1. Introduction
The significant aerothermal effects of high-speed aircraft, such as rockets and so on, create an urgent demand for high-temperature structural materials. As one of the primary classes of high-temperature resin matrix composites, thermosetting polyimide (PI) resin matrix composites offer outstanding advantages, including excellent high-temperature stability, lightweight properties, and high strength [
1,
2]. Consequently, they are widely used in military and civilian engineering applications as well as in aerospace transportation vehicles. However, in recent years, the continuous increase in the flight speed of aerospace vehicles has led to a sharp rise in the temperature of the composites on the fuselage’s surface during high-speed flight. The elevated temperature and thermal stress can result in changes or even damage to the material structure, significantly affecting its mechanical properties [
3]. To address the requirements for structural strength and thermal deformation control of aerospace aircraft under aerodynamic heating (high-temperature) conditions, it is essential to conduct research on high-performance resin matrix composites with high-temperature resistance [
4,
5,
6,
7].
Research on the thermal oxidation stability, processing performance, and comprehensive mechanical properties of thermosetting polyimide composite materials has received wide attention [
8,
9]. Various modification methods, including chemical structure modification methods, physical doping modification methods, and other approaches, have been developed to enhance thermal oxidation stability. Chemical structure modification is the most direct method to improve polyimide’s thermal oxidation stability. However, the improvement is limited based on the molecular structure, and the introduction of the carbon borane molecular backbone unit structure has been reported as the most effective modification method to enhance polyimide resin’s thermal oxidation stability [
10], despite the high cost of monomer preparation. Physical doping modification methods, such as incorporating alumina [
11], carbon nanotubes [
12,
13], graphene [
14,
