Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges
Abstract
1. Introduction
2. Classification, Formulation, Characteristics, Bioapplications and Degradation of Polymeric Nanoparticles
2.1. Classification of Polymeric Nanoparticles
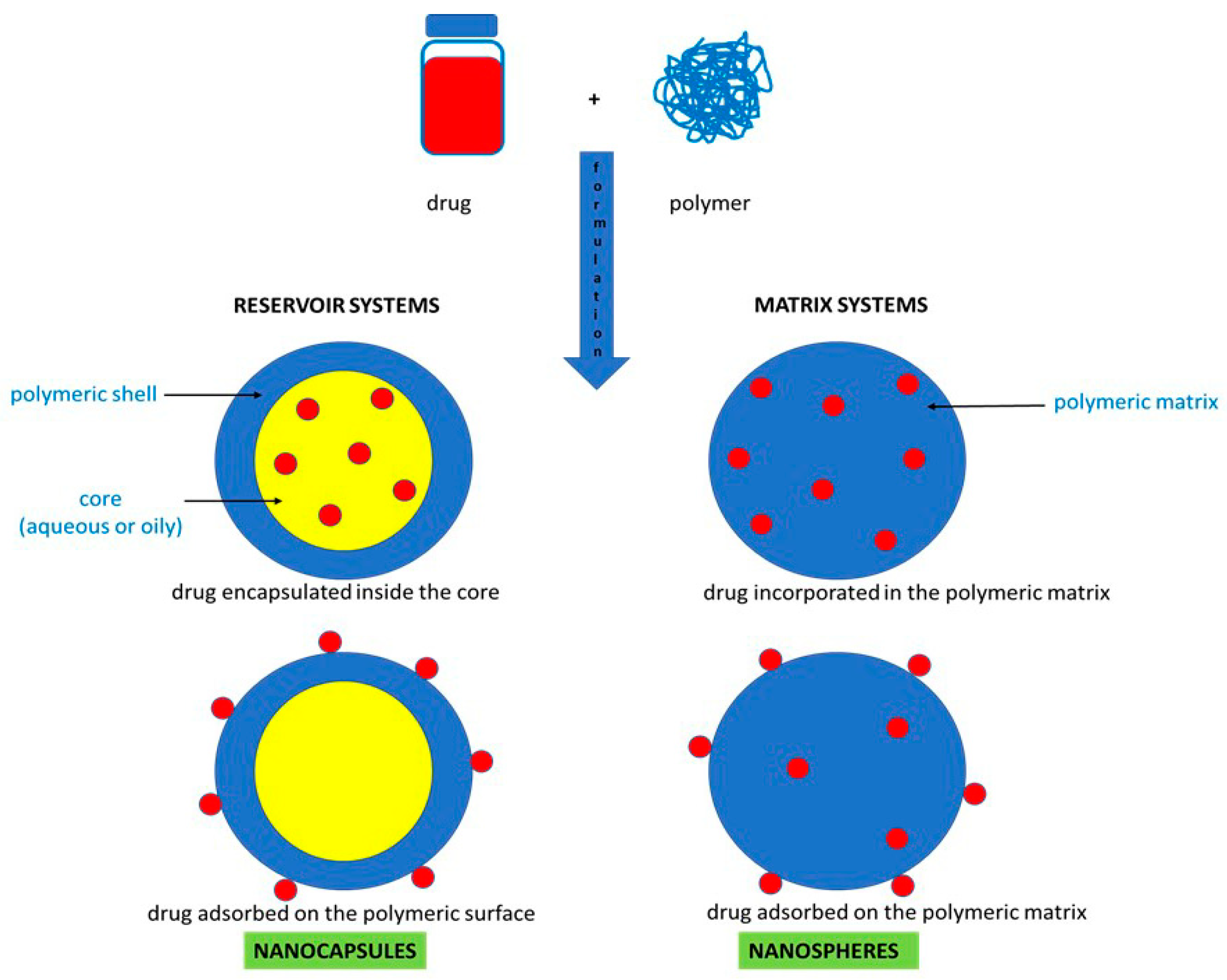
2.2. Formulation of Polymeric Nanoparticles
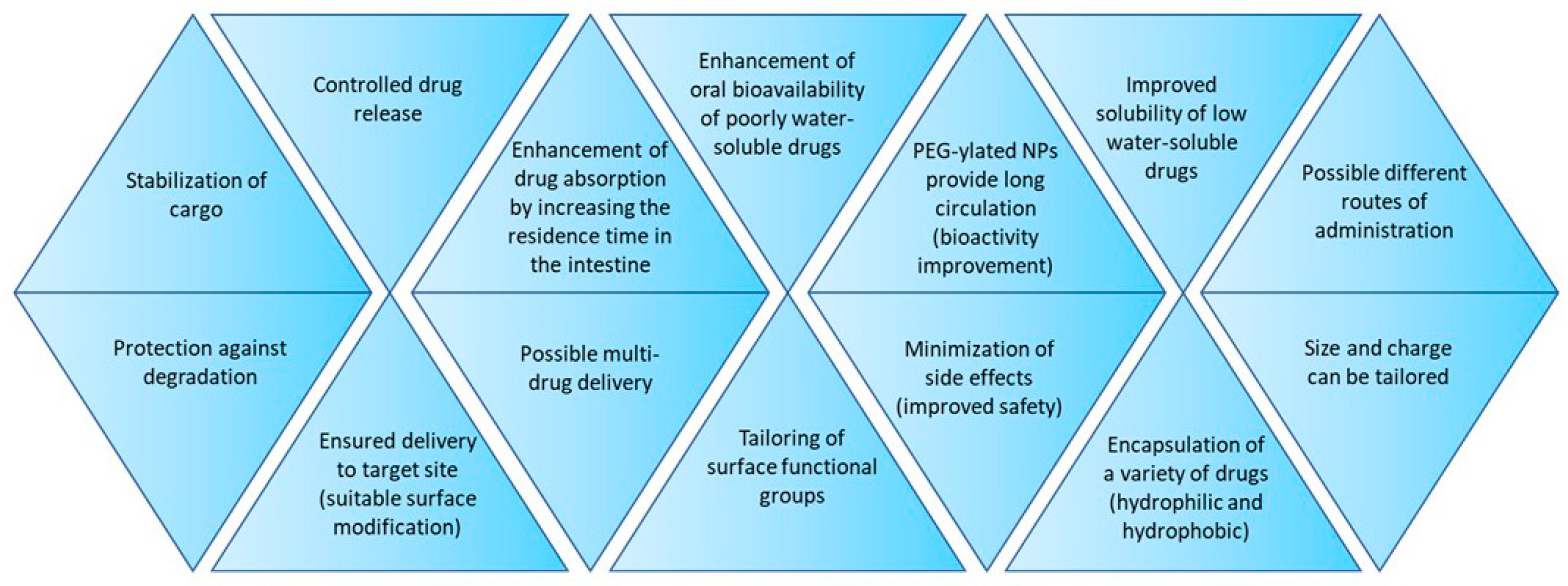
2.3. Characteristics of Polymeric Nanoparticles
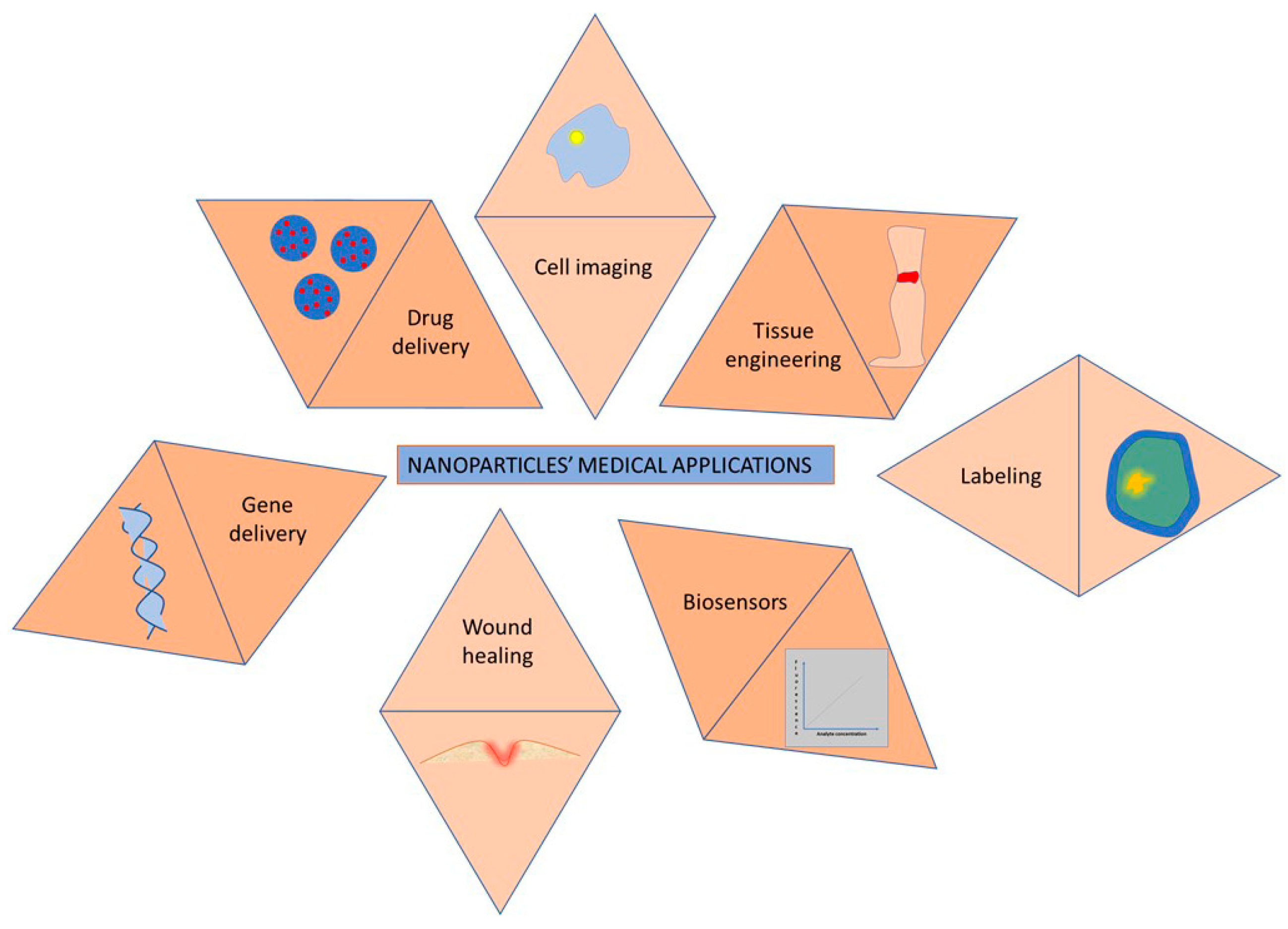
2.4. Bioapplications of Polymeric Nanoparticles
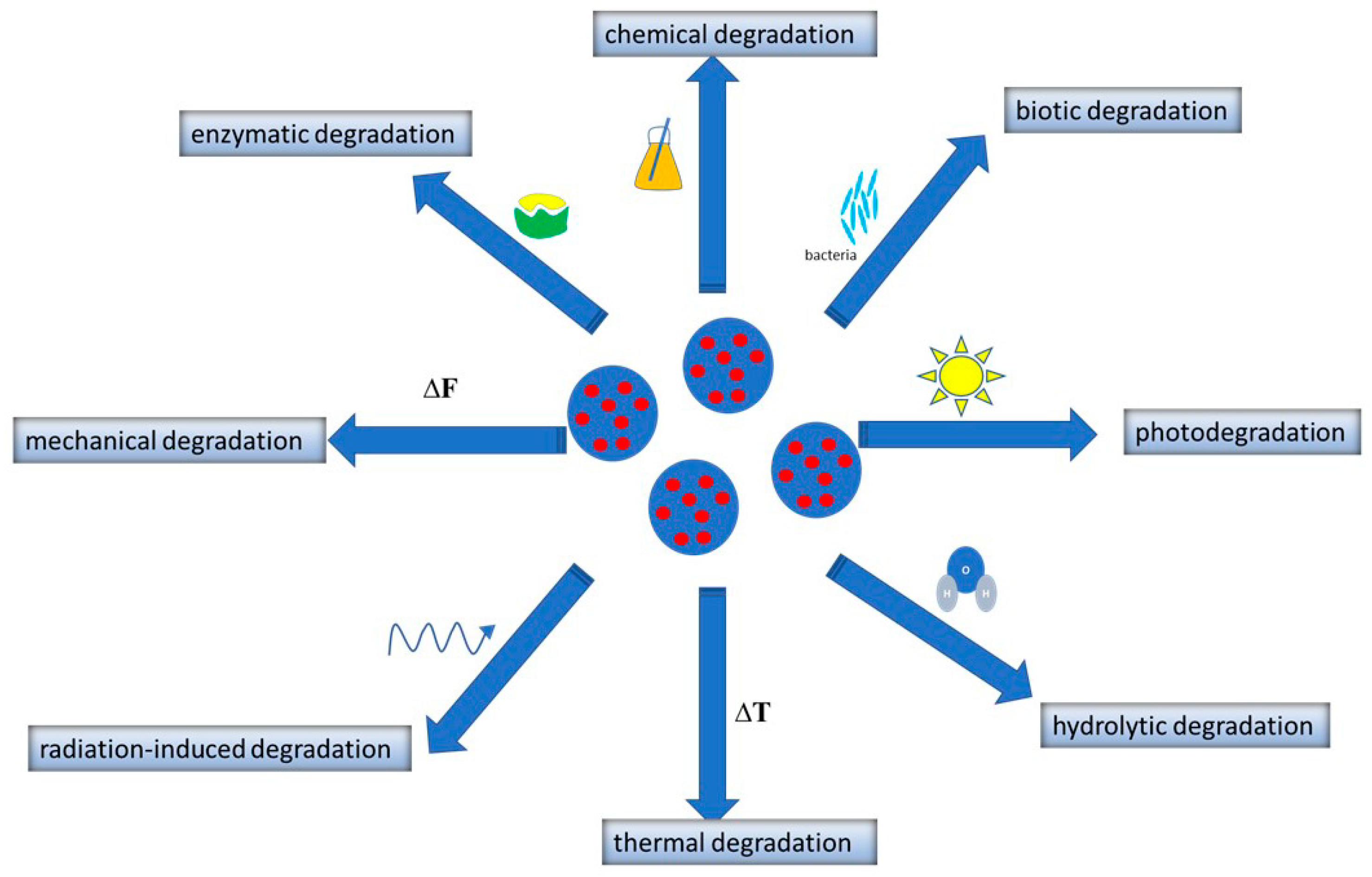
2.5. Degradation of Polymeric Nanoparticles
3. Perspectives and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric nanoparticles for delivery of natural bioactive agents: Recent advances and challenges. Polymer 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Sreena, R.; Nathanael, A.J. Biodegradable biopolymeric nanoparticles for biomedical applications—Challenges and future oulook. Materials 2023, 16, 2364. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Shi, W.; Fuad, A.R.M.; Li, Y.; Wang, Y.; Huang, J.; Du, R.; Wang, G.; Wang, Y.; Yin, T. Biodegradable polymeric nanoparticles increase risk of cardiovascular diseases by inducing endothelium dysfunction and inflammation. Nanobiotechnol 2023, 21, 65. [Google Scholar]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable biodegradable biopolymer-based nanoparticles for healthcare applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zuo, P.; Wang, Y.-L. Enhanced antiproliferative effect of carboplatin in cervical cancer cells utilizing folate-grafted polymeric nanoparticles. Nanoscale Res. Lett. 2015, 10, 453. [Google Scholar] [CrossRef]
- Li, L.; Tao, R.; Song, M.; Zhang, Y.; Chen, K.; Wang, H.; Gong, R. Fabrication of self-assembled folate-biotin-quaternized starch nanoparticles as co-carrier of doxorubicin and siRNA. Biomater. Appl. 2017, 32, 587–597. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen biodegradable nanoparticles: One step closer towards a better ocular interaction study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Madhali, O.A. Drug delivery of gelatin nanoparticles as a biodegradable polymer for the treatment of infectious diseases: Perspectives and challenges. Polymer 2023, 15, 4327. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef]
- Voigt, N.; Henrich-Noack, P.; Kockentiedt, S.; Hintz, W.; Tomas, J.; Sabel, B.A. Toxicity of polymeric nanoparticles in vivo and in vitro. Nanoparticle Res. 2014, 16, 2379. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, A.L.; Pangua, C.; Reboredo, C.; Campión, R.; Morales-Gracia, J.; Irache, J.M. Protein-based nanoparticles for drug delivery purposes. Int. J. Pharm. 2020, 581, 119289. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Materials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate nanoparticles as ocular drug delivery carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Cruz, M.A.E.; Tovani, C.B.; Lopes, H.B.; Beloti, M.M.; Ciancaglini, P.; Bottini, M.; Ramos, A.P. Curcumin-loaded carrageenan nanoparticles: Fabrication, characterization, and assessment of the effects on osteoblasts mineralization. Colloids Surf. B 2022, 217, 112622. [Google Scholar] [CrossRef]
- Venkatesan, J.; Murugan, S.S.; Seong, G.H. Fucoidan-based nanoparticles: Preparations and applications. Int. J. Biol. Macromol. 2022, 217, 652–667. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Duan, J.; Yang, X.-D. Targeted treatment of colon cancer with aptamer-guided albumin nanoparticles loaded with docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef]
- Chen, L.; Wei, J.; An, M.; Zhang, L.; Lin, S.; Shu, G.; Yuan, Z.; Lin, J.; Peng, G.; Liang, X.; et al. Casein nanoparticles as oral delivery carriers of mequindox for the improved bioavailability. Colloids Surf. B 2020, 195, 111221. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmer 2021, 13, 1686. [Google Scholar] [CrossRef]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen nanoparticles in drug delivery systems and tissue engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mater. Sci. Eng. C 2021, 123, 112027. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, C.; Varchi, G. Keratin-based nanoparticles as drug delivery carriers. Appl. Sci. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Pandey, V.; Haider, T.; Chandak, A.R.; Chakraborty, A.; Banerjee, S.; Soni, V. Surface modified silk fibroin nanoparticles for inproved delivery of doxorubicin: Development, characterization, in-vitro studies. Int. J. Biol. Macromol. 2020, 164, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Naveed, M.; Azeem, I.; Faisal, A.; Nazar, M.F.; Yameen, B. Colon specific enzyme responsive oligoester crosslinked dextran nanoparticles for controlled release of 5-fluorouracil. Int. J. Pharm. 2020, 586, 119605. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, U. Formulation and characterization of atenolol-loaded gellan gum nanoparticles. Indian. J. Pharm. Sci. 2021, 83, 60–68. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, E.; Oh, H.; Choi, W.I.; Koo, H. Levan nanoparticles with intrinsic CD44-targeting ability for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2023, 234, 123634. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, J.; Ni, C. Preparation of redox responsive modified xanthan gum nanoparticles and the drug controlled release. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 994–1001. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Wang, J.; Li, H.; Yang, H. Glycyrrhetinic acid-cyclodextrin grafted pullulan nanoparticles loaded doxorubicin as a liver targeted delivery carrier. Int. J. Biol. Macromol. 2022, 216, 789–798. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, P.; Kush, P. Amphotericin B loaded ethyl cellulose nanoparticles with magnified oral bioavailability for safe and effective treatment of fungal infection. Biomed. Pharmacother. 2020, 128, 110297. [Google Scholar] [CrossRef] [PubMed]
- Freitag, T.L.; Podojil, J.R.; Pearson, R.M.; Fokta, F.J.; Sahl, C.; Messing, M.; Andersson, L.C.; Leskinen, K.; Saavalainen, P.; Hoover, L.I.; et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology 2020, 158, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qin, H.; Zheng, Y.; Chen, J.; Liang, X.; Huang, J.; Luo, W.; Yang, R.; Guan, Y.-Q. Construction of a double-responsive modified guar gum nanoparticles and its application in oral insulin administration. Colloids Surf. B 2022, 220, 112858. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, X.; Gu, P.; Cheng, W.; Zhang, R.; Hu, K. Curcumin-loaded zein/pectin nanoparticles: Caco-2 cellular uptake and the effects on cell cycle arrest and apoptosis of human hepatoma cells (HepG2). J. Drug Deliv. Sci. Technol. 2022, 74, 103497. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.-H. Smart drug delivery of p-coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int. J. Biol. Macromol. 2022, 195, 22–29. [Google Scholar] [CrossRef]
- de Almeida Campos, L.A.; Neto, A.F.S.; Noronha, M.C.S.; de Lima, M.F.; Cavalcanti, I.M.F.; Santos-Magalhães, N.S. Zein nanoparticles for drug delivery: Preparation methods and biological applications. Int. J. Pharm. 2023, 635, 122754. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone nanoparticles as promising candidates for nanocarriers in novel nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- Guido, C.; Testini, M.; D’Amone, S.; Cortese, B.; Grano, M.; Gigli, G.; Palamà, I.E. Capsid-like biodegradable poly-glycolic acid nanoparticles for a long-time release of nucleic acid molecules. Mater. Adv. 2021, 2, 310–321. [Google Scholar] [CrossRef]
- Salama, A.H.; Abdelkhalek, A.A.; Elkasagby, N.A. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int. J. Pharm. 2020, 578, 119081. [Google Scholar] [CrossRef]
- Cabeza, L.; El-Hammadi, M.M.; Ortiz, R.; Cayero-Otero, M.D.; Jiménez-López, J.; Perazzoli, G.; Martin-Banderas, L.; Baeyens, J.M.; Melguizo, C.; Prados, J. Evaluation of poly(lactic-co-glycolic acid) nanoparticles to improve the therapeutic efficacy of paclitaxel in breast cancer. Bioimpacts 2022, 12, 515–531. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ridardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef] [PubMed]
- Spósito, L.; Fonseca, D.; Carvahlo, S.G.; Sábio, R.M.; Marena, G.D.; Bauab, T.M.; Meneguin, A.B.; Parreira, P.; Martins, M.C.L.; Chorili, M. Engineering resveratrol-loaded chitosan nanoparticles for potential use against Helicobacter pylori infection. Eur. J. Pharm. Biopharm. 2024, 199, 114280. [Google Scholar] [CrossRef]
- Yang, X.; Lv, Z.; Han, C.; Zhang, J.; Duan, Y.; Guo, Q. Stability and encapsulation properties of daidzein in zein/carrageenan/sodium alginate nanoparticles with ultrasound treatment. Int. J. Biol. Macromol. 2024, 262, 130070. [Google Scholar] [CrossRef] [PubMed]
- Chacon, W.D.C.; Menteiro, A.R.; Verruck, S.; Valencia, G.A. Optimization model of starch nanoparticles production loaded with phenolic compounds from green propolis extract. J. Polym. Environ. 2024, 32, 3946–3960. [Google Scholar] [CrossRef]
- Balistreri, G.N.; Campbell, I.R.; Li, X.; Amorim, J.; Zhang, S.; Nance, E.; Roumeli, E. Bacterial cellulose nanoparticles as a sustainable drug delivery platform for protein-based therapeutics. RSC Appl. Polym. 2024, 2, 172–183. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Liu, L.; Chu, X.; Zhong, M.; Li, H.; Zhao, C.; Fu, H.; Sun, Y.; Li, Y. Hyaluronic acid nanoparticles for targeted oral delivery of doxorubicin: Lymphatic transport and CD44 engagement. Int. J. Biol. Macromol. 2024, 273, 133063. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Bansal, R.; Vishwakarma, V.K.; Yadav, H.N.; Dinda, A.K.; Gupta, Y.K. pH responsive dextran nanoparticles loaded with doxorubicin and RITA against cancer cells: Synergistic inhibitory effects. J. Nanoparticle Res. 2024, 26, 135. [Google Scholar] [CrossRef]
- El-Sawah, A.A.; El-Naggar, N.E.; Eldegla, H.E.; Soliman, H.M. Bionanofactory for green synthesis of collagen nanoparticles, characterization, optimization, in-vitro and in-vivo anticancer activities. Scientific Res. 2024, 14, 6328. [Google Scholar] [CrossRef]
