin Delivery for Improving Diabetic Treatment. Appl. Sci. 2020, 10, 2649. [Google Scholar] [CrossRef]
- Muselík, J.; Komersová, A.; Kubová, K.; Matzick, K.; Skalická, B. A Critical Overview of FDA and EMA Statistical Methods to Compare In Vitro Drug Dissolution Profiles of Pharmaceutical Products. Pharmaceutics 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Zhang, H.; Ryu, S. Elastic modulus measurement of hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Rafiee, A.; Mozafari, N.; Fekri, N.; Memarpour, M.; Azadi, A. Preparation and Characterization of a Nanohydroxyapatite and Sodium Fluoride Loaded Chitosan-Based in Situ Forming Gel for Enamel Biomineralization. Heliyon 2024, 10, e24217. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Usta, D.; Teksin, Z.S.; Tugcu-Demiroz, F. Evaluation of Emulgel and Nanostructured Lipid Carrier-Based Gel Formulations for Transdermal Administration of Ibuprofen: Characterization, Mechanical Properties, and Ex-Vivo Skin Permeation. AAPS PharmSciTech 2024, 25, 124. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Sohn, J.S.; Choi, J.S. Development of a tadalafil transdermal formulation and evaluation of its ability to in vitro transdermal permeate using Strat-M® membrane. Eur. J. Pharm. Sci. 2024, 192, 106615. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Dinçer, C.; Topuz, O.K. Modeling of Release Mechanism of Sage (Salvia fruticosa Miller) Phenolics Encapsulated in Alginate Capsule: Physicochemical Properties. J. Food. Process. Pres. 2024, 2024, 7598455. [Google Scholar] [CrossRef]
- Baggi, R.B.; Kilaru, N.B. Calculation of predominant drug release mechanism using Peppas-Sahlin model, Part-I (substitution method): A linear regression approach. Asian J. Pharm. Technol. 2016, 6, 223–230. [Google Scholar] [CrossRef]
- Meena, P.; Singh, P.; Warkar, S.G. Fabrication and Evaluation of Stimuli-Sensitive Xanthan Gum-Based Hydrogel as a Potential Carrier for a Hydrophobic Drug Ibuprofen. Colloid. Polym. Sci. 2024, 302, 377–391. [Google Scholar] [CrossRef]
- Parfenyuk, E.V.; Dolinina, E.S.; Kraev, A.S. Synthesis and study of organo-modified silica based hydrogels: Rheological properties and drug release kinetics. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35418. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef] [PubMed]
