Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion (Part 2: Operational Conditions of Vibrational Technology)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Strain and Biomass Production
2.2.2. Encapsulation Process
Preparing Solutions
Probiotic Encapsulation
Cell Release
Cell Viability
Encapsulation Efficiency (EE)
2.2.3. Beads Characterization
Particle Size
Raman Spectroscopy
Scanning Electron Microscopy (SEM)
Live/Dead Assay of Cells by Confocal Laser Scanning Microscopy (CLSM)
Mechanical Properties
2.2.4. Probiotic Cell Viability under INFOGEST Simulated Gastrointestinal Model
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Optimal Design
3.2. Characterization of Beads
3.3. Probiotic Cell Viability under INFOGEST Simulated Gastrointestinal Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cueto, C.; Aragón, S. Evaluación del potencial probiótico de bacterias ácido lácticas para reducir el colesterol in vitro Evaluation of probiotic potential of lactic acid bacteria to reduce in vitro cholesterol. Sci. Agropecu. 2012, 1, 45–50. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Muhammad, N.; Hak, B.; Yoo, J.J. Probiotic delivery systems: A brief overview Probiotic delivery systems: A brief overview. J. Pharm. Investig. 2016, 46, 377–386. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Vignolles, M.L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Altintas, Z.; Sharma, S. Effect of Alginate-Microencapsulated Hydrogels on the Survival of Lactobacillus rhamnosus under Simulated. Foods 2021, 10, 1999. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibsonbc, D.L.; Hoorfar, M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021, 3, 2699. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT—Food Sci. Technol. 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Sun, Q.; Yin, S.; He, Y.; Cao, Y.; Jiang, C. Biomaterials and Encapsulation Techniques for Probiotics: Current Status and Future Prospects in Biomedical Applications. Nanomaterials 2023, 13, 2185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiménez-Martín, E.; Gharsallaoui, A.; Rojas, T.A. Suitability of Using Monolayered and Multilayered Emulsions for Microencapsulation of ω-3 Fatty Acids by Spray Drying: Effect of Storage at Different Temperatures. Food Bioprocess Technol. 2015, 8, 100–111. [Google Scholar] [CrossRef]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.J.; Cho, Y. Microencapsulation of probiotic lactobacillus acidophilus kbl409 by extrusion technology to enhance survival under simulated intestinal and freeze-drying conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Żelaziński, T. Materials Used for the Microencapsulation of Probiotic Bacteria in the Food Industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ergin, F.; Atamer, Z.; Göcer, E.M.C.; Demir, M.; Hinrichs, J.; Kucukcetin, A. Optimization of Salmonella bacteriophage microencapsulation in alginate-caseinate formulation using vibrational nozzle technique. Food Hydrocoll. 2021, 113, 106456. [Google Scholar] [CrossRef]
- Whelehan, M.; Marison, I.W. Microencapsulation using vibrating technology. J. Microencapsul. 2011, 28, 669–688. [Google Scholar] [CrossRef]
- Kailasapathy, K. Microencapsulation of Probiotic Bacteria: Technology and Potential Applications. Curr. Issues Intest. Microbiol. 2002, 3, 39–48. [Google Scholar]