- Desai, S.K.; Bera, S.; Mondal, D. Gelatin nanoparticles as carrier for effective antituberculosis drug delivery in combination therapy. BioNanoScience 2024. [Google Scholar] [CrossRef]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin nanoparticle-based drug delivery systems. Int. J. Nanomed. 2024, 19, 6945–6980. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Phipps, M.L.; Magurudeniya, H.D.; Pedersen, C.A.; Rojale, T.; Sheenan, C.J.; Courtney, S.J.; Bradfute, S.B.; Hraber, P.; Rush, M.N.; et al. Formulation of stabilizer-free, nontoxic PLGA and elastin-PLGA nanoparticle delivery systems. Int. J. Pharm. 2021, 597, 120340. [Google Scholar] [CrossRef]
- Moin, A.; Wani, S.U.D.; Osmani, R.A.; Lila, A.S.A.; Khafagy, E.-S.; Arab, H.H.; Gangadharappa, H.V.; Allam, A.N. Formulation, characterization, and cellular toxicity essessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. J. Drug Deliv. 2021, 28, 1626–1636. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable polymeric bio(nano)materials and their biomedical applications: A comprehensive overview and recent uptades. Polym. J. 2024, 16, 206. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Weigmann, B. Biodegradable polymeric nanoparticles loaded with flavonoids: A promising therapy for inflammatory bowel disease. Int. J. Mol. Sci. 2023, 24, 4454. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alberti, S.; Gagger, G.; Ferretti, M.; Botter, R.; Vivini, S.; Castellano, M. An up-to-date review on alginate nanoparticles and nanofibers for biomedical and pharmaceutical applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Design 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J.; Khan, W. Biodegradable polymers as drug carrier system. In Polymeric Biomaterials: Structure and Function; Dumitriu, S., Popa, V., Eds.; Taylor & Francis Group: Abingdon, UK, 2013; Volume 1, pp. 135–175. [Google Scholar]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polym. J. 2021, 13, 2623. [Google Scholar] [CrossRef]
- Cunha, A.; Gaubert, A.; Latxague, L.; Dehay, B. PLGA-based nanoparticles for neuroprotective drug delivery in neurodegenerative diseases. J. Pharm. 2021, 13, 1042. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. J. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Hemant, K.S.J.; Ayez, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles - A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Reboredo, C.; González-Navarro, C.J.; Martínez-Oharriz, C.; Martínez-López, A.L.; Irache, J.M. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int. J. Pharm. 2021, 597, 120287. [Google Scholar] [CrossRef] [PubMed]
- Szczęch, M.; Szczepanowicz, K. Polymeric core-shell nanoparticles prepared by spontaneous emulsification solvent evaporation and functionalized by the layer-by-layer method. J. Mater. 2020, 10, 496. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Fan, B.; Wang, Y.; Mao, Z.; Wang, W.; Wu, J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 2020, 321, 463–474. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral drug-loaded biocompatible polymeric nanoparticles obtained by non-aqueous emulsion polymerization. Polym. J. 2020, 12, 1018. [Google Scholar] [CrossRef]
- Baby, T.; Liu, Y.; Yang, G.; Chen, D.; Zhao, C.-X. Microfluidic synthesis of curcumin loaded polymer nanoparticles with tunable drug loading and pH-triggered release. J. Coll. Int. Sci. 2021, 594, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of poly(vinyl alcohol) on nanoencapsulation of budesonide in chitosan nanoparticles via ionic gelation and its improved bioavailability. Polym. J. 2020, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Ziora, Z.M.; Tamer, T.M.; Khalifa, R.E.; Hassan, M.A.; Mohy-Eldin, M.S.; Blaskovich, M.A.T. Formulation of quaternized aminated chitosan nanoparticles for efficient encapsulation and slow release of curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]
- Han, F.Y.; Liu, Y.; Kumar, V.; Xu, W.; Yang, G.; Zhao, C.-X.; Woodruff, T.M.; Whittaker, A.K.; Smith, M.T. Sustained-release ketamine-loaded nanoparticles fabricated by sequential nanoprecipitation. Int. J. Pharm. 2020, 581, 119291. [Google Scholar] [CrossRef]
- Romeo, A.; Musumeci, T.; Carbone, C.; Bonaccorso, A.; Corvo, S.; Lupo, G.; Anfuso, C.D.; Puglisi, G.; Pignatello, R. Ferulic acid-loaded polymeric nanoparticles for potential ocular delivery. J. Pharm. 2021, 13, 687. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, V.M.; de Brito, N.A.P.; Ferreira, N.N.; Boni, F.I.; Ferreira, L.M.B.; Carvahlo, S.G.; Gremião, M.P.D. Design of mucoadhesive gellan gum and chitosan nanoparticles intended for colon-specific delivery of peptide drugs. Colloids Surf. A 2021, 628, 127321. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, Y.; Wen, Q.; Luo, J.; Lu, Y.; Wu, Z.X.; Wang, B.Q.; Chen, Y.; Zhao, L.; Fu, S.Z. Co-delivery of paclitaxel and curcumin by biodegradable polymeric nanoparticles for breast cancer chemotherapy. Int. J. Pharm. 2020, 589, 119875. [Google Scholar] [CrossRef] [PubMed]