- Ortan, A.; Dinu-Parvu, C.; Ghica, M.V.; Popescu, L.M.; Ionita, L. Rheological study of a liposomal hydrogel based on carbopol. Rom. Biotechnol. Lett. 2011, 16, 47–54. [Google Scholar]
- Forge, V.; Mathevon, C.; Pignon, F. Thixotropic α-lactalbumin hydrogels, method for preparing same and uses thereof. US Patent 9,724,423, 10 April 2012. [Google Scholar]
- Cid, Y.P.; Pedrazzi, V.; De Sousa, V.P.; Pierre, M.B.R. In Vitro Characterization of Chitosan Gels for Buccal Delivery of Celecoxib: Influence of a Penetration Enhancer. AAPS PharmSciTech 2011, 13, 101–111. [Google Scholar] [CrossRef]
- do Amaral Sobral, P.J.; Gebremariam, G.; Drudi, F.; De Aguiar Saldanha Pinheiro, A.C.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Rheological and Viscoelastic Properties of Chitosan Solutions Prepared with Different Chitosan or Acetic Acid Concentrations. Foods 2022, 11, 2692. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E. Dynamic Rheological Properties of Concentrated Chitosan Soltions. Appl. Rheol. 2004, 14, 140–147. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Ghannam, M.; Alsayyed Ahmed, K.; Djama, M. The Effect of Biopolymer Chitosan on the Rheology and Stability of Na-Bentonite Drilling Mud. Polymers 2021, 13, 3361. [Google Scholar] [CrossRef]
- Tovar, C.A.; Gómez-Guillén, M.C.; Montero, M.P. Effect of Chitosan Concentration on the Rheological Properties of Acetic and Lactic Acid Solutions. In Proceedings of the Iberian Meeting on Rheology (IBEREO 2019); Springer Proceedings in Materials. Springer: Berlin/Heidelberg, Germany, 2020; pp. 20–24. [Google Scholar] [CrossRef]
- Perez Bravo, J.J.; Francois, N.J. Chitosan/starch matrices prepared by ionotropic gelation: Rheological characterization, swelling behavior and potassium nitrate release kinetics. J. Polym. Environ. 2020, 28, 2681–2690. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Tugcu-Demiröz, F. Vaginal delivery of benzydamine hydrochloride through liposomes dispersed in mucoadhesive gels. Chem. Pharm. Bull. 2017, 65, 660–667. [Google Scholar] [CrossRef]
