Thermal and Catalytic Recycling of Plastics from Waste Electrical and Electronic Equipment—Challenges and Perspectives
Abstract
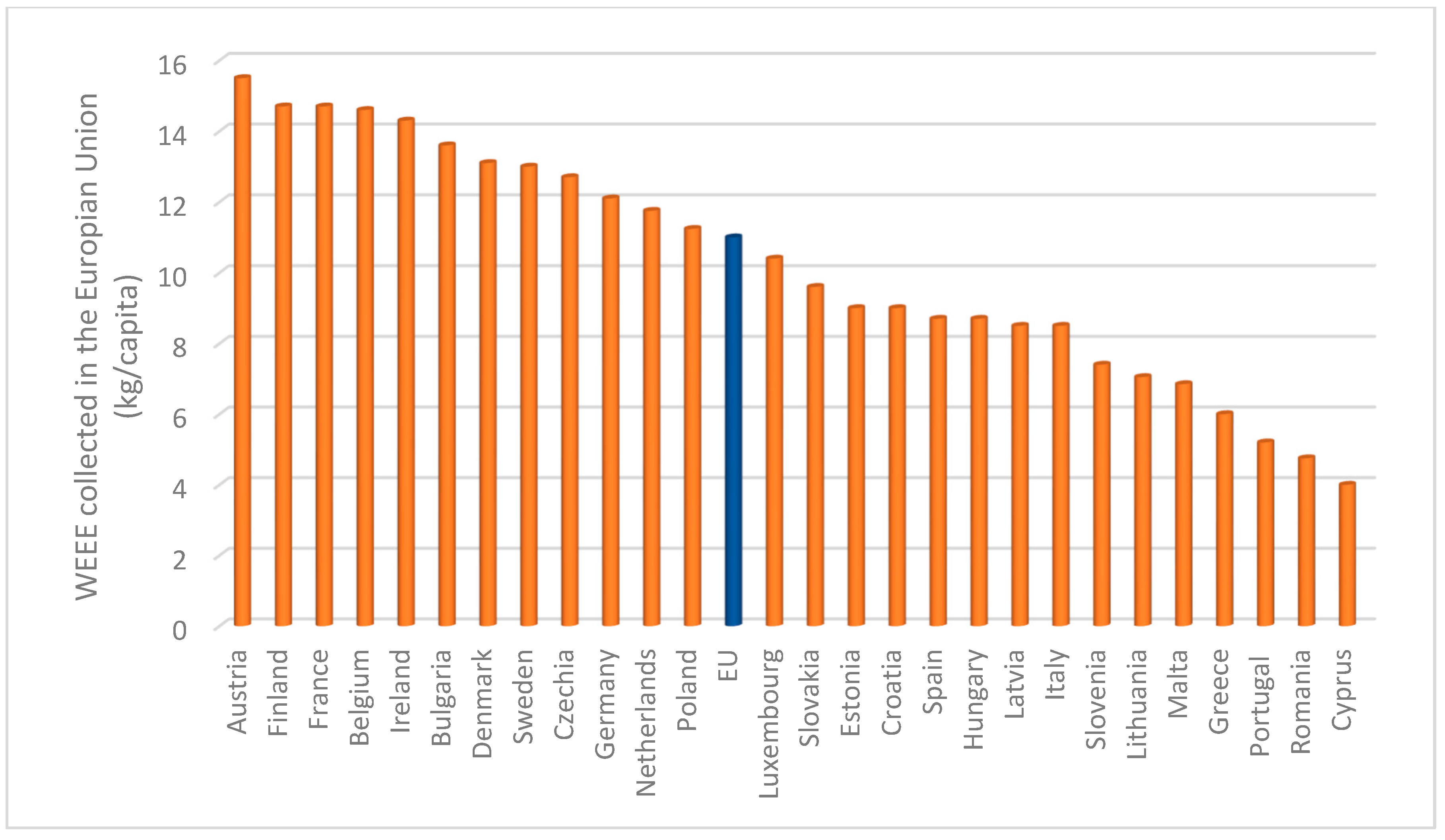
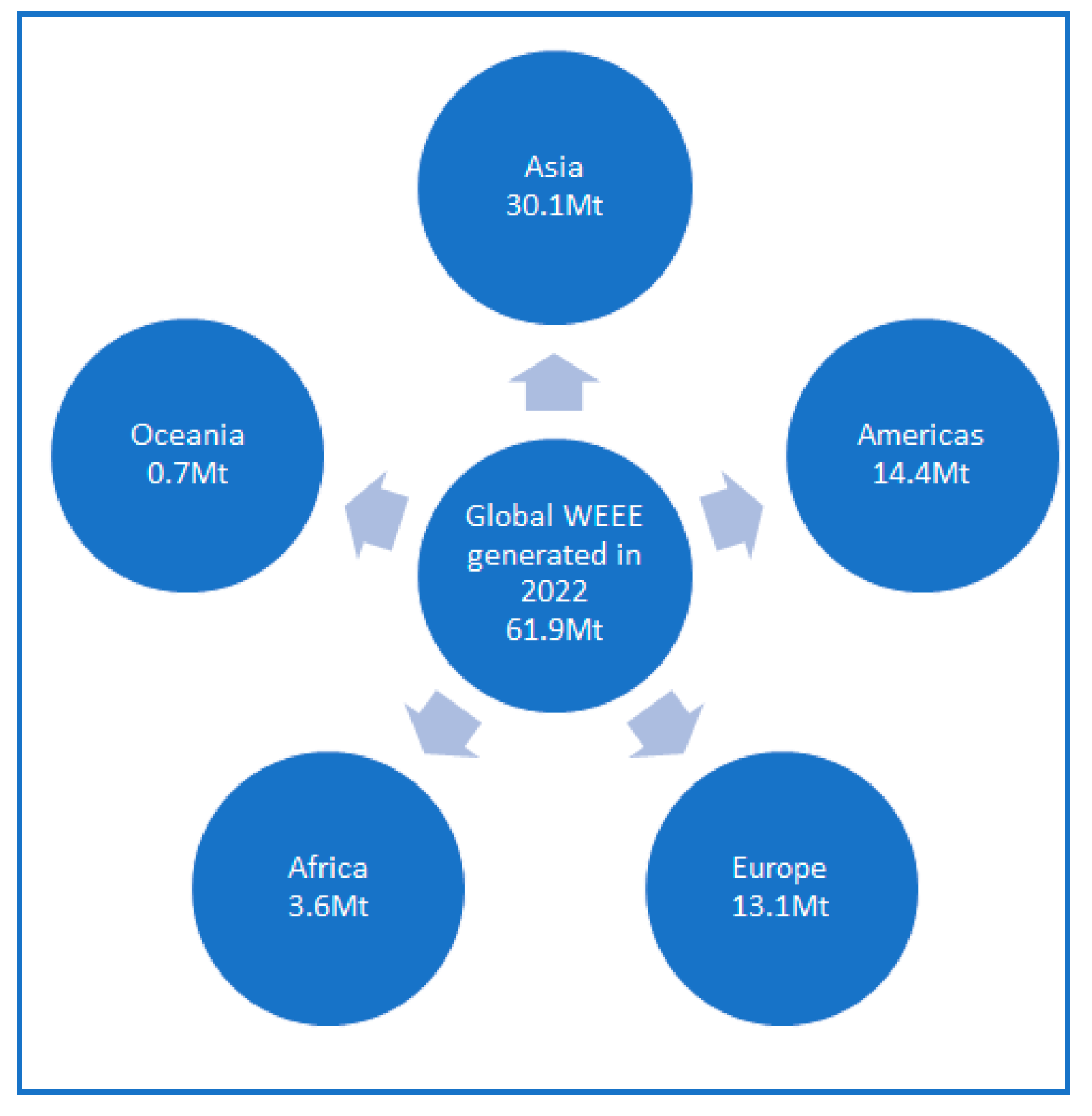
1. Introduction
2. Definition, Categories and Composition of WEEE
2.1. Definition of EEE and WEEE
2.2. Categories of EEE
- Large household appliances (including devices large in size, such as refrigerators, washing machines, electric heating appliances, air conditioner appliances, etc.);
- Small household appliances (including devices smaller in size, such as irons, toasters, coffee machines, electric knives, etc.);
- IT and telecommunications equipment (such as personal and laptop computers, with all accessories: CPU, mouse, screen and keyboard included, printers, copying equipment, pocket and desk calculators, telephones and cellular telephones, etc.);
- Consumer equipment and photovoltaic panels (such as radio sets, television sets, video cameras, photovoltaic panels, etc.);
- Lighting equipment (different types of lamps, such as straight fluorescent or compact fluorescent, and others);
- Electrical and electronic tools (such as drills, saws, sewing machines, etc., with the exception of large-scale stationary industrial tools);
- Toys and leisure and sports equipment (for instance, electric trains, video games, etc., and sports equipment with electric or electronic components);
- Medical devices (for instance, radiotherapy equipment, cardiology equipment, etc., with the exception of all implanted and infected products);
- Monitoring and control instruments (such as smoke detectors, heating regulators, thermostats and others);
- Automatic dispensers (for instance, automatic dispensers for hot drinks, automatic dispensers for money, and all appliances that deliver automatically all kinds of products).