- Pedrozo, R.C.; Antônio, E.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing the flavonoid rutin produced by nano spray drying. Braz. J. Pharm. Sci. 2020, 56, e17692. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Anjum, M.M.; Patel, K.K. Gemcitabine cationic polymeric nanoparticles against ovarian cancer: Formulation, characterization, and targeted drug delivery. J. Drug Deliv. 2022, 29, 1060–1074. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Forys, A.; Trzebicka, B.; Pispas, S. Structure-based evaluation of hybrid lipi-polymer nanoparticles: The role of the polymeric guest. Polym. J. 2024, 16, 290. [Google Scholar]
- Meng, F.; Yun, Z.; Yan, G.; Wang, G.; Lin, C. Engineering of anticancer drugs entrapped polymeric nanoparticles for the treatment of colorectal cancer therapy. Process Biochem. 2021, 111, 36–42. [Google Scholar] [CrossRef]
- Bolla, P.K.; Gote, V.; Singh, M.; Yellepeddi, V.K.; Patel, M.; Pal, D.; Gong, X.; Sambalingam, D. Preparation and characterization of lutein loaded folate conjugated polymeric nanoparticles. J. Microencapsul. 2020, 37, 502–516. [Google Scholar] [CrossRef]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In vitro release study of the polymeric drug nanoparticles: Development and validation of a novel method. J. Pharm. 2020, 12, 732. [Google Scholar] [CrossRef]
- de Oliveira Junior, E.R.; Santos, L.C.R.; Salomão, M.A.; Nascimento, T.L.; Oliveira, G.A.R.; Lião, L.M.; Lima, E.M. Nose-to-brain drug delivery mediated by polymeric nanoparticles: Influence of PEG surface coating. J. Drug Deliv. Transl. Res. 2020, 10, 1688–1699. [Google Scholar] [CrossRef]
- Abdollahi, A.; Alidaei-Sharif, H.; Roghani-Mamaqani, H.; Herizchi, A. Photoswitchable fluorescent polymer nanoparticles as high-security anticounterfeiting materials for authentication and optical pattering. J. Mater. Chem. C 2020, 8, 5476–5493. [Google Scholar] [CrossRef]
- Alberg, I.; Kramer, S.; Schinnerer, M.; Hu, Q.; Seidl, C.; Leps, C.; Drude, N.; Möckel, D.; Rijcken, C.; Lamers, T.; et al. Polymeric nanoparticles with neglectable protein corona. Small 2020, 16, 1907574. [Google Scholar] [CrossRef]
- Kolouchova, K.; Groborz, O.; Cernochova, Z.; Skarkova, A.; Brabek, J.; Rosel, D.; Svec, P.; Starcuk, Z.; Slouf, M.; Hruby, M. Thermo- and ROS-responsive self-assembled polymer nanoparticle tracers for 19F MRI theranostics. J. Biol. Macromol. 2021, 22, 2325–2337. [Google Scholar] [CrossRef]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-modified polymeric nanoparticles for drug delivery to cancer cells. Expert. Opin. J. Drug Deliv. 2020, 18, 1–24. [Google Scholar] [CrossRef]
- Cornel, E.J.; Smith, G.N.; Rogers, S.E.; Hallett, J.E.; Growney, D.J.; Smith, T.; O’Hora, P.S.; Meurs, S.; Mykhaylyk, O.O.; Armes, S.P. Time-resolved small-angle neutron scattering studies of the thermally-induced exchange of copolymer chains between spherical diblock copolymer nanoparticles prepared via polymerization-induced self-assembly. J. Soft Matter. 2020, 16, 3657–3668. [Google Scholar] [CrossRef]
- Smith, G.N.; Canning, S.L.; Derry, M.J.; Jones, E.R.; Neal, T.J.; Smith, A.J. Ionic and nonspherical polymer nanoparticles in nonpolar solvents. Macromolecules 2020, 53, 3148–3156. [Google Scholar] [CrossRef]
- Wen, K.; Wu, L.; Wu, X.; Lu, Y.; Duan, T.; Ma, H.; Peng, A.; Shi, Q.; Huang, H. Precisely tuning photothermal and photodynamic effects of polymeric nanoparticles by controlled copolymerization. Angew. Chem. 2020, 132, 12856–12861. [Google Scholar] [CrossRef]
- Raula, J.; Eerikäinen, H.; Kauppinen, E.I. Influence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticles. Int. J. Pharm. 2004, 284, 13–21. [Google Scholar] [CrossRef]
- Gosecka, M.; Gosecki, M. Characterization methods of polymer core-shell particles. Colloid. Polym. Sci. 2015, 293, 2719–2740. [Google Scholar] [CrossRef]
- Bunio, P.; Zielińska, K.; Chlebicki, J.; Wilk, K. Preparation of polymeric nanoparticles using a new polymerizable surfactant. Cent. Eur. J. Phys. 2011, 9, 570–575. [Google Scholar] [CrossRef]
- Nimesh, S.; Manchanda, R.; Kumar, R.; Saxena, A.; Chaudhary, P.; Yadav, V.; Mozumdar, S.; Chandra, R. Preparation, characterization and in vitro drug release studies of novel polymeric nanoparticles. Int. J. Pharm. 2006, 323, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.R. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Fisher, J.P.; Mikos, A.G.; Hogan, K.J. Polymeric nanomaterials in 3D bioprinting for tissue engineering and drug delivery applications. Bioprinting 2024, 40, e00345. [Google Scholar] [CrossRef]
- Khan, I.N.; Navaid, S.; Waqar, W.; Hussein, D.; Ullah, N.; Khan, M.U.A.; Hussain, Z.; Javed, A. Chitosan-based polymeric nanoparticles as an efficient gene delivery system to cross blood brain barrier: In vitro and in vivo evaluations. J. Pharm. 2024, 17, 169. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. J. Mater. 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Haq, I.; Cowen, T.; Masi, S.D.; Trivedi, S.; Alanazi, K.; Piletska, E.; Mujahid, A.; Piletsky, S.A. Design and fabrication of a smart sensor using in silico epitope mapping and electro-responsive imprinted polymer nanoparticles for determination of insulin levels in human plasma. Biosens. Bioelectron. 2020, 169, 112536. [Google Scholar]