- Sita, V.G.; Vavia, P.R. Bromocriptine Nanoemulsion-Loaded Transdermal Gel: Optimization Using Factorial Design, In Vitro and In Vivo Evaluation. AAPS Pharm. Sci. Tech. 2020, 21, 80. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.; de Freitas, O.; Lara, E.H. Semisolid systems containing propolis for the treatment of periodontal disease: In vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A.C.C. Design of a Dual Nanostructured Lipid Carrier Formulation Based on Physicochemical, Rheological, and Mechanical Properties. J. Nanopart. Res. 2013, 15, 1993. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Yao, H.; Wu, M.; Lin, L.W.; Wu, Z.L.; Bae, M.J.; Park, S.M.; Wang, S.L.; Zhang, W.; Gao, J.F.; Wang, D.G.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio 2022, 16, 100429. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.M.; Shen, T.T.; Wu, D.Y. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Zanchetta, F.C.; De Wever, P.; Morari, J.; Gaspar, R.C.; Prado, T.P.D.; De Maeseneer, T.; Cardinaels, R.; Araújo, E.P.; Lima, M.H.M.; Fardim, P. In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications. Bioengineering 2024, 11, 168. [Google Scholar] [CrossRef]
- Hardman, D.; George Thuruthel, T.; Iida, F. Self-Healing Ionic Gelatin/Glycerol Hydrogels for Strain Sensing Applications. NPG Asia Mater. 2022, 14, 11. [Google Scholar] [CrossRef]
- dos Santos Carvalho, J.D.; Rabelo, R.S.; Hubinger, M.D. Thermo-rheological properties of chitosan hydrogels with hydroxypropyl methylcellulose and methylcellulose. Int. J. Biol. Macromol. 2022, 209, 367–375. [Google Scholar] [CrossRef]
- Zgoda, M.M.; Kołodziejska, J. Effect of rheological parameters on pharmaceutical availability of ketoprofen from hydrogel products made on Carbopol base. Polim. Med. 2006, 36, 11–25. [Google Scholar] [PubMed]
- Sanad, W.G.; Bader, Q.A.; Mahdi, F.M.S.; Kabbani, F. Formulation and in Vitro Evaluation of Moxifloxacin-Lidocaine Base as A Topical Hydrogel Dressing. J. Nat. Sc. Biol. Med. 2023, 14, 152. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Jadach, B.; Ancukiewicz, K.; Gadziński, P.; Wagner, D.; Białas, W. Badania reologiczne i analiza tekstury termowrażliwych hydrożeli dopochwowych z chlorowodorkiem benzydaminy. Farm. Współczesna 2018, 11, 72–82. [Google Scholar]
- Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The Role of Viscosity on Skin Penetration from Cellulose Ether-Based Hydrogels. Ski. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Mondal, B.; Sharma, A.; Murugesan, P.; Arora, M.; Saini, D.; Mandal, D.; Ghosh, D. A self-powered, anti-bacterial, moist-wound dressing made with electroactive free-flowing hydrogel particles, encourage faster wound closure. Chem. Eng. J. 2024, 494, 153063. [Google Scholar] [CrossRef]
- Markovic, M.D.; Spasojevic, P.M.; Pantic, O.J.; Savic, S.I.; Savkovic, M.M.S.; Panic, V.V. Status and future scope of hydrogels in wound healing. J. Drug Deliv. Sci. Technol. 2024, 98, 105903. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Deng, Y.; Zou, G.; Xu, J. Effects of topical insulin on wound healing: A meta-analysis of animal and clinical studies. Endocr. J. 2021, 68, 969–979. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J. Effects of Topical Insulin on Wound Healing: A Review of Animal and Human Evidences. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 719. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Przybyła, M.; Wójcik, W.; Birówka, K.; Majczyna, M.; Dolińska, B. Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing. Med. Sci. Forum 2023, 21, 17. [Google Scholar] [CrossRef]
- Przybyła, M.; Dolińska, B.; Ostróżka-Cieślik, A. Research Progress on Insulin Dressings to Promote Wound Healing. Eng. Proc. 2023, 56, 21. [Google Scholar] [CrossRef]
- Azevedo, F.; Pessoa, A.; Moreira, G.; Dos Santos, M.; Liberti, E.; Araujo, E.; Carvalho, C.; Saad, M.; Lima, M.H. Effect of Topical Insulin on Second-Degree Burns in Diabetic Rats. Biol. Res. Nurs. 2016, 18, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, M.H.S.; Yassin, G.E.; Ghorab, D.M.; Morsi, N.M. Insulin Mucoadhesive Liposomal Gel for Wound Healing: A Formulation with Sustained Release and Extended Stability Using Quality by Design Approach. AAPS PharmSciTech 2019, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Meis, C.M.; Salzman, E.E.; Maikawa, C.L.; Smith, A.A.A.; Mann, J.L.; Grosskopf, A.K.; Appel, E.A. Self-Assembled, Dilution-Responsive Hydrogels for Enhanced Thermal Stability of Insulin Biopharmaceuticals. ACS Biomater. Sci. Eng. 2021, 13, 4221–4229. [Google Scholar] [CrossRef]