15], inorganic compounds, and polyimide compounds, have been reported as low-cost and high-efficiency approaches to enhance heat resistance. However, the increase in thermal oxidation stability is relatively limited, making them suitable for specific scenarios with functional requirements. Despite efforts to enhance the temperature tolerance of polyimide materials, the practical engineering applications may still face limitations. For instance, oxidation at high temperatures can result in chain fracture of polyimide molecules and thermal degradation of the resin. Prolonged exposure to high-temperature environments may lead to a decline in the mechanical properties of polyimide composite resin-based materials [
16]. The long-term reuse at high temperatures is a fundamental requirement for aviation structural composite materials. Currently, the aviation industry is testing resistance at 350 °C and 400 °C, which imposes more stringent demands on the long-term thermal oxygen aging ability and toughness of matrix resin. Therefore, the thermal oxidation stability (TOS) of polyimide composites has become a key index for evaluating their performance. Studying the thermo-oxidative aging process of polyimide is of great significance to understand the aging characteristics of polyimide.
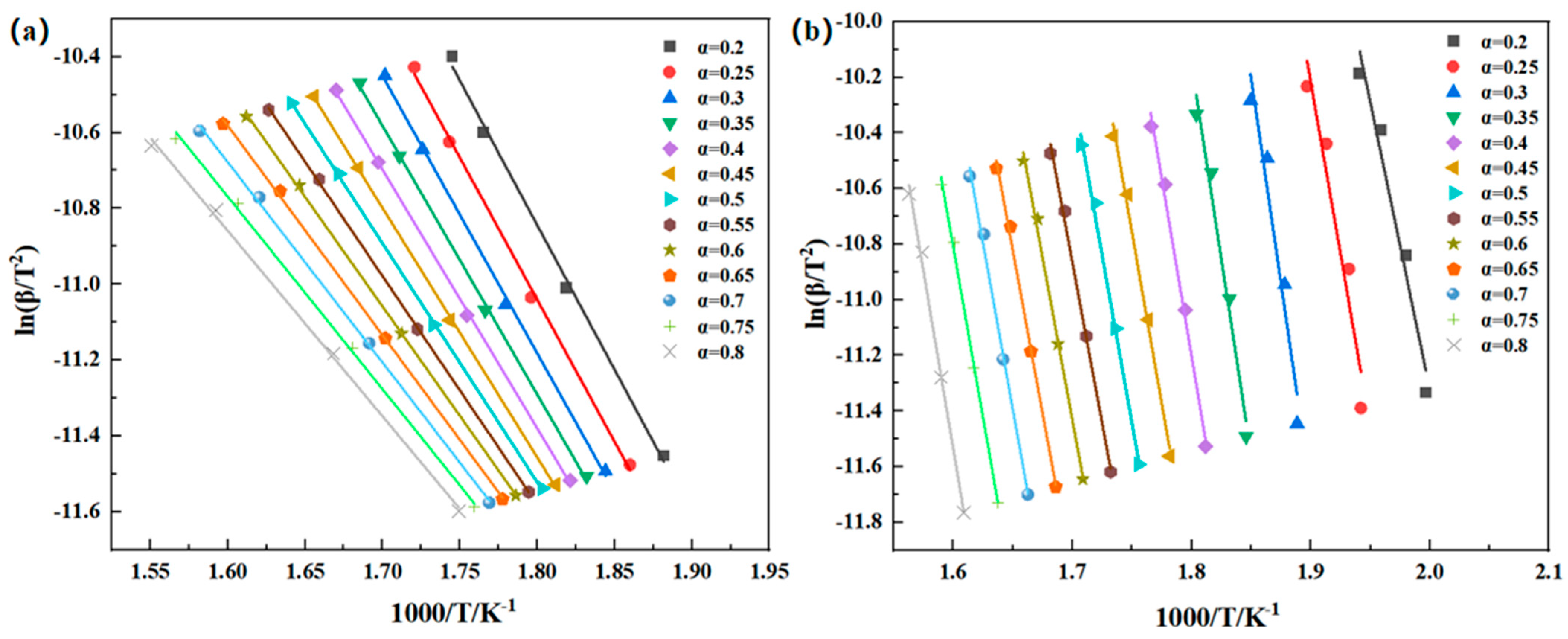
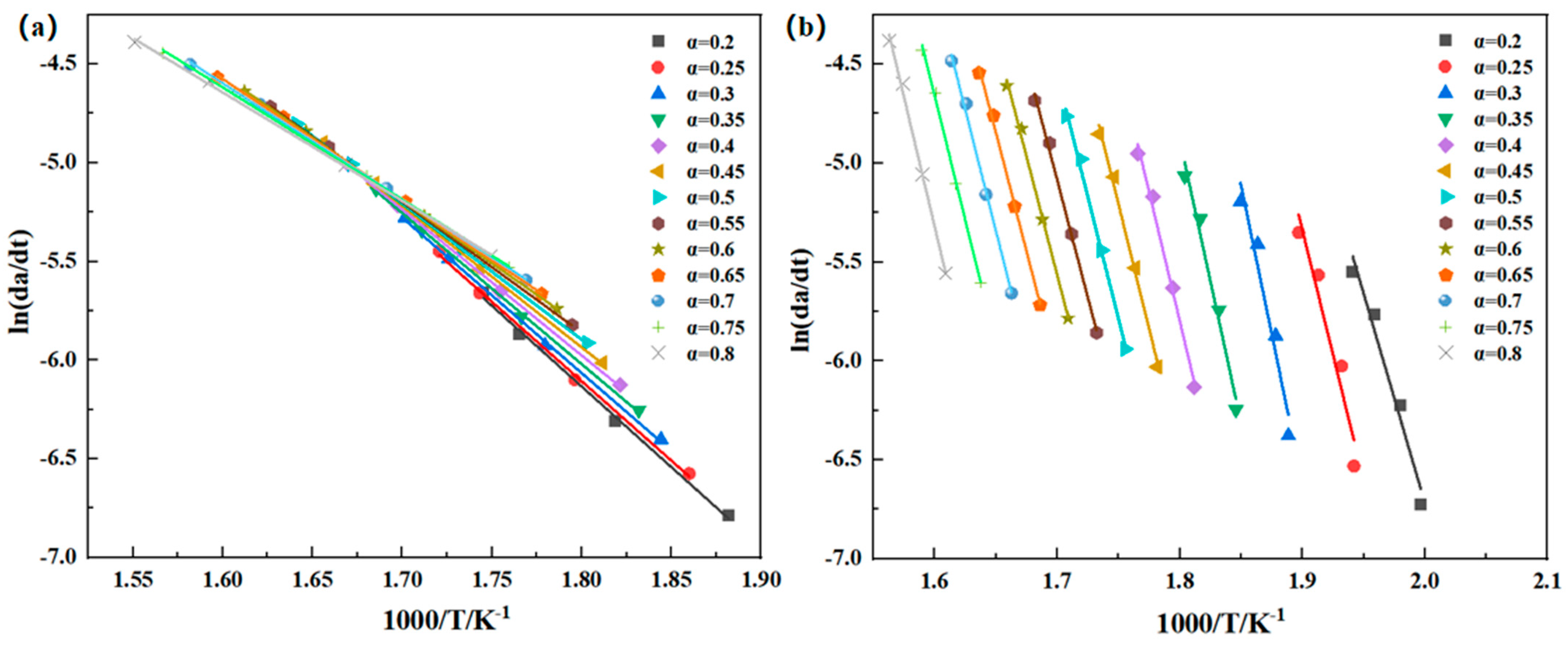
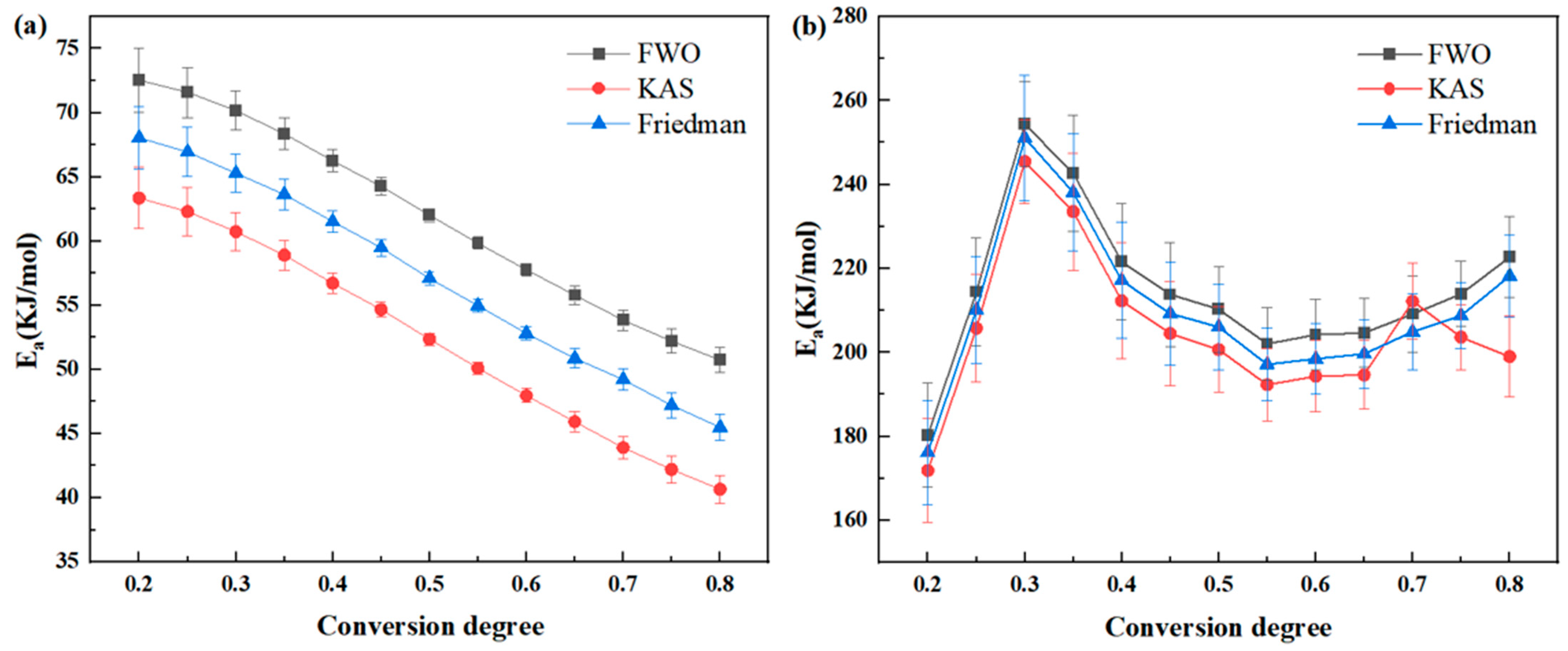
Thermal oxidative degradation of thermosetting polyimide resins is very complex, involving multiple chemical reaction processes. Currently, there is no unified theoretical model established for this process. The use of thermal degradation kinetics has emerged as an effective tool for studying resin degradation in recent years, with commonly used methods including the Kissinger, Friedman, Flynn–Wall–Ozawa, and Kissinger–Akahira–Sunose methods, and others [
17]. The Kissinger method [
18,
19] is favored in the study of the degradation behavior of polyimide. Chang et al. [
20] conducted a detailed study on the thermal oxidation degradation behavior of siloxide-containing structured polyimide. Through this method, the thermal degradation mechanism can be better understood by analyzing the polyimide degradation process. Gu et al. [