- Rojas-Muñoz, Y.V.; Santagapita, P.R.; Quintanilla-Carvajal, M.X. Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion. Polymers 2023, 15, 4296. [Google Scholar] [CrossRef]
- Aragón-Rojas, S.; Quintanilla-Carvajal, M.X.; Hernández-Sánchez, H. Multifunctional Role of the Whey Culture Medium in the Spray-Drying Microencapsulation of Lactic Acid Bacteria. Food Technol. Biotechnol. 2018, 56, 381–397. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods 2020, 9, 1051. [Google Scholar] [CrossRef]
- Graff, S.; Hussain, S.; Chaumeil, J.; Charrueau, C. Increased Intestinal Delivery of Viable Saccharomyces boulardii by Encapsulation in Microspheres. Pharm. Res. 2008, 25, 1290–1296. [Google Scholar] [CrossRef]
- Ricaurte, L.; Santagapita, P.R.; Díaz, L.E.; Quintanilla-Carvajal, M.X. Edible gelatin-based nanofibres loaded with oil encapsulating high-oleic palm oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124673. [Google Scholar] [CrossRef]
- Zazzali, I.; Rocio, T.; Calvo, A.; Manuel, V.; Ruíz-henestrosa, P.; Santagapita, P.R.; Perullini, M. Effects of pH, extrusion tip size and storage protocol on the structural properties of Ca (II)-alginate beads. Carbohydr. Polym. 2019, 206, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Gaona-Sánchez, V.A.; Calderón-Domínguez, G.; Morales-Sánchez, E.; Moreno-Ruiz, L.A.; Terrés-Rojas, E.; Salgado-Cruz, M.D.L.P.; Escamilla-García, M.; Barrios-Francisco, R. Physicochemical and superficial characterization of a bilayer film of zein and pectin obtained by electrospraying. Appl. Polym. Sci. 2020, 138, 50045. [Google Scholar] [CrossRef]