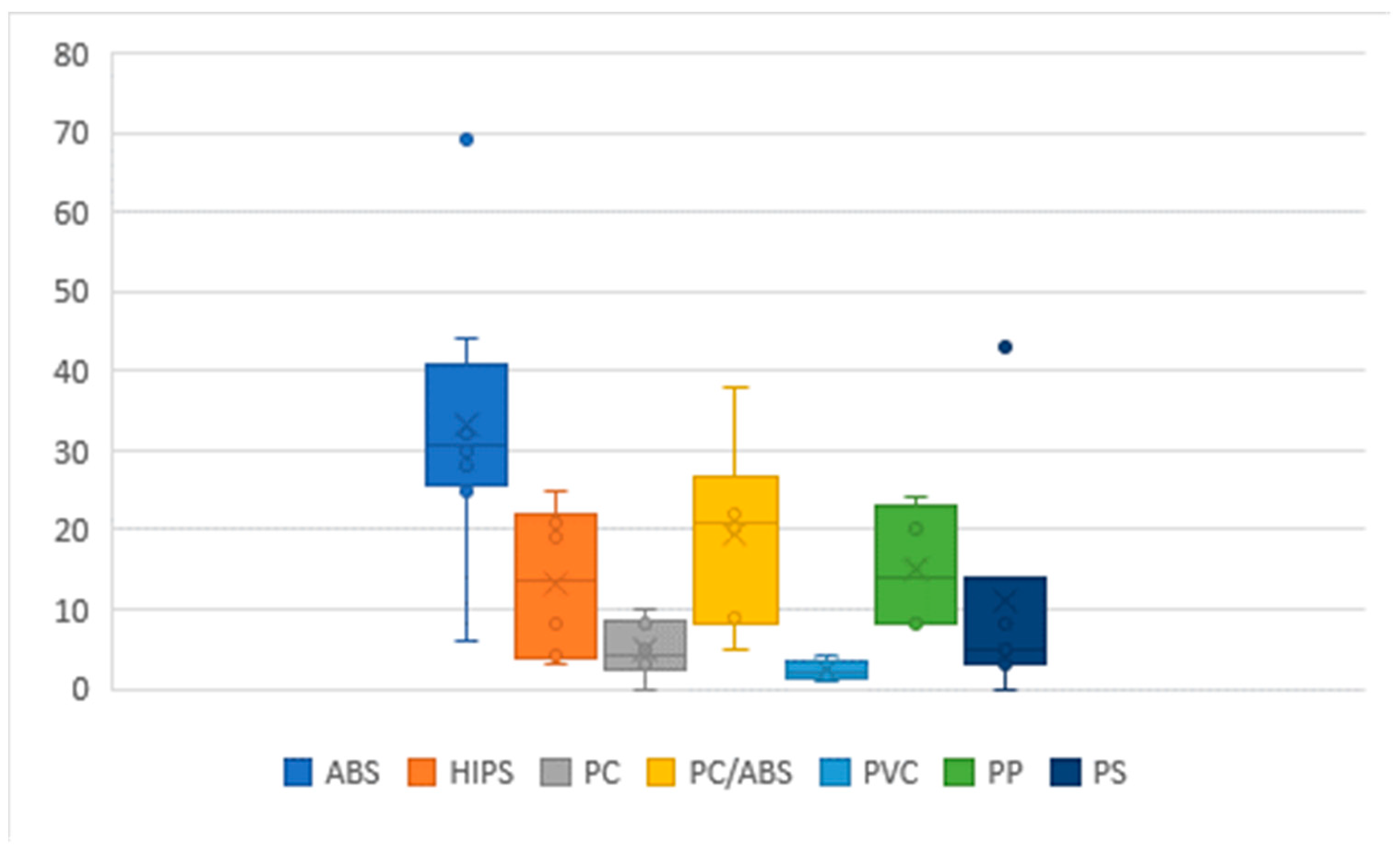
2.3. Composition of WEEE
- Ferrous metals;
- Non-ferrous metals;
- Glass;
- Plastics;
- Other materials.