21] utilized the Kissinger method to investigate the kinetic parameters of the thermal degradation of aromatic polyimide, aiming to comprehensively understand the thermal degradation mechanism and establish a foundation for its high-temperature applications. The Kissinger method may not be suitable when the resin decomposition involves multiple step reactions and multiple peaks, as the temperature point corresponding to the peak needs to be selected during the calculation process. The Friedman method, a differential method commonly used to determine the kinetic parameters of thermal decomposition reactions, has been employed more frequently [
22,
23] in the degradation studies of phenolic resin and polyether ether ketone resin. The Kissinger–Akahira–Sunose method, similar to the Flynn–Wall–Ozawa method, is an integration method. When using these two methods to determine the thermal decomposition kinetic parameters, there is no need to understand the thermal decomposition mechanism or introduce thermal loss differential data. The two methods are often used together to verify the reliability of the calculated data [
24,
25]. In order to speculate the thermal degradation mechanism of the resin, people often use the Coats-Redfern method to compare the test data with the standard curve to predict the chemical reaction of the resin degradation process [
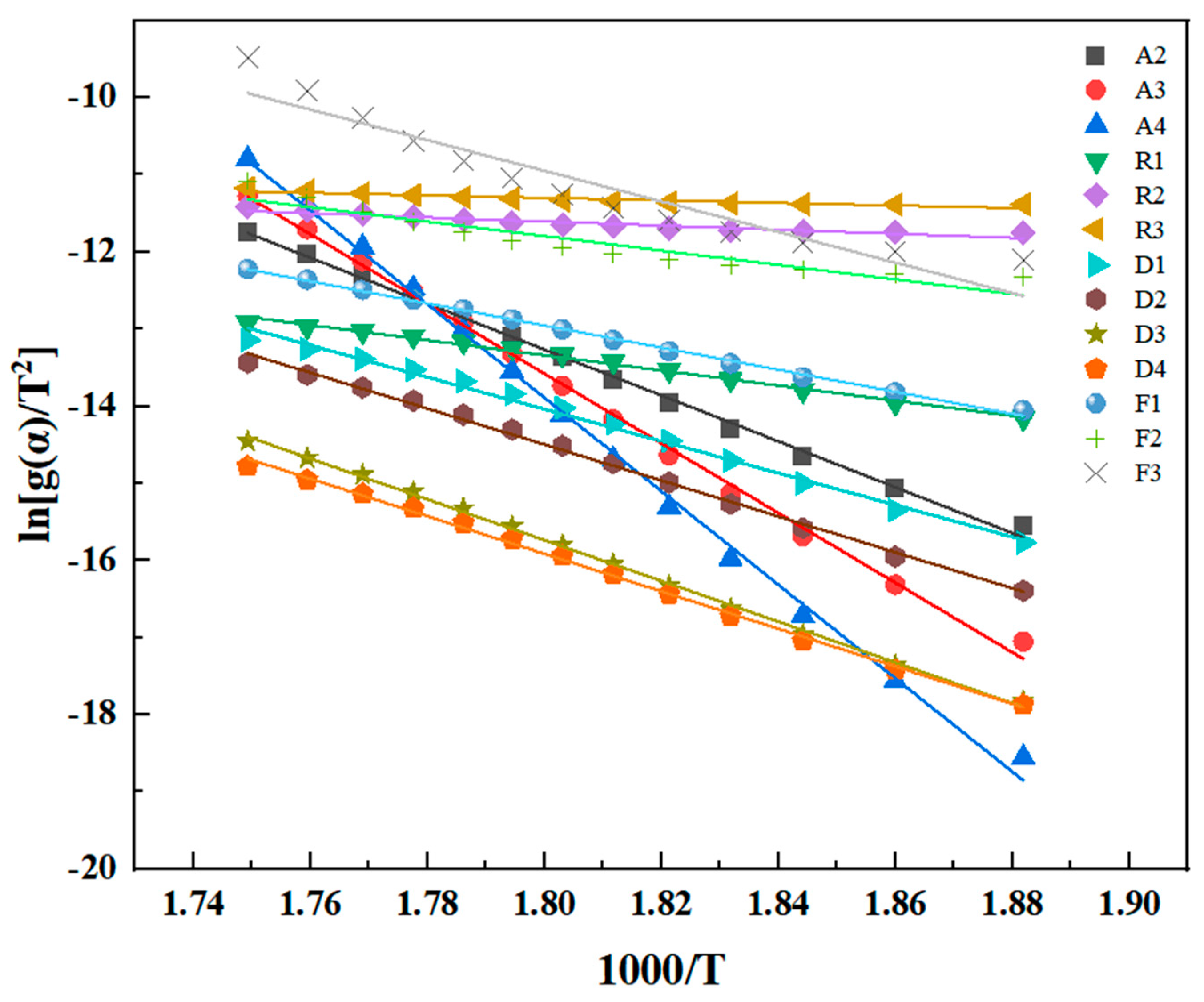
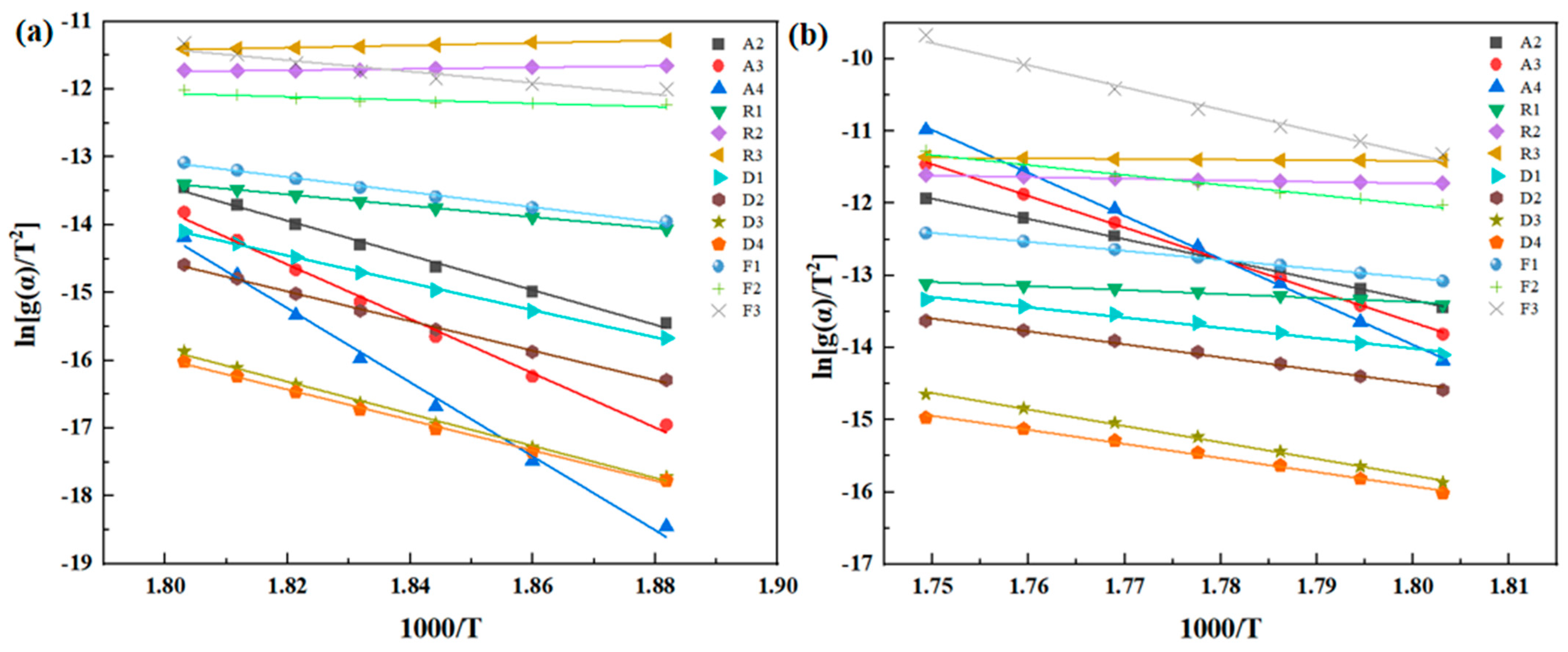
26].
Phenylacetylene group-sealing end polyimide resin (PMR350) is a newly explored high-performance polyimide resin with improved temperature resistance. The analysis of its thermal properties has not been reported. To evaluate its temperature resistance, TGA and isothermal oxygen aging methods were used to test the thermal oxidative degradation dynamics based on the classical theories of Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose, and Friedman, and analysis. This is the first systematic work to analyze the thermal stability characteristics of PMR350 resin, providing key theoretical and data support for understanding the thermal stability modification mechanism of PMR350 and its engineering application.
2. Experiment and Characterization
2.1. Experimental Material
Phenylene-capped polyimide prepolymer (PMR350), a brown–green liquid, was developed by the Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences. The specific parameters are detailed in