- Hernández-Varela, J.D.; Villaseñor-Altamirano, S.L.; Chanona-Pérez, J.J.; González Victoriano, L.; Perea Flores, M.d.J.; Cervantes Sodi, F.; Calderón Benavides, H.A.; Morgado Aucar, P. Effect of cellulose nanoparticles from garlic waste on the structural, mechanical, thermal, and dye removal properties of chitosan/alginate aerogels. J. Polym. Res. 2022, 29, 133. [Google Scholar] [CrossRef]
- González-Quijano, G.K.; Dorantes-Alvarez, L.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E.; de Jesús Perea-Flores, M.; Vera-Ponce de León, A.; Hernández-Rodríguez, C. Halotolerance and survival kinetics of lactic acid bacteria isolated from jalapeño pepper (Capsicum annuum L.) fermentation. J. Food Sci. 2014, 79, M1545–M1553. [Google Scholar] [CrossRef]
- Rajmohan, D.; Bellmer, D. Characterization of Spirulina-Alginate Beads Formed Using Ionic Gelation. Int. J. Food Sci. 2019, 2019, 7101279. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Gañan-Calvo, A.M.; Riesco-Chueca, P. Jetting–dripping transition of a liquid jet in a lower viscosity co-flowing immiscible liquid: The minimum flow rate in flow focusing. J. Fluid Mech. 2006, 553, 75–84. [Google Scholar] [CrossRef]
- Fangmeier, M.; Lehn, D.N.; Maciel, M.J.; Volken de Souza, C.F. Encapsulation of Bioactive Ingredients by Extrusion with Vibrating Technology: Advantages and Challenges. Food Bioprocess Technol. 2019, 12, 1472–1486. [Google Scholar] [CrossRef]
- Olivares, A.; Silva, P.; Altamirano, C. Microencapsulation of probiotics by efficient vibration technology. J. Microencapsul. 2017, 34, 667–674. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Li, D.; Xu, M.; Chen, H.; Zhang, Z.; Tang, Z. Encapsulation of probiotic Lactobacillus bulgaricus in alginate—Milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Nemethova, V.; Lacik, I.; Razga, F. Vibration technology for microencapsulation: The restrictive role of viscosity. J. Bioprocess. Biotech. 2014, 5, 1–3. [Google Scholar] [CrossRef]