Hazardous Substances in WEEE and Legislation
3. Recycling of Plastics from WEEE
3.1. Mechanical Recycling
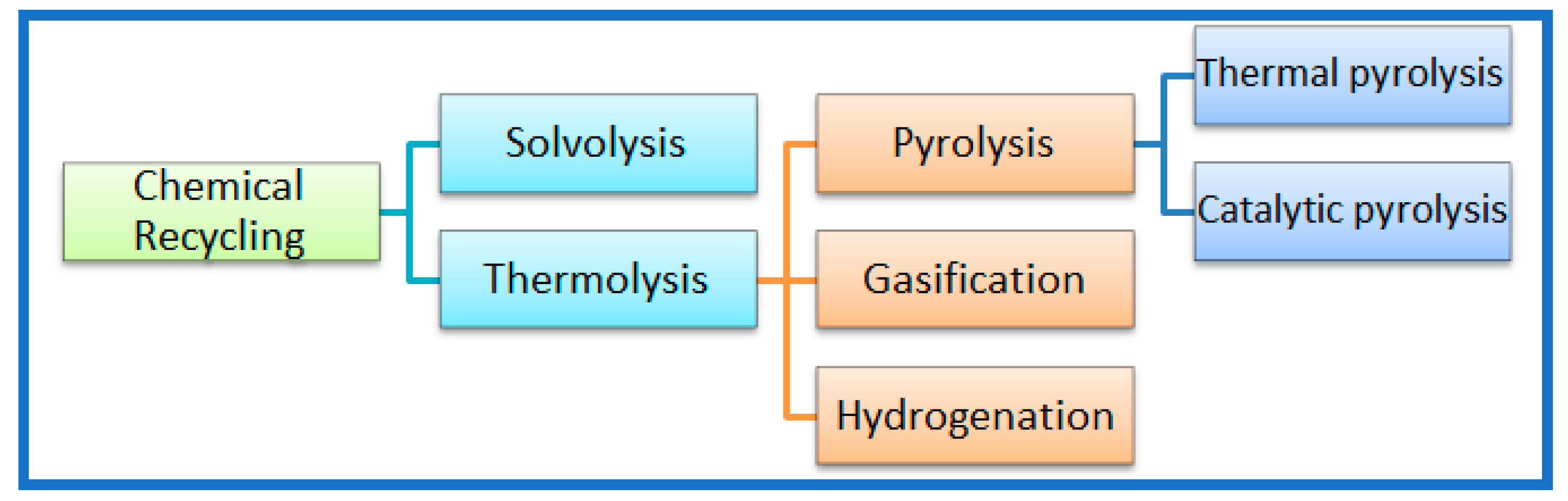
3.2. Thermo-Chemical Recycling
3.2.1. Thermal Pyrolysis
3.2.2. Gasification
3.2.3. Hydrogenation
3.3. Catalytic Pyrolysis
3.4. Emerging Technologies
3.5. Combined Approaches
4. Discussion
4.1. Challenges
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Removal of Bromine from Polymer Blends with a Composition Simulating That Found in Waste Electric and Electronic Equipment through a Facile and Environmentally Friendly Method. Polymers 2023, 15, 709. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex, Official Journal of the European Union (Directive 2012/19/EU). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012L0019 (accessed on 7 April 2024).
- Shittu, O.S.; Williams, I.D.; Shaw, P.J. Global E-waste management: Can WEEE make a difference? A review of e-waste trends, legislation, contemporary issues and future challenges. Waste Manag. 2021, 120, 549–563. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_electrical_and_electronic_equipment#Electrical_and_electronic_equipment_.28EEE.29_put_on_the_market_and_WEEE_processed_in_the_EU (accessed on 6 April 2024).
- EUR-Lex, Official Journal of the European Union (Commission Recommendation (EU) 2023/2585). Available online: http://data.europa.eu/eli/reco/2023/2585/oj (accessed on 6 April 2024).
- Baldé, C.P.; Kuehr, R.; Yamamoto, T.; McDonald, R.; D’Angelo, E.; Althaf, S.; Bel, G.; Deubzer, O.; Fernandez-Cubillo, E.; Forti, V.; et al. The Global E-Waste Monitor 2024; International Telecommunication Union (ITU): Geneva, Switzerland; United Nations Institute for Training and Research (UNITAR): Bonn, Germany, 2024; ISBN 978-92-61-38791-4. [Google Scholar]
- Cardamone, F.G.; Ardolino, F.; Arena, U. About the environmental sustainability of the European management of WEEE plastics. Waste Manag. 2021, 126, 119–132. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, Y.; Zhong, Y.; Dong, H.; Liu, Z. A bibliometric analysis on waste electrical and electronic equipment research. Environ. Sci. Pollut. Res. 2019, 26, 21098–21108. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sustain. Energy Rev. 2016, 61, 433–450. [Google Scholar] [CrossRef]
- Nnorom, I.C.; Osibanjo, O. Sound management of brominated flame retarded (BFR) plastics from electronic wastes: State of the art and options in Nigeria. Resour. Conserv. Recycl. 2008, 52, 1362–1372. [Google Scholar] [CrossRef]
- Li, J.; Lopez, B.N.N.; Liu, L.; Zhao, N.; Yu, K.; Zheng, L. Regional or global WEEE recycling. Where to go? Waste Manag. 2013, 33, 923–934. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-electrical-and-electronic-equipment-weee_en (accessed on 7 April 2024).