Table 1.
2.2. Material Characterization
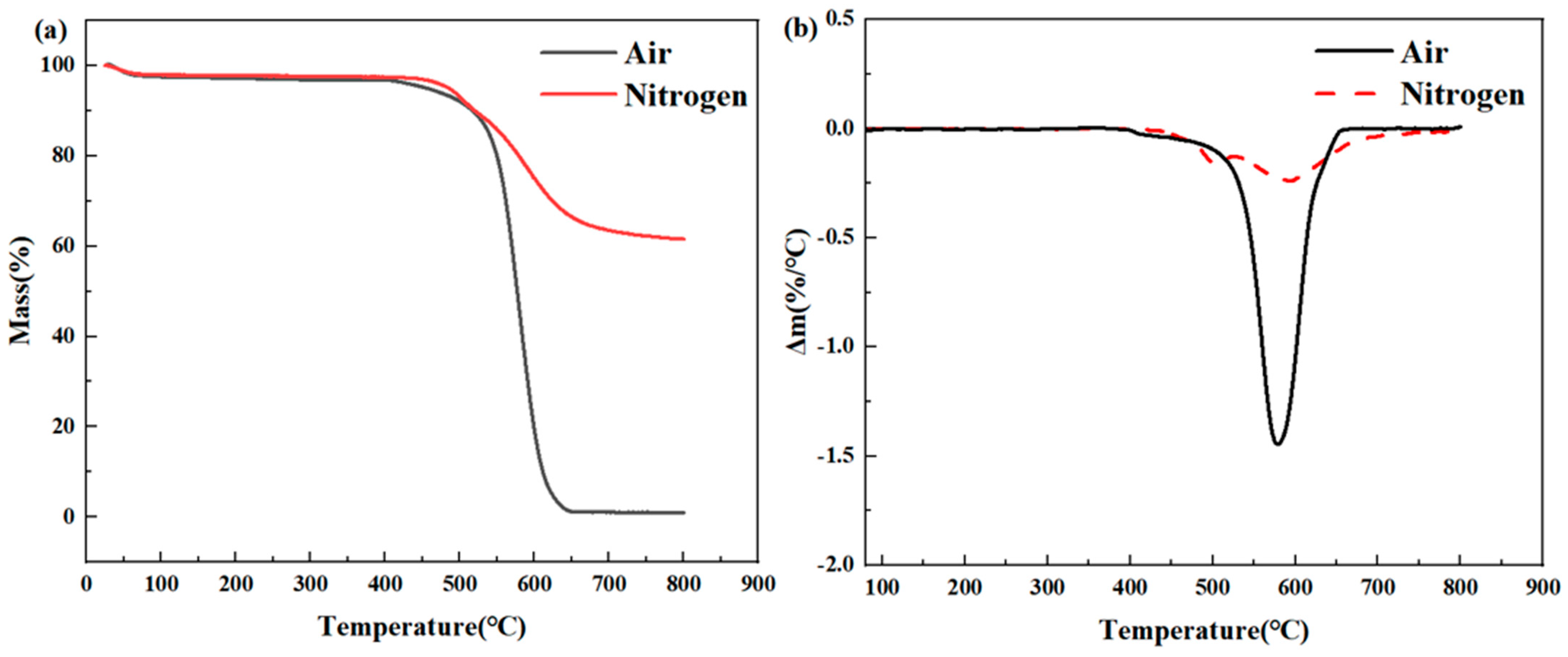
(1) TGA analysis: The STA499 F3 thermal analyzer by Netzsch (Waldkraiburg, Germany) was utilized in both nitrogen and air atmospheres. The test temperature ranged from room temperature to 800 °C, with heating rates of 3, 5, 8, and 10 °C/min. This was performed to examine the TGA curve of PMR350 polyimide resin under various heating rates.
(2) Fourier transform infrared spectroscopy (FT-IR): The Vector-22 infrared spectrometer from Bruker (Berlin, Germany) was utilized. The scanning range was 4000~400 cm−1 with 128 scanning times at a resolution of 4 cm−1, and testing was conducted on a sample powder and KBr mixed tablet.
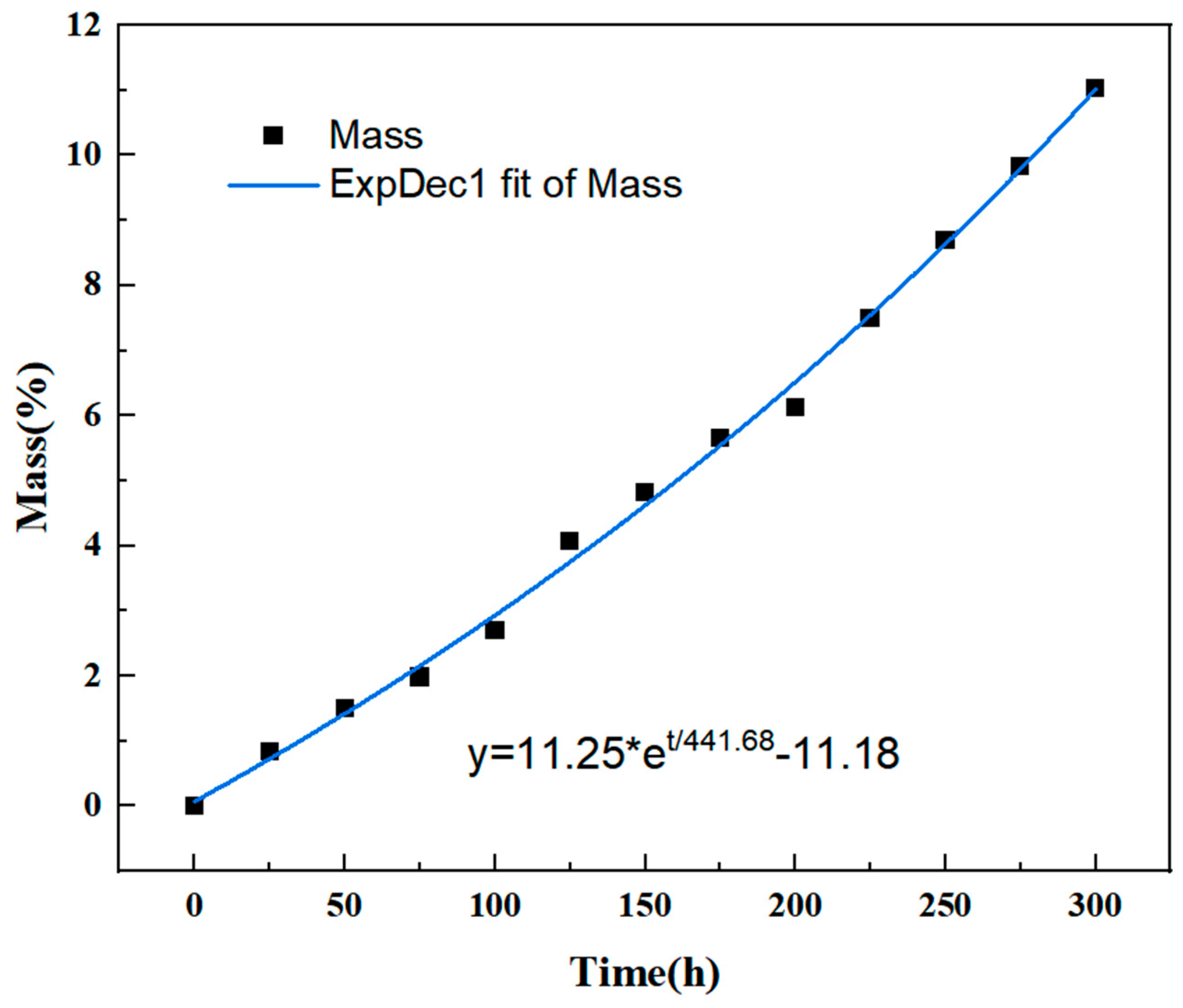
(3) Hot oxidation experiment: The high-temperature oven of DHG-9039A of Heheng (Yibin, China) is used as a hot oxygen aging test equipment, and the gas flow rate of the oven is the fixed value of the equipment. Initially, the PMR350 polyimide resin cured sample was placed in the high-temperature oven, heated to 200 °C for 1 h, then removed and cooled to room temperature in a dryer. It was weighed using an analytical balance (accurate to 0.00001 g), and the recorded value was considered the initial weight (m
0). Subsequently, the composite sample was placed back into the high-temperature oven, heated to 350 °C at a rate of 3 °C/min, and maintained at a constant temperature, and the time was recorded. The sample was removed and weighed every 25 h, recorded as m
tn (n = 25, 50, 75, 100...). The weight loss rate at that time is calculated according to Equation (1):
(4) Scanning electron microscopy (SEM): The JSM-7610FPlus scanning electron microscope (Japan) was used to analyze and characterize the surface and cross-section of the resin samples before and after thermal aging.
(5) Micromechanical properties test: The hardness and modulus of the resin samples before and after thermal examination were tested using the Hysitron T1950 nanoindentation instrument manufactured by Bruker (Germany). The loading mode selects the loading force control mode and sets the maximum loading force to 10,000 mN.