- Yudhistira, T.; da Silva, E.C.; Combes, A.; Lehmann, M.; Reisch, A.; Klymchenko, A.S. Biotinylated fluorescent polymeric nanoparticles for enhanced immunostaining. Small Methods 2023, 7, 2201452. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Ren, Y.; Yuan, Q.; Wang, Y.; Bao, B.; Li, M.; Tang, Y. Chemiluminescent conjugated polymer nanoparticles for deep-tissue inflammation imaging and photodynamic therapy of cancer. J. Am. Chem. Soc. 2024, 146, 5927–5939. [Google Scholar] [CrossRef]
- Simpson, E.; Sarwar, H.; Jack, I.; Lowry, D. Evaluation of the potential of chitosan nanoparticles as a delivery vehicle for gentamicin for the treatment of osteomyelitis. J. Antibiot. 2024, 13, 208. [Google Scholar] [CrossRef]
- Latronico, T.; Rizzi, F.; Panniello, A.; Laquintana, V.; Arduino, I.; Denora, N.; Fanizza, E.; Milella, S.; Mastroianni, C.M.; Striccoli, M.; et al. Luminescent PLGA nanoparticles for delivery of darunavir to the brain and inhibition of matrix metalloproteinase-9, a relevant therapeutic target of HIV-associated neurological disorders. ACS Chem. Neurosci. 2021, 12, 4286–4301. [Google Scholar] [CrossRef]
- Endo, E.H.; Makimori, R.Y.; Companhoni, M.V.P.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Ketoconazole-loaded poly(lactic acid) nanoparticles: Characterization and improvement of antifungal efficacy in vitro against Candida and dermatophytes. J. Mycol. Med. 2020, 30, 101003. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Dehaghi, H.; Ghaemi, A.; Maleki, H.; Yazdian, F.; Rahdar, A.; Pandey, S. Polymeric nanoparticles as delivery vehicles for targeted delivery of chemotherapy drug fludarabine to treat hematological cancers. Inorg. Chem. Commun. 2024, 167, 112819. [Google Scholar] [CrossRef]
- Bensouiki, S.; Belaib, F.; Sindt, M.; Magri, P.; Rup-Jacques, S.; Bensouici, C.; Meniai, A.-H. Evaluation of anti-inflammatory activity and in vitro drug release of ibuprofen-loaded nanoparticles based on sodium alginate and chitosan. Arab. J. Sci. Eng. 2020, 45, 7599–7609. [Google Scholar] [CrossRef]
- Mohsen, A.M. Cationic polymeric nanoparticles for improved ocular delivery and antimycotic activity of terconazole. J. Pharm. Sci. 2022, 111, 458–468. [Google Scholar] [CrossRef]
- Pechanova, O.; Dayar, E.; Cebova, M. Therapeutic potential of polyphenols-loaded polymeric nanoparticles in cardiovascular system. Molecules 2020, 25, 3322. [Google Scholar] [CrossRef]
- Jummes, B.; Sganzerla, W.G.; da Rosa, C.G.; Noronha, C.M.; Nunes, M.R.; Bertoldi, F.C.; Baretto, P.L.M. Antioxidant and antimicrobial poly-ε-caprolactone loaded with Cymbopogon martinii essential oil. Biocatal. Agric. Biotechnol. 2020, 23, 101499. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Formulation and characterisation of insulin-loaded chitosan nanoparticles capable of inducing glucose uptake in skeletal muscle cells in vitro. J. Drug Deliv. Sci. Technol. 2020, 57, 101738. [Google Scholar] [CrossRef]
- Shinde, U.; Ahmed, M.H.; Singh, K. Development of dorzolamide loaded 6-O-carboxymethyl chitosan nanoparticles for open angle glaucoma. J. Drug Deliv. 2013, 2013, 562727. [Google Scholar] [CrossRef]
- Khan, F.U.; Nasir, F.; Iqbal, Z.; Neau, S.; Khan, I.; Hassan, M.; Iqbal, M.; Ullah, A.; Khan, S.I.; Sakhi, M. Improved ocular bioavailability of moxifloxacin HCl using PLGA nanoparticles: Fabrication, characterization, in-vitro and in-vivo evaluation. Iran. J. Pharm. Res. 2021, 20, 592–608. [Google Scholar]
- Hanafy, A.F.; Abdalla, A.M.; Guda, T.K.; Gabr, K.E.; Royall, P.G.; Alqurshi, A. Ocular anti-inflammatoryactivity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int. J. Nanomed. 2019, 14, 3679–3689. [Google Scholar] [CrossRef] [PubMed]
- Toudeshchouei, M.G.; Zahedi, P.; Shavandi, A. Microfluidic-assisted preparation of 5-fluorouracil-loaded PLGA nanoparticles as a potential system for colorectal cancer therapy. J. Mater. 2020, 13, 1483. [Google Scholar] [CrossRef]
- El-Said Azzazy, H.M.; Abdelnaser, A.; Mulla, H.A.; Sawy, A.M.; Shamma, S.N.; Elhusseiny, M.; Alwahibi, S.; Mahdy, N.K.; Fahmy, S.A. Essential oils extracted from Boswelia sacra oleo gum resin loaded into PLGA-PCL nanoparticles: Enhanced cytotoxic and apoptotic effects against breast cancer cells. ACS Omega 2023, 8, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Muntimadugu, E.; Rafeeqi, T.A.; Domb, A.J.; Khan, W. Co-delivery of rapamycin and piperine-loaded polymeric nanoparticles for breast cancer treatment. J. Drug Deliv. 2016, 23, 2608–2616. [Google Scholar] [CrossRef]
- Gamil, Y.; Hamed, M.G.; Elsayed, M.; Essawy, A.; Medhat, S.; Zayed, S.O.; Ismail, R.M. The anti-fungal effect of miconazole and miconazole-loaded chitosan nanoparticles gels in diabetic patients with oral candidiasis - randomized control clinical trial and microbiological analysis. BMC Oral. Health 2024, 24, 196. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Rhazi, M.; Taourirte, M. Chitosan nanoparticles as a new delivery system for the anti-HIV drug zidovudine. Bull. Korean Chem. Soc. 2013, 34, 1333. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, M.; Ullah, Q.; Khan, M.Z.; Sohail, A.; Islam, R.; Bilal, H.; Ullah, S.; Iqbal, A. In vitro and in vivo effects of conventional and chitosan nanoparticle-encapsulated miltefosine drug for treatment of cutaneous leishmaniasis. Med. Sci. Forum 2023, 21, 19. [Google Scholar] [CrossRef]