- Antonakou, Ε. Development of Advanced Thermochemical Recycling Techniques for Polymers. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2014. [Google Scholar]
- Charitopoulou, M.A.; Kalogiannis, K.G.; Lappas, A.A.; Achilias, D.S. Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environ. Sci. Pollut. Control Ser. 2021, 28, 59190–59213. [Google Scholar] [CrossRef] [PubMed]
- Delva, L.; Hubo, S.; Cardon, L.; Ragaert, K. On the role of flame retardants in mechanical recycling of solid plastic waste. Waste Manag. 2018, 82, 198–206. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Arato, S.; Persson, H.; Kantarelis, E.; Yang, W. Reduction of brominated flame retardants (BFRs) in plasticsfrom waste electrical and electronic equipment (WEEE) by solvent extraction and the influence on their thermal decomposition. Waste Manag. 2019, 94, 165–171. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Lappas, A.A.; Achilias, D.S. Thermo-chemical recycling of plastics retrieved from waste electric and electronic equipment (WEEE) by pyrolysis: Identification of the polymer type, removal of bromine compounds from plastics based on an environmentally-friendly process and characterization of the pyrolysates. Sustain. Chem. Pharm. 2023, 35, 101210. [Google Scholar] [CrossRef]
- Caballero, B.M.; de Marco, I.; Adrados, A.; López-Urionabarrenechea, A.; Solar, J.; Gastelu, N. Possibilities and limits of pyrolysis for recycling plastic rich waste streams rejected from phones recycling plants. Waste Manag. 2016, 57, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Acomb, J.C.; Nahil, M.A.; Williams, P.T. Thermal processing of plastics from waste electrical and electronic equipment for hydrogen production. J. Anal. Appl. Pyrolysis 2013, 103, 320–327. [Google Scholar] [CrossRef]
- Williams, I.D. Global metal reuse, and formal and informal recycling from electronics and other high-tech wastes. In Metal Sustainability: Global Challenges, Consequences, and Prospects; Izatt, R.M., Ed.; Wiley: Oxford, UK, 2016; pp. 23–51. [Google Scholar]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Buekens, A.; Yang, J. Recycling of WEEE plastics: A review. J. Mater. Cycles Waste Manag. 2014, 16, 415–434. [Google Scholar] [CrossRef]
- Jia, C.; Das, P.; Kim, I.; Yoon, Y.J.; Tay, C.Y.; Lee, J.M. Applications, treatments, and reuse of plastics from electrical and electronic equipment. J. Ind. Eng. Chem. 2022, 110, 84–99. [Google Scholar] [CrossRef]
- European Union. Available online: https://eur-lex.europa.eu/search.html?scope=EURLEX&text=composition+of+WEEE&lang=en&type=quick&qid=1712553840948 (accessed on 8 April 2024).
- López, J.; Amodio, L.; del Mar Alonso-Doncel, M.; Cueto, J.; Hernando, H.; Mazur, M.; Čejka, J.; Pizarro, P.; Serrano, D.P. Enhanced oil dehalogenation during catalytic pyrolysis of WEEE-derived plastics over Fe- and Ca-modified zeolites. J. Environ. Chem. Eng. 2024, 12, 111790. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Alexopoulou, E.; Alexiou, P.; Achilias, D.S. Current Topics in Plastic Recycling. In Waste Material Recycling in the Circular Economy—Challenges and Developments; Achilias, D.S., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Achilias, D.; Antonakou, E. Chemical and thermochemical recycling of polymers from waste electrical and electronic equipment. In Recycling Materials Based on Environmentally Friendly Techniques; Achilias, D.S., Ed.; InTech Open: London, UK, 2015. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef]
- Martinho, G.; Pires, A.; Saraiva, L.; Ribeiro, R. Composition of plastics from waste electrical and electronic equipment (WEEE) by direct sampling. Waste Manag. 2012, 32, 1213–1217. [Google Scholar] [CrossRef]
- European Commission, RoHS Directive. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/rohs-directive_en (accessed on 8 April 2024).
- Mtibe, A.; Mokhena, T.C.; John, M.J. Sustainable valorization and conversion of e-waste plastics into value-added products. Curr. Opin. Green Sustain. Chem. 2023, 40, 100762. [Google Scholar] [CrossRef]
- Kike, O.; Sindiku, O.; Osibanjo, O.; Weber, R. Polybrominated diphenyl ethers (PBDEs) concentrations in soil, sediment andwater samples around electronic wastes dumpsites in Lagos, Nigeria. Emerg. Contam. 2022, 8, 206–215. [Google Scholar] [CrossRef]
- Chaine, C.; Hursthouse, A.S.; McLean, B.; McLellan, I.; McMahon, B.; McNulty, J.; Miller, J.; Viza, E. Recycling Plastics from WEEE: A Review of the Environmental and Human Health Challenges Associated with Brominated Flame Retardants. Int. J. Environ. Res. Public Health 2022, 19, 766. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, R.; Wang, J.; Chen, X.; Ge, X.; Chen, M. Waste-to energy: Dehalogenation of plastic-containing wastes. Waste Manag. 2016, 49, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Aurisano, N.; Weber, R.; Fantke, P. Enabling a circular economy for chemicals in plastics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100513. [Google Scholar] [CrossRef]
- Wiesinger, H.; Wang, Z.; Hellweg, S. Deep Dive into Plastic Monomers, Additives, and Processing Aids. Environ. Sci. Technol. 2021, 55, 9339–9351. [Google Scholar] [CrossRef] [PubMed]
- EERA-European Electronic Recyclers Association. Figures on the State of Play on Collection of Plastics from WEEE in EU May 2020. 2020. Available online: www.eera-recyclers.com/publications (accessed on 2 May 2024).