| Kinetics Models | Equation | Parameters Definition |
|---|---|---|
| Zero-order | f = k0 × t | f, amount of the drug released; t, time; k0, reaction rate coefficient. |
| First-order | f = 100 × [1 − e−k1 × t] | f, amount of the drug released; t, time; k1, rate constant. |
| Higuchi | f = kH × t0.5 | f, amount of the drug released; t, time; kH, dissolution constant. |
| Korsmeyer–Peppas | f = kKP × tn | f, amount of the drug released; t, time; kKP, constant depicting the experimental parameters based on geometry and dosage forms; n, release exponent; n ≤ 0.45 Fickian diffusion; 0.45 < n < 0.89 non-Fickian transport; n = 0.89 case II (relaxation) transport; n > 0.89 super case II transport mechanism. |
| Peppas–Sahlin | f = kPS1 × tm + kPS2 × t(2 × m) | f, amount of the drug released; t, time; kPS1, Peppas–Sahlin release constant (constant for Fickian diffusion); kPS2, constant for case II relaxational mechanism; m, diffusion exponent. |
| Hixson–Crowell | f = 100 × [1 − (1 − kHC × t)3] | f, amount of the drug released; t, time; kHC, Hixson–Crowell release constant. |
| Hopfenberg | f = 100 × [1 − (1 − kHB × t)n] | f, amount of the drug released; t, time; n, release exponent; kHB, Hopfenberg release constant. |
| Baker–Lonsdale | 3/2 × [1 − (1 − F/100)2/3] − F/100= kBL × t | f, amount of the drug released; t, time; kBL, Baker–Lonsdale release constant. |
| Rheological Models | Equation | Parameters Definition |
|---|---|---|
| Ostwald–de Waele | τ = K × n | τ, shear stress [Pa]; K, consistency coefficient [Pa]1/2[s]n; , shear rate [s−1]; n, flow behavior index. |
| Herschel–Bulkley | τ = τ0 + K × n | τ, shear stress [Pa]; τ0, yield stress or yield point; K, consistency coefficient [Pa]1/2[s]n; , shear rate [s−1]; n, flow behavior index. |
| Bingham | τ = τ0 + η × | τ, shear stress [Pa]; τ0, yield stress or yield point; γ, viscosity [Pa·s]; , shear rate [s−1]. |