- Tzeyung, A.; Md, S.; Bhattamisra, S.K.; Madheswaran, T.; Alhakamy, N.A.; Aldawsari, M.M.; Radhakrishnan, A. RETRACTED: Fabrication, optimization, and evaluation of rotigotine-loaded chitosan nanoparticles for nose-to-brain delivery. J. Pharm. 2019, 11, 26. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, S.K.; Girotra, P. Targeting silymarin for improved hepatoprotective activity through chitosan nanoparticles. Int. J. Pharm. Invest. 2014, 4, 156–163. [Google Scholar]
- Bhokare, S.G.; Marathe, R.P. Rosuvastatin calcium loaded chitosan nanoparticles: Preparation, evaluation and in vitro release studies. Int. J. Appl. Pharm. 2020, 12, 95–102. [Google Scholar] [CrossRef]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhai, T.; Sun, J.; Yu, Q.; Feng, Y.; Li, R.; Wang, H.; Ouyang, Q.; Yang, T.; Zhan, Q.; et al. Mucus-permeable polymyxin B-hyaluronic acid/poly(lactic-co-glycolic acid) nanoparticle platform for the nebulized treatment of lung infections. J. Colloid. Interface Sci. 2022, 624, 307–319. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Taha, G.M.; El-Alfy, E.A.; El-Bisi, M.K. Enhancing antibacterial action of gauze by adding gelatin nanoparticles loaded with spectinomycin and chloramphenicol. Cellulose 2022, 29, 5677–5688. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Soltaninejad, H.; Ghorani-Azam, A.; Forouzani-Moghaddam, M.J.; Mozafri, S.; Akhoundi-Meybodi, Z.; Ferdosian, F.; Jabinian, F. Investigating the antimicrobial activity of vancomycin-loaded soy protein nanoparticles. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 5709999. [Google Scholar] [CrossRef]
- Jose, S.; Juna, B.C.; Cinu, T.A.; Jyoti, H.; Aleykutty, N.A. Carboplatin loaded surface modified PLGA nanoparticles: Optimization, characterization, and in vivo brain targeting studies. Colloids Surf. B 2016, 142, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, V.; Katiyar, S.S.; Dora, C.P.; Agrawal, A.K.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Co-delivery of doxetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018, 73, 424–436. [Google Scholar] [CrossRef]
- Jamwal, S.; Ram, B.; Ranote, S.; Dharela, R.; Chauhan, G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int. J. Biol. Macromol. 2019, 123, 968–978. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Verma, R.K.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H. Metformin-loaded alginate nanoparticles as an effective antidiabetic agent for controlled drug release. Pharm. Pharmacol. 2017, 69, 143–150. [Google Scholar] [CrossRef]
- Nahar, M.; Mishra, D.; Dubey, V.; Kumar, N. Development, characterization, and toxicity evaluation of amphotericin B-loaded gelatin nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 252–261. [Google Scholar] [CrossRef]
- John, J.E.; Tytler, B.A.; Habila, J.; Apeji, Y.E.; Olayemi, O.; Isimi, C.Y. Cross-linking with multifunctional excipients and its effect on the physicochemical properties and release profile of ibuprofen-loaded Digitaria exilis starch nanoparticles. J. Res. Pharm. 2022, 25, 1190–1201. [Google Scholar]
- Ghaffarzadegan, R.; Khoee, S.; Rezazadeh, S. Fabrication, characterization and optimization of berberine-loaded PLA nanoparticles using coaxial electrospray for sustained drug release. DARU J. Pharm. Sci. 2020, 28, 237–252. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS Pharm. Sci. Tech. 2019, 20. [Google Scholar] [CrossRef]
- Zanetti, M.; Mazon, L.R.; de Meneses, A.C.; Silva, L.L.; de Araújo, P.H.H.; Fiori, M.A.; de Oliveira, D. Encapsulation of geranyl cinnamate in polycaprolactone nanoparticles. Mater. Sci. Eng. C 2019, 97, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Tang, K.; Hu, X.; Zou, G. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2007, 107, 891–897. [Google Scholar] [CrossRef]
- Geng, T.; Zhao, X.; Ma, M.; Zhu, G.; Yin, L. Resveratrol-loaded albumin nanoparticles with prolonged blood circulation and improved biocompatibility for highly effective targeted pamcreatic tumor therapy. Nanoscale Res. Lett. 2017, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Nesalin, J.A.J.; Smith, A.A. Preparation and evaluation of stavudine loaded chitosan nanoparticles. J. Pharm. Res. 2013, 6, 268–274. [Google Scholar]
- Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Antibacterial and antibiofilm activities of chitosan nanoparticles loaded with Ocimum basilicum L. essential oil. Int. J. Biol. Macromol. 2022, 202, 122–129. [Google Scholar] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the antimicrobial activity of oregano oil by encapsulation in chitosan-alginate nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef] [PubMed]
- Niclas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17β-estradiol-hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef]
- Parente, J.F.; Sousa, V.I.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Biodegradable Polym. J for microencapsulation systems. Adv. Polym. Technol. 2022, 2022, 4640379. [Google Scholar] [CrossRef]