- De Meester, S.; Nachtergaele, P.; Debaveye, S.; Vos, P.; Dewulf, J. Using material flow analysis and life cycle assessment in decision support: A case study on WEEE valorization in Belgium. Resour. Conserv. Recy. 2019, 142, 1–9. [Google Scholar] [CrossRef]
- Lase, I.S.; Ragaert, K.; Dewulf, J.; De Meester, S. Multivariate input-output and material flow analysis of current and future plastic recycling rates from waste electrical and electronic equipment: The case of small household appliances. Resour. Conserv. Recy. 2021, 174, 105772. [Google Scholar] [CrossRef]
- Sahin, O.; Kirim, Y. Material recycling. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cafiero, L.; Fabbri, D.; Trinca, E.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and spectroscopic (TG/DSC-FTIR) characterization of mixed plastics for materials and energy recovery under pyrolytic conditions. J. Therm. Anal. Calorim. 2015, 121, 1111–1119. [Google Scholar] [CrossRef]
- Cafiero, L.; Castoldi, E.; Tuffi, R.; Vecchio Ciprioti, S. Identification and characterization of plastics from small appliances and kinetic analysis of their thermally activated pyrolysis. Polym. Degrad. Stab. 2014, 109, 307–318. [Google Scholar] [CrossRef]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Vecchio Ciprioti, S. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vinu, R.; Ojha, D.K.; Nair, V. Polymer pyrolysis for resource recovery. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier Inc.: Waltham, MA, USA, 2016. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Rieger, T.; Oey, J.; Palchyk, V.; Hofmann, A.; Franke, M.; Hornung, A. Chemical Recycling of WEEE Plastics—Production of High Purity Monocyclic Aromatic Chemicals. Processes 2021, 9, 530. [Google Scholar] [CrossRef]
- Menad, N.-E.; Guignot, S.; Göklap, I.; Bostyn, S.; Graz, Y.; Poirier, J. Method for Recycling Waste Electrical and Electronic Equipment. U.S. Patent 15,539,570, 21 December 2017. [Google Scholar]
- Sun, S.; Long, L.; Zhong, S. The Separation of Each Component Material and Recovery Method in a Kind of Waste and Old Printed Circuit Board. Chinese Patent 1,01,612,628, 30 December 2009. [Google Scholar]
- Riedewald, F. Process for the Recycling of Waste Batteries and Waste Printed Circuit Boards in Molten Salts or Molten Metals. Int. Patent 2014, 167, 139. [Google Scholar]
- Brandhorst, H.W.; Engel, U.H., Jr.; Ludwig, C.T.; Zavoral, E.J. Multistage Thermolysis Method for Safe and Efficient Conversion of E-Waste Materials. U.S. Patent 9,850,433, 6 July 2017. [Google Scholar]
- Hornung, A.; Hense, P.; Aigner, J.; Reh, K.; Franke, M. Rohrofen und Verfahrenzur Chemischen Umsetzung. Int. Patent 2016, 189, 138. [Google Scholar]
- Colantonio, S.; Cafiero, L.; De Angelis, D.; Ippolito, N.M.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and catalytic pyrolysis of a synthetic mixture representative of packaging plastics residue. Front. Chem. Sci. Eng. 2020, 14, 288–303. [Google Scholar] [CrossRef]
- Altwaiq, A.M.; Wolf, M.; Eldik, R.V. Extraction of brominated flame retardants from polymeric waste material using different solvents and supercritical carbondioxide. Anal. Chim. Acta. 2003, 491, 111–123. [Google Scholar] [CrossRef]
- Covaci, A.; Voorspoels, S.; de Boer, J. Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples—A review. Environ. Int. 2003, 29, 735–756. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Microwave-assisted extraction for qualitative and quantitative determination of brominated flame retardants in styrenic plastic fractions from waste electrical and electronic equipment (WEEE). Talanta 2009, 78, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Abnisa, F.; Daud, W.M.A.W. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Ma, C.; Sun, L.; Jin, L.; Zhou, C.; Xiang, J.; Hu, S.; Su, S. Effect of polypropylene on the pyrolysis of flame retarded high impact polystyrene. Fuel Process. Technol. 2015, 135, 150–156. [Google Scholar] [CrossRef]