| Casson | τ0.5 = τ00.5 + η0.5 × 0.5 | τ, shear stress [Pa]; τ0, yield stress or yield point; γ, viscosity [Pa·s]; , shear rate [s−1]. |
| Formula Code | f1 | f2 | Dissolution Profile |
|---|---|---|---|
| CS/MC-INS | |||
| vs. CS/HEC-INS | 6.17 | 84.21 | Similar |
| CS/MC-INS | |||
| vs. CS/HPMC-INS | 24.02 | 58.95 | Dissimilar |
| CS/HEC-INS | |||
| vs. CS/HPMC-INS | 18.34 | 64.67 | Dissimilar |
| Kinetics Models | Hydrogel | Parameters | R2 Adjusted | AIC | MSC |
|---|---|---|---|---|---|
| Zero-order | CS/MC-INS | k0 = 0.106 | 0.9398 | 99.6577 | 2.5555 |
| CS/HEC-INS | k0 = 0.113 | 0.9531 | 98.1674 | 2.8163 | |
| CS/HPMC-INS | k0 = 0.132 | 0.9332 | 109.6961 | 2.4551 | |
| First-order | CS/MC-INS | k1 = 0.001 | 0.9768 | 82.5242 | 3.5073 |
| CS/HEC-INS | k1 = 0.001 | 0.9820 | 80.9338 | 3.7737 | |
| CS/HPMC-INS | k1 = 0.002 | 0.9780 | 89.7122 | 3.5653 | |
| Higuchi | CS/MC-INS | kH = 1.849 | 0.9139 | 106.0903 | 2.1981 |
| CS/HEC-INS | kH = 1.955 | 0.8975 | 112.2405 | 2.0344 | |
| CS/HPMC-INS | kH = 2.297 | 0.9064 | 115.7729 | 2.1175 | |
| Korsmeyer–Peppas | CS/MC-INS CS/HEC-INS CS/HPMC-INS | kKP = 0.434 n = 0.757 kKP = 0.352 n = 0.803 kKP =0.536 n = 0.758 | 0.9725 0.9720 0.9650 | 86.4733 89.8124 98.9581 | 3.2879 3.2804 3.0517 |
| Peppas–Sahlin | CS/MC-INS CS/HEC-INS CS/HPMC-INS | kPS1 = −20.419 kPS2 = 11.999 m = 0.175 kPS1 = −15.056 kPS2 = 7.978 m = 0.206 kPS1 = −30.198 kPS2 = 18.100 m = 0.165 | 0,9898 0.9865 0.9858 | 69.5147 77.4582 83.5061 | 4.2301 3.9668 3.9101 |
| Hixson–Crowell | CS/MC-INS CS/HEC-INS CS/HPMC-INS | kHC = 0.000 kHC = 0.000 kHC = 0.001 | 0.9678 0.9761 0.9689 | 88.3766 86.0249 95.9302 | 3.1822 3.4909 3.2199 |
| Hopfenberg | CS/MC-INS CS/HEC-INS CS/HPMC-INS | kHB = 0.000 n = 275.349 kHB = 0.000 n = 645.651 kHB = 0.0 n = 1114.896 | 0.9752 0.9809 0.9766 | 84.5870 82.9537 91.7268 | 3.3927 3.6615 3.4534 |
| Baker–Lonsdale model | CS/MC-INS | kBL = 0.0 | 0.8975 | 109.2393 | 2.0232 |
| CS/HEC-INS | kBL = 0.0 | 0.8780 | 115.3791 | 1.8601 | |
| CS/HPMC-INS | kBL = 0.0 | 0.8847 | 119.5170 | 1.9095 |
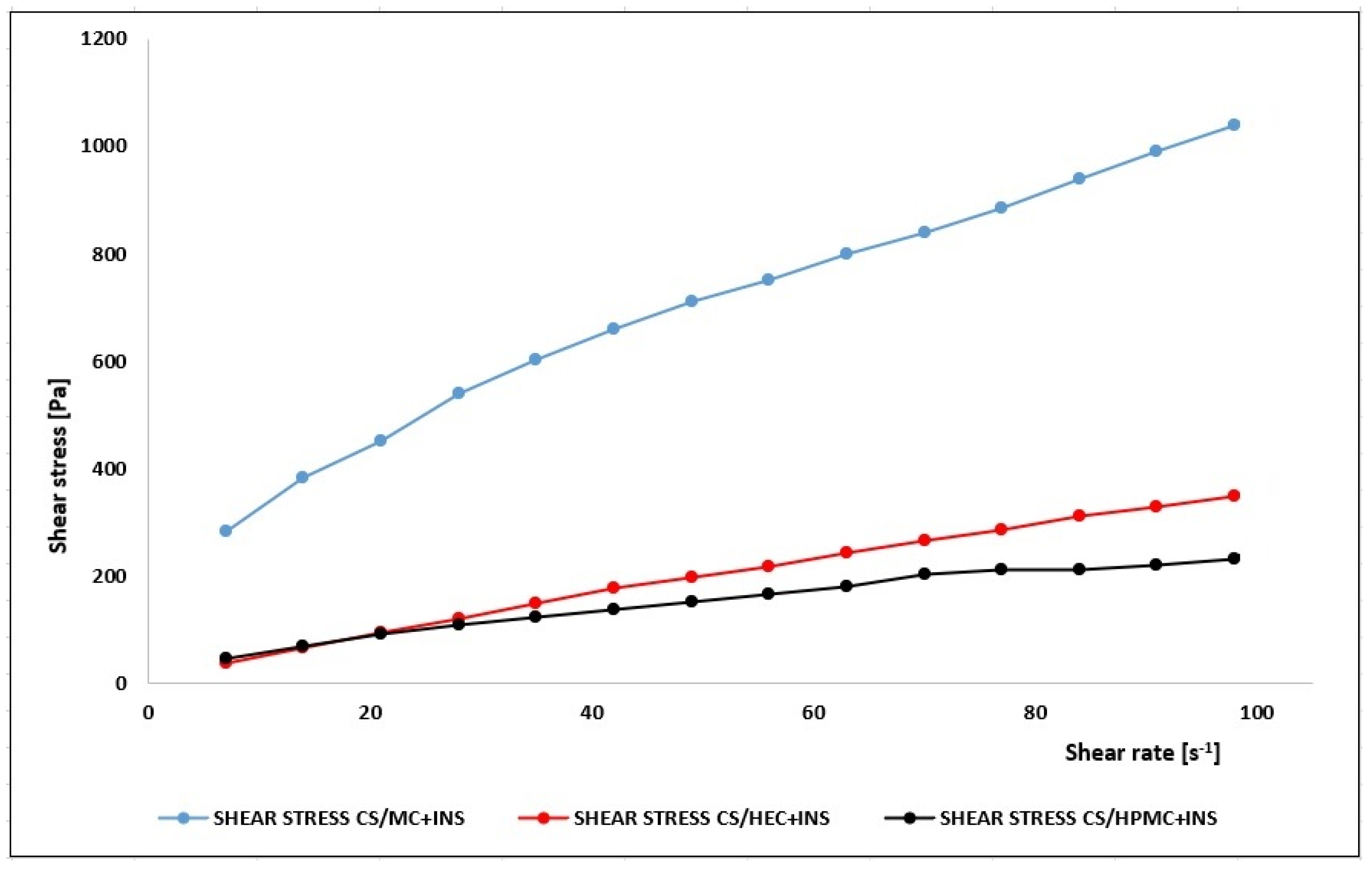
| Hydrogel | η (30 s−1) [Pa·s] | η (50 s−1) [Pa·s] | η (100 s−1) [Pa·s] |
|---|---|---|---|
| CS/MC + INS | 14.0 ± 0.201 | 8.08 ± 0.423 | 5.84 ± 0.467 |