- Anju, S.; Prajitha, N.; Sukanya, V.S.; Mohanan, P.V. Complicity of degradable polymers in health-care applications. Mater. Today Chem. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Chen, C.-K.; Huang, P.-K.; Law, W.-C.; Chu, C.-H.; Chen, N.-T.; Lo, L.-W. Biodegradable polymers for gene-delivery applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef] [PubMed]


| Type of Polymeric Nanoparticles | Origin | Polymeric Matrix | Ref. |
|---|---|---|---|
| Natural | Algae | Alginate | [16] |
| Carageenan | [17] | ||
| Fucoidan | [18] | ||
| Animals | Albumin | [19] | |
| Casein | [20] | ||
| Chitosan | [21] | ||
| Collagen | [22] | ||
| Gelatin | [23] | ||
| Keratin | [24] | ||
| Silk fibroin | [25] | ||
| Bacteria | Dextran | [26] | |
| Gellan gum | [27] | ||
| Levan | [28] | ||
| Xanthan gum | [29] | ||
| Fungi | Pullulan | [30] | |
| Plants | Cellulose | [31] | |
| Gliadin | [32] | ||
| Guar gum | [33] | ||
| Pectin | [34] | ||
| Starch | [35] | ||
| Zein | [36] | ||
| Synthetic | --- | Poly-ε-caprolactone (PCL) | [37] |
| Polyglycolic acid (PGA) | [38] | ||
| Polylactic acid (PLA) | [39] | ||
| Poly(lactic-co-glycolic acid) (PLGA) | [40] | ||
| Polyvinyl alcohol (PVA) | [41] |
| Polymer Type/Chemical Composition | Polymeric Material | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Natural | Polysaccharides | Alginate | Water-solubility Biodegradability Biocompatibility Mucoadhesion Gel-forming capability Low immunogenicity Low cost Non-toxicity | Low mechanical properties Sterilization is difficult | [2,5,54,55,56] |
| Carageenan | Forms highly viscous solutions or elastic gels Protein-binding properties Emulsion stabilizer | Low gel strength Anti-coagulant properties | [53,55] | ||
| Cellulose | Abundant in nature Biocompatibility Low toxicity Low cost | Insolubility in many common solvents (difficult processing) Lack of flexibility Lack of thermoplasticity | [55] | ||
| Chitosan | Mucoadhesion In situ gelation Biocompatibility Anti-bacterial properties Biodegradability | High in vivo degradation rate Low mechanical strength Hard to control NP size Low flexibility Not easy to process Insoluble in neutral solutions (dissolves in diluted acidic solutions) | [2,55] | ||
| Chitin | Abundant in nature Biodegradability Biocompatibility Non-toxicity Mucoadhesive Easy chemical modification | The content of impurities depends on the chitin source and preparation method Poor solubility at physiological pH | [53] | ||
| Dextran | Biocompatibility Anti-thrombotic properties Good water solubility Easy functionalization Biodegradability Good rheological and thermal properties | High cost Non-available Encapsulated drugs are released very fast | [53,55] | ||
| Fucoidan | Non-toxicity Biodegradability Biocompatibility Certain biological properties (anti-oxidant, anti-inflammatory, anticoagulant) | The quality of fucoidan depends on the species from which it is extracted | [53] | ||
| Hyaluronic acid | Easy chemical modification Interacts with cells (cell proliferation, angiogenesis, matrix organization) | Absorbs large amount of water Rapid degradation Brittle High cost Poor mechanical properties | [53,54] | ||
| Pullulan | Biodegradability Biocompatibility Non-toxicity Water-solubility Forms stable, viscous non-hygroscopic solutions Adhesion properties Non-immunogenicity | Highly expensive Brittle Low mechanical strength | [55] | ||
| Starch | Biodegradability Low cost Biocompatibility Easily available Swelling properties | Very high viscosity Low mechanical properties Fragile (very high water uptake) Lack of flexibility Brittle Degrades before its melting temperature Difficult processability | [2,55] | ||
| Proteins | Albumin | Biodegradability Non-toxicity Highly abundant Biocompatibility Non-cytotoxicity Water-solubility | Possible immunogenic reactions Expensive | [2,55] | |
| Collagen | Low immunogenicity Excellent cell adhesion Biocompatibility Biodegradability Abundant in human body | Low mechanical strength Variability depending on collagen source | [2,55] | ||
| Elastin | Abundant in human body It can retain its original shape even after stretching Ability to self-assemble (response to various temperatures) | Not always biocompatible Difficult to alter | [53] | ||
| Gelatin | Biocompatibility Biodegradability Thermo-reversible gelation properties Ability to form hydrogels Eco-friendly Low cost Water-solubility Easily available Great stability Non-immunogenic The isoelectric point can be modified to optimize the loading of charged drugs | Fast degradation in physiological fluids Brittle | [2,9,53] | ||
| Silk fibroin | Biocompatibility Good elastic properties Very high mechanical strength Controlled degradation rate | Degradation with immunogenic reactions | [2,53] | ||
| Synthetic | Poly-ε-caprolactone (PCL) | Biocompatibility Non-toxicity Good mechanical properties Flexibility Slow biodegradation (useful in controlled drug release) Good rheological properties | Low bioactivity Hydrophobicity (poor cellular adhesion) Use of toxic solvents during synthesis | [53,54,57] | |