- Ma, C.; Yan, Q.; Yu, J.; Chen, T.; Wang, D.; Liu, S.; Bikane, K.; Sun, L. The behavior of heteroatom compounds during the pyrolysis of waste computer casing plastic under various heating conditions. J. Clean. Prod. 2019, 219, 461–470. [Google Scholar] [CrossRef]
- Hennebert, P.; Filella, M. WEEE plastic sorting from bromine essential to enforce EU regulation. Waste Manag. 2018, 74, 390–399. [Google Scholar] [CrossRef]
- Hermoso-Orzáez, M.J.; Mota-Panizio, R.; Carmo-Calado, L.; Brito, P. Thermochemical and Economic Analysis for Energy Recovery by the Gasification of WEEE Plastic Waste from the Disassembly of Large-Scale Outdoor Obsolete Luminaires by LEDs in the Alto Alentejo Region (Portugal). Appl. Sci. 2020, 10, 4601. [Google Scholar] [CrossRef]
- Díaz-Perete, D.; Hermoso-Orzáez, M.J.; Terrados-Cepeda, J.; Silva-Romano, P.; Martin-Doñate, C. WEEE polymers valorization, its use as fuel in the gasification process and revaluation of the inert by-products obtained: Sustainable mortars as a solution. Heliyon 2023, 9, 20194. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. A holistic review on biomass gasification modified equilibrium models. Energies 2019, 12, 160. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Park, S.J.; Binns, M. Biomass to Syngas: Modified Stoichiometric Thermodynamic Models for Downdraft Biomass Gasification. Energies 2020, 13, 5383. [Google Scholar] [CrossRef]
- Carmo-Calado, L.; Hermoso-Orzáez, M.J.; Mota-Panizio, R.; Brito, P. Co-combustion of waste tires and plastic-rubber wastes with biomass technical and environmental analysis. Sustainability 2020, 12, 1036. [Google Scholar] [CrossRef]
- Kasper, A.C.; Berselli, G.B.T.; Freitas, B.D.; Tenório, J.A.S.; Bernardes, A.M.; Veit, H.M. Printed wiring boards for mobile phones: Characterization and recycling of copper. Waste Manag. 2011, 31, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, P.; Nowosielski, R.; Sakiewicz, P. Recycling of waste electrical and electronic Recycling of waste electrical and electronic equipment. J. Achiev. Mater. Manuf. Eng. 2015, 20, 535–538. [Google Scholar]
- Mota-Panizio, R.; Hermoso-Orzáez, M.J.; Carmo-Calado, L.; Campos, V.A.F.d.; Silveira, J.L.; Gonçalves, M.M.; Brito, P. Energy Recovery via Thermal Gasification from Waste Insulation Electrical Cables (WIEC). Appl. Sci. 2020, 10, 8253. [Google Scholar] [CrossRef]
- Qi, P.; Su, Y.; Yang, L.; Wang, J.; Jiang, M.; Xiong, Y. Catalytic pyrolysis of rice husk to co-produce hydrogen-rich syngas, phenol-rich bio-oil and nanostructured porous carbon. Energy 2024, 298, 131427. [Google Scholar] [CrossRef]
- Benedetti, M.; Cafiero, L.; De Angelis, D.; Dell’Era, A.; Pasquali, M.; Stendardo, S.; Tuffi, R.; Vecchio Ciprioti, S. Pyrolysis of WEEE plastics using catalysts produced from fly ash of coal gasification. Front. Environ. Sci. Eng. 2017, 11, 11. [Google Scholar] [CrossRef]
- Dufaud, V.; Basset, J.-M. Catalytic Hydrogenolysis at Low Temperature and Pressure of Polyethylene and Polypropylene to Diesels or Lower Alkanes by a Zirconium Hydride Supported on Silica-Alumina: A Step Toward Polyolefin Degradation by the Microscopic Reverse of Ziegler ± Natta Polymerization. Angew. Chem. Int. Ed. 1998, 37, 806–810. [Google Scholar]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Catalytic upgrading of plastic wastes. In Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; Scheirs, J., Kaminsky, W., Eds.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Garcia, R.A.; Serrano, D.P.; Otero, D. Catalytic cracking of HDPE over hybrid zeolitic-mesoporous materials. J. Anal. Appl. Pyrolysis 2005, 74, 379–386. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; SanMiguel, G.; Castro, M.C. Madrid, Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Muhammad, C.; Onwudili, J.; Williams, P. Catalytic pyrolysis of waste plastic from electrical and electronic equipment. J. Anal. Appl. Pyrolysis 2015, 113, 332–339. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Yan, Q.; Song, Z.; Wang, K.; Wang, B.; Sun, L. Pyrolysis-catalytic upgrading of brominated high impact polystyrene over Fe and Ni modified catalysts: Influence of HZSM-5 and MCM-41 catalysts. Polym. Degrad. Stab. 2017, 146, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Yang, F.; Li, S.; Wu, J.; Liu, J.; Zhong, S.; Zeng, J. The effects of activated Al2O3 on the recycling of light oil from the catalytic pyrolysis of waste printed circuit boards. Process Saf. Environ. Prot. 2015, 98, 276–284. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, F.; Qiu, Y.; Chen, N.; Lin, W.; Sun, S. The dibrominated and lightweight oil generated from two stage pyrolysis of WPCBs by using compound chemical additives. Process Saf. Environ. Prot. 2018, 116, 654–662. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Tae Uk, H.; Kim, S.; Jae, J.; Jeon, J.-K.; Jung, S.-C.; Park, Y.-K. Catalytic co-pyrolysis of epoxy-printed circuit board and plastics over HZSM-5 and HY. J. Clean. Prod. 2017, 168, 366–374. [Google Scholar] [CrossRef]
- Ma, C.; Kamo, T. Enhanced debromination by Fe particles during the catalytic pyrolysis of non-metallic fractions of printed circuit boards over ZSM-5 and Ni/SiO2-Al2O3 catalyst. J. Anal. Appl. Pyrolysis. 2019, 138, 170–177. [Google Scholar] [CrossRef]
- Park, Y.-K.; Han, T.U.; Jeong, J.; Kim, Y.-M. Debrominated high quality oil production by the two-step catalytic pyrolysis of phenolic printed circuit boards (PPCB) using natural clays and HY. J. Hazard. Mater. 2019, 367, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, J.; Ma, C.; Bikane, K.; Sun, L. Catalytic performance and debromination of Fe–Ni bimetallic MCM-41 catalyst for the two-stage pyrolysis of waste computer casing plastic. Chemosphere 2020, 248, 125964. [Google Scholar] [CrossRef]
- Tejaswini, M.S.S.R.; Pathak, P.; Ramkrishna, S.; Ganesh, P.S. A comprehensive review on integrative approach for sustainable management of plastic waste and its associated externalities. Sci. Total Environ. 2022, 825, 153973. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Shon, H.K.; Ni, B.-J. Designing bifunctional catalysts for urea electrolysis: Progress and perspectives. Green Chem. 2024, 26, 631–654. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Chen, X.; Liu, Y.; Shen, Y.; Ni, B.-J. Upcycling of plastic wastes for hydrogen production: Advances and perspectives. Renew. Sustain. Energy Rev. 2024, 195, 114333. [Google Scholar] [CrossRef]
- Huo, E.; Lei, H.; Liu, C.; Zhang, Y.; Xin, L.; Zhao, Y.; Qian, M.; Zhang, Q.; Lin, X.; Wang, C.; et al. Jet fuel and hydrogen produced from waste plastics catalytic pyrolysis with activated carbon and MgO. Sci. Total Environ. 2020, 727, 138411. [Google Scholar] [CrossRef] [PubMed]
- Muniyappan, D.; Lima, G.R.; Pereira, A.O.; Gopi, R.; Ramanathan, A. Multivariate combined optimization strategy and comparative life-cycle assessment of biomass and plastic residues via microwave co-pyrolysis approach towards a sustainable synthesis of renewable hydrocarbon fuel. J. Environ. Chem. Eng. 2023, 11, 111436. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achilias, D.S.; Charitopoulou, M.-A.; Ciprioti, S.V. Thermal and Catalytic Recycling of Plastics from Waste Electrical and Electronic Equipment—Challenges and Perspectives. Polymers 2024, 16, 2538. https://doi.org/10.3390/polym16172538
Achilias DS, Charitopoulou M-A, Ciprioti SV. Thermal and Catalytic Recycling of Plastics from Waste Electrical and Electronic Equipment—Challenges and Perspectives. Polymers. 2024; 16(17):2538. https://doi.org/10.3390/polym16172538
Chicago/Turabian StyleAchilias, Dimitris S., Maria-Anna Charitopoulou, and Stefano Vecchio Ciprioti. 2024. "Thermal and Catalytic Recycling of Plastics from Waste Electrical and Electronic Equipment—Challenges and Perspectives" Polymers 16, no. 17: 2538. https://doi.org/10.3390/polym16172538
APA StyleAchilias, D. S., Charitopoulou, M.-A., & Ciprioti, S. V. (2024). Thermal and Catalytic Recycling of Plastics from Waste Electrical and Electronic Equipment—Challenges and Perspectives. Polymers, 16(17), 2538. https://doi.org/10.3390/polym16172538