| CS/HEC + INS | 5.81 ± 0.343 | 3.22 ± 0.190 | 2.68 ± 0.201 |
| CS/HPMC + INS | 4.23 ± 0.131 | 2.94 ± 0.153 | 2.12 ± 0.303 |
| Hydrogel | Herschel–Bulkley | Ostwald–de Waele | Bingham | Casson | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| τ0 | N | K | R2 | n | K | R2 | τ0 | R2 | τ0 | R2 | |
| CS/MC + INS | 90.40 | 0.596 | 60.90 | 0.998 | 0.495 | 104.70 | 0.996 | 300.2 | 0.985 | 145.3 | 0.995 |
| CS/HEC + INS | 0.05 | 0.930 | 4.98 | 0.999 | 0.930 | 4.98 | 0.999 | 12.3 | 0.998 | 0.792 | 0.999 |
| CS/HPMC + INS | 11.00 | 0.694 | 9.60 | 0.997 | 0.621 | 13.90 | 0.996 | 47.8 | 0.979 | 17.0 | 0.991 |
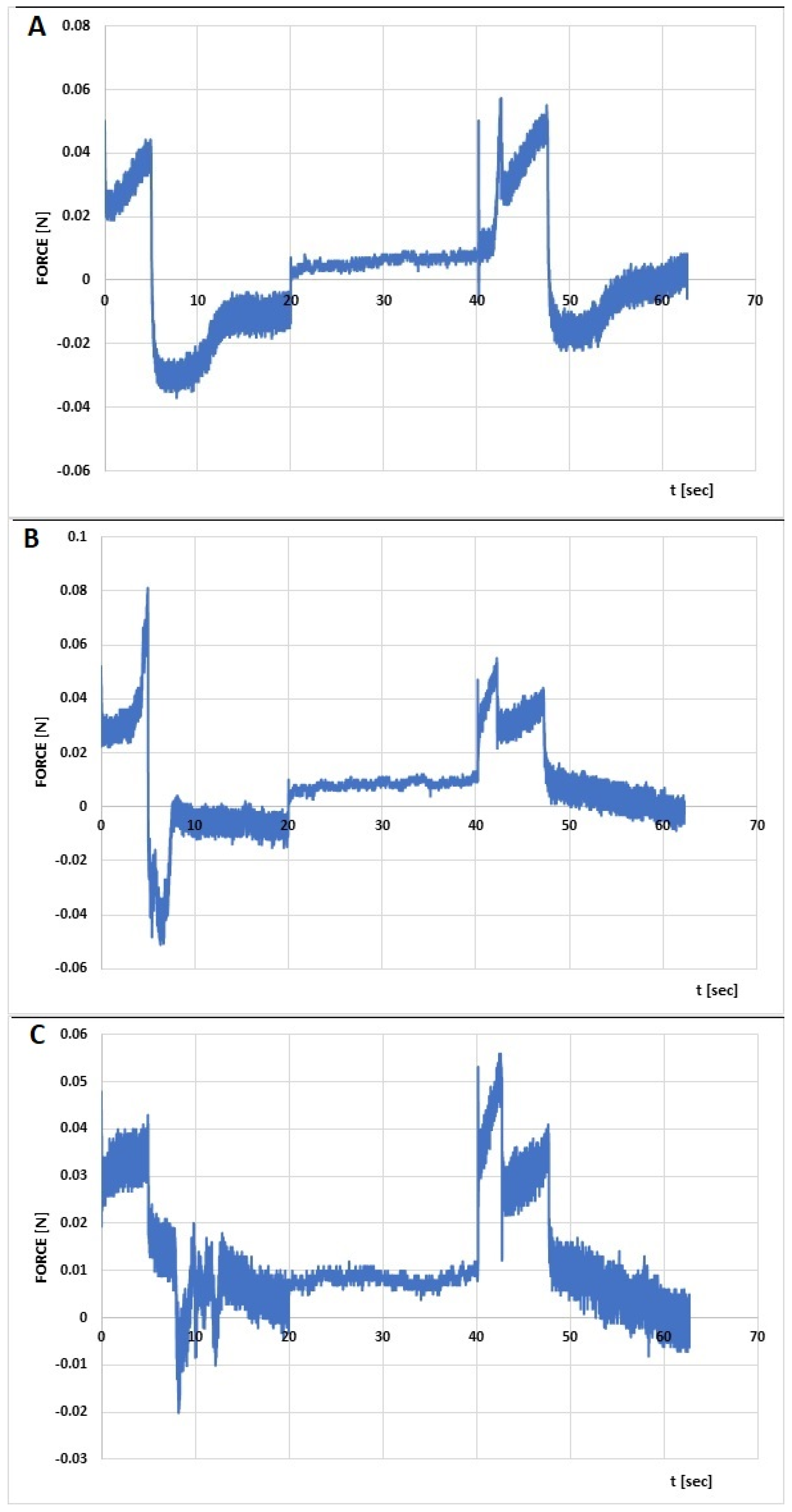
| Formula Code | Relaxation [%] | Hardness 1 [N] | Hardness 2 [N] | Cohesiveness | Adhesiveness [mJ] | Elasticity |
|---|---|---|---|---|---|---|
| CS/MC + INS | 89.1 | 0.050 | 0.057 | 1.373 | 0.3 | 0.952 |
| CS/HEC + INS | 82.2 | 0.081 | 0.055 | 1.478 | 0.2 | 0.747 |
| CS/HPMC + INS | 49.4 | 0.048 | 0.056 | 1.000 | 0.1 | 1.046 |
| p | <0.05 | <0.05 | NS | <0.05 | <0.05 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A.; Strasser, C.; Dolińska, B. Insulin-Loaded Chitosan–Cellulose-Derivative Hydrogels: In Vitro Permeation of Hormone through Strat-M® Membrane and Rheological and Textural Analysis. Polymers 2024, 16, 2619. https://doi.org/10.3390/polym16182619
Ostróżka-Cieślik A, Strasser C, Dolińska B. Insulin-Loaded Chitosan–Cellulose-Derivative Hydrogels: In Vitro Permeation of Hormone through Strat-M® Membrane and Rheological and Textural Analysis. Polymers. 2024; 16(18):2619. https://doi.org/10.3390/polym16182619
Chicago/Turabian StyleOstróżka-Cieślik, Aneta, Claire Strasser, and Barbara Dolińska. 2024. "Insulin-Loaded Chitosan–Cellulose-Derivative Hydrogels: In Vitro Permeation of Hormone through Strat-M® Membrane and Rheological and Textural Analysis" Polymers 16, no. 18: 2619. https://doi.org/10.3390/polym16182619
APA StyleOstróżka-Cieślik, A., Strasser, C., & Dolińska, B. (2024). Insulin-Loaded Chitosan–Cellulose-Derivative Hydrogels: In Vitro Permeation of Hormone through Strat-M® Membrane and Rheological and Textural Analysis. Polymers, 16(18), 2619. https://doi.org/10.3390/polym16182619