| Polyglycolic acid (PGA) | Excellent mechanical properties Long-term stability Soft Biodegradability High melting point | Short biocompatibility (in contact with biological fluids) Hydrophobicity (difficulties in interaction with cells) Insolubility in many common solvents Rapid degradation rate | [58] | ||
| Polylactic acid (PLA) | Biodegradability Biocompatibility High mechanical strength Eco-friendly | No cell adhesion Expensive Chemically inert Poor stability in heat Very brittle | [3,55] | ||
| Poly(lactic-co-glycolic acid) (PLGA) | Biodegradability Biocompatibility High stability Low toxicity | Before degradation the polymer remains in the circulation and then accumulates in the main organs like liver, lung, and spleen | [3,4,54,59,60] | ||
| Polivinyl alcohol (PVA) | Biocompatibility Water-solubility Flexibility Low cost | Very high water uptake No cell adhesion | [2] | ||
| Technique | Type of Polymeric Nanoparticles. | Loaded Drug | Ref. |
|---|---|---|---|
| Desolvation | Zein | --- | [62] |
| Emulsification solvent evaporation | Poly(caprolactone) (PCL), poly(lactic acid) (PLA), poly(lactide-co-glycolic acid) (PLGA) | Coumarin-6 | [63] |
| Poly(lactide-co-glycolic acid) (PLGA | Sparfloxacin, tacrolimus | [64] | |
| Emulsion polymerization | Poly(ε-caprolactone) (PCL), poly(vinylpyrrolidone) (PNVP) | Cisplatin | [65] |
| Microfluidization | Shellac | Curcumin | [66] |
| Ionic gelation | Chitosan | Budenoside | [67] |
| Chitosan | Curcumin | [68] | |
| Nanoprecipitation | Poly(ethylene glycol) (PEG)-block-poly(lactic-co-glycolic acid) (PLGA) | Ketamine | [69] |
| Polylactic acid (PLA), poly(lactic-co-glycolic acid) (PLGA) | Ferulic acid | [70] | |
| Polyelectrolyte complexation | Gellan gum, chitosan | --- | [71] |
| Self-assembly | Tri-block copolymer poly (ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL-PEG-PCL, PCEC) | Paclitaxel, curcumin | [72] |
| Spray-drying | Bovine serum albumin (BSA) | Rutin | [73] |
| Technique | Knowledge Obtained | Ref. |
|---|---|---|
| Atomic force microscopy (AFM) | Surface texture Roughness Particle size distribution Aggregation | [74] |
| Cryo-transmission electron microscopy (cryo-TEM) High-resolution transmission electron microscopy (HR-TEM) Transmission electron microscopy (TEM) | Structure Size Size distribution Shape heterogeneity Aggregation | [74,75,76] |
| Differential scanning colorimetry (DSC) | Drug–polymer interaction Physicochemical state | [74,77] |
| Dynamic light scattering (DLS) Photon correlation spectroscopy (PCS) | Size Shape Polydispersity Surface charge | [12,78,79] |
| Fluorimetry High-performance liquid chromatography (HPLC) UV-Vis spectrophotometry | Drug content In vitro drug release | [78,80] |
| Fourier-transform infrared spectroscopy (FT-IR) Raman spectroscopy | Chemical composition Functional groups | [74,77] |
| Mass spectrometry (MS) | Molecular weight Composition Structure Surface properties | [81] |
| Nuclear magnetic resonance (NMR) | Chemical composition Structure Purity | [82] |
| Scanning electron microscopy (SEM) Scanning tunneling microscopy (STM) | Geometry Topography Composition Size Size distribution Aggregation | [74,80,83] |
| Small-angle neutron scattering (SANS) Small-angle X-ray diffraction (SAXS) | Shape Core/shell morphology | [84,85] |
| X-ray photoelectron spectroscopy (XPS) | Elemental and chemical composition at the surface | [86] |
| Group of Drugs | Encapsulated Active Agent | Polymeric Matrix | Ref. |
|---|---|---|---|
| Antibiotics | Ciprofloxacin Gentamycin Tetracycline | Chitosan | [120] |
| Polymyxin B | Hyaluronic acid Poly(lactic-co-glycolic acid) | [121] | |
| Spectinomycin and chloramphenicol | Gelatin | [122] | |
| Vancomycin | Soy protein | [123] | |
| Anticancers | Carboplatin | Poly(lactic-co-glycolic acid) | [124] |
| Docetaxel Gemcitabine | Albumin | [125] | |
| Doxorubicin siRNA | Starch | [7] | |
| Antidiabetics | Insulin | Dextran | [126] |
| Metformin | Alginate | [127] | |
| Antifungals | Amphotericin A | Gelatin | [128] |
| Anti-inflammatory drugs | Ibuprofen | Starch | [129] |
| Antioxidants | Berberine | Polylactic acid | [130] |
| Curcumin | Chitosan | [131] | |
| Geranyl cinnamate | Poly-ε-caprolactone | [132] | |
| Quercetin | Chitosan | [133] | |
| Resveratrol | Albumin | [134] | |
| Antivirals | Stavudine | Chitosan | [135] |
| Essential oils | Basil essential oil | Chitosan | [136] |
| Green tea essential oil Peppermint essential oil | Chitosan | [137] | |
| Oregano essential oil | Alginate Chitosan | [138] | |
| Hormones | 17β-Estradiol hemihydrate | Collagen | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. https://doi.org/10.3390/polym16172536
Geszke-Moritz M, Moritz M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers. 2024; 16(17):2536. https://doi.org/10.3390/polym16172536
Chicago/Turabian StyleGeszke-Moritz, Małgorzata, and Michał Moritz. 2024. "Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges" Polymers 16, no. 17: 2536. https://doi.org/10.3390/polym16172536
APA StyleGeszke-Moritz, M., & Moritz, M. (2024). Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers, 16(17), 2536. https://doi.org/10.3390/polym16172536