- Eckert, C.; Agnol, W.D.; Dallé, D.; Serpa, V.G.; Maciel, M.J.; Lehn, D.N.; Souza, C.F.V. Development of alginate-pectin microparticles with dairy whey using vibration technology: Effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Res. Int. 2018, 113, 65–73. [Google Scholar] [CrossRef]
- Kroneková, Z.; Pelach, M.; Mazancová, P.; Uhelská, L.; Treľová, D.; Rázga, F.; Némethová, V.; Szalai, S.; Chorvát, D.; McGarrigle, J.J.; et al. Structural changes in alginate-based microspheres exposed to in vivo environment as revealed by confocal Raman microscopy. Sci. Rep. 2018, 8, 1637. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT—Food Sci. Technol. 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT—Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]

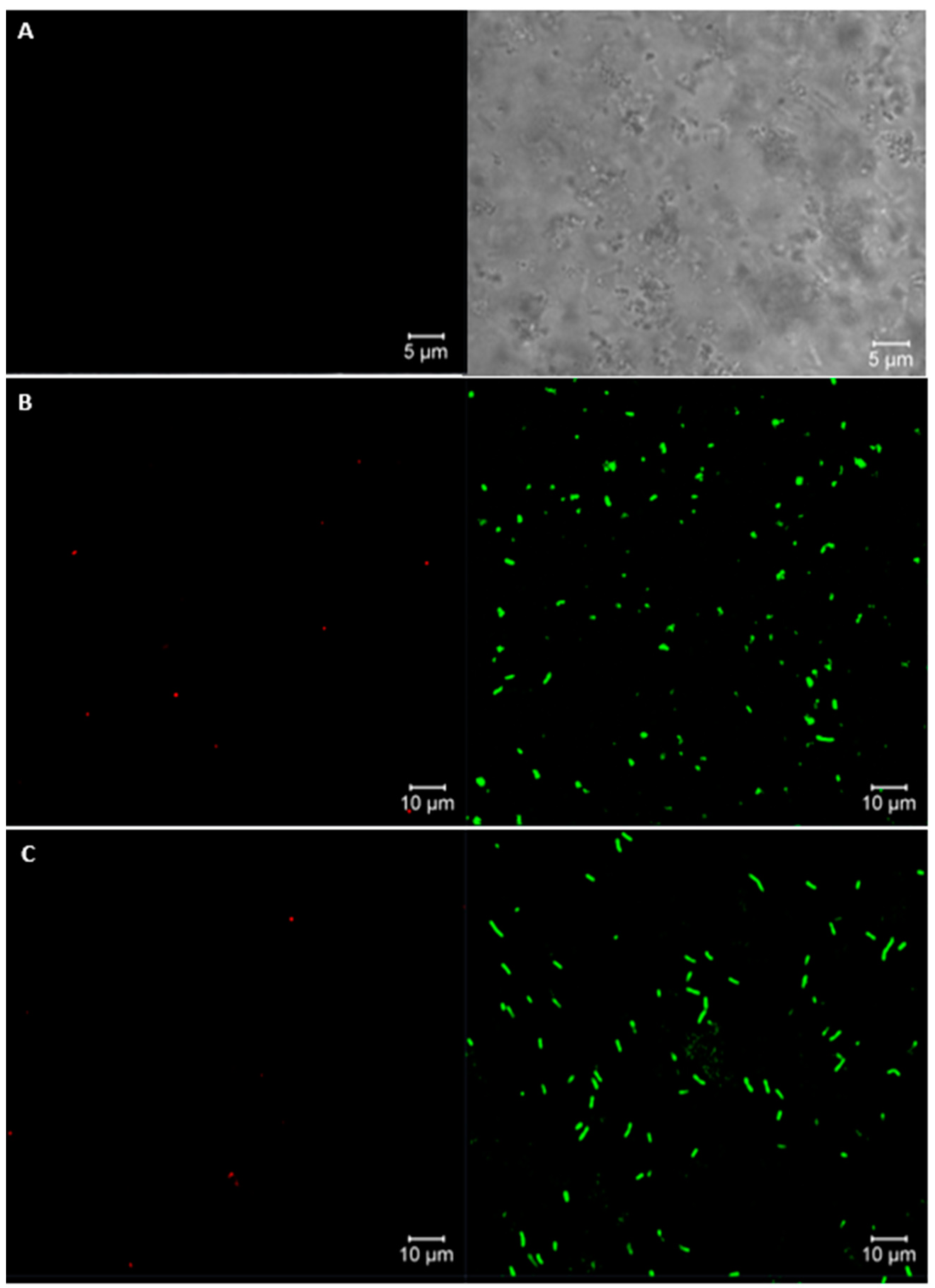

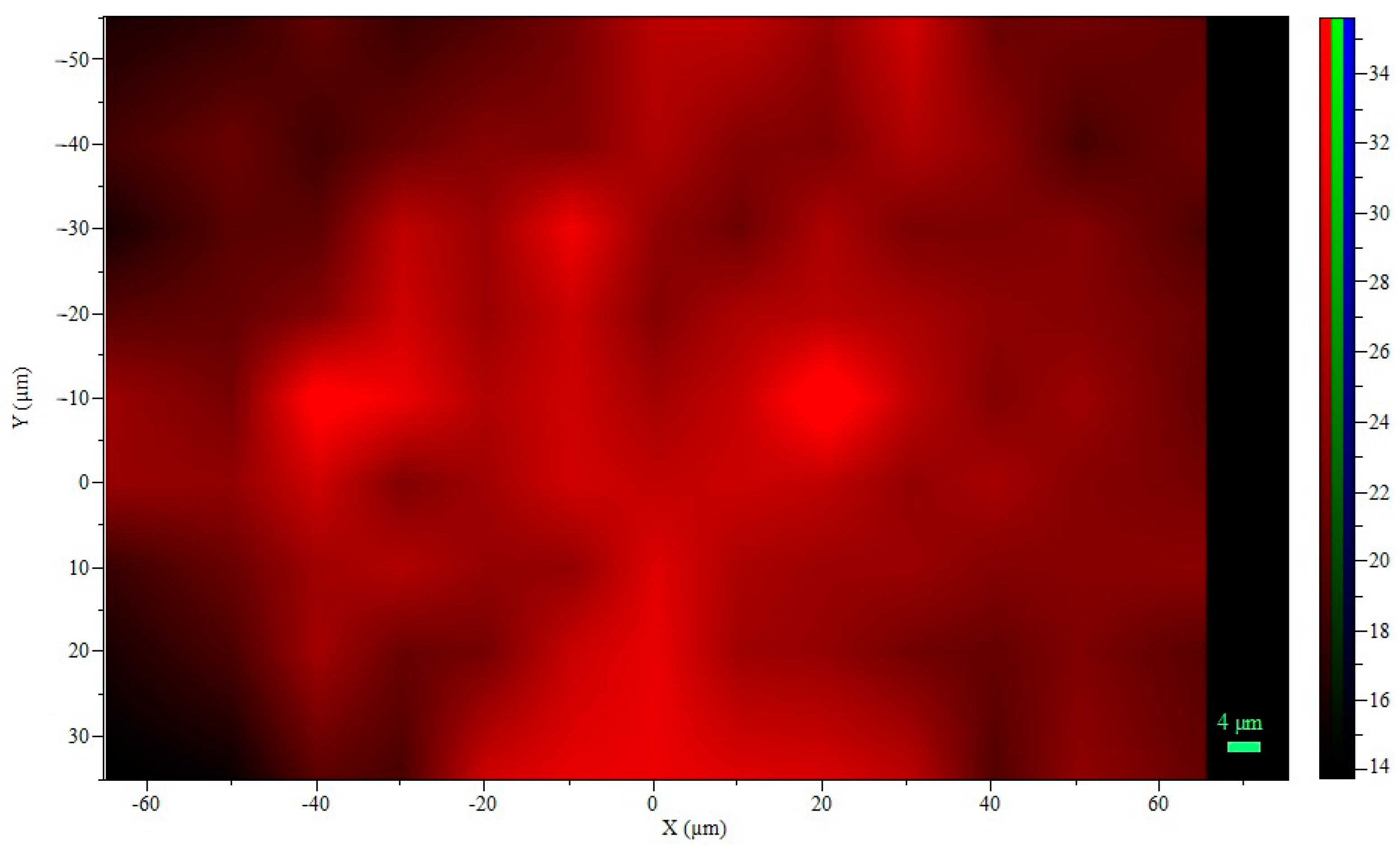
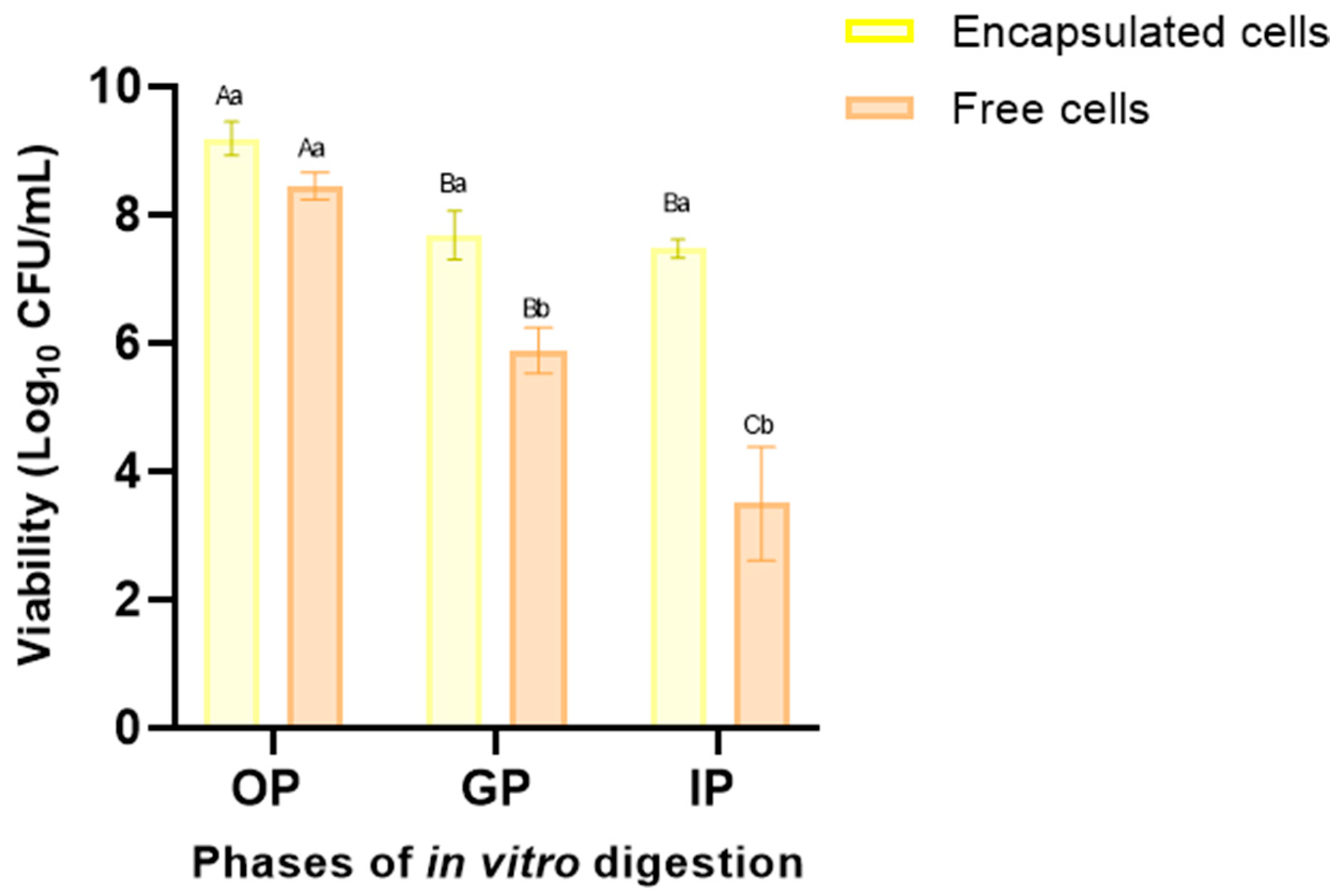
| Corrida | Factors | Response Variable | |||
|---|---|---|---|---|---|
| Frequency (Hz) | E. Tension (V) | Pump Rate (mL/min) | Viability (Log10 (CFU/mL)) | EE (%) | |
| 1 | 4000.0 | 250.0 | 20.0 | 8.21 | 88.82 |
| 2 | 2428.0 | 250.0 | 11.6 | 7.83 | 86.54 |
| 3 | 2428.0 | 250.0 | 11.6 | 7.99 | 88.42 |
| 4 | 1484.8 | 2500.0 | 6.0 | 8.35 | 92.32 |
| 5 | 2801.4 | 1678.8 | 11.2 | 7.87 | 87.07 |
| 6 | 1425.9 | 1217.5 | 6.0 | 7.91 | 87.50 |
| 7 | 70.0 | 250.0 | 6.0 | 7.97 | 88.12 |
| 8 | 4000.0 | 2500.0 | 6.0 | 8.34 | 92.27 |
| 9 | 2801.4 | 1678.8 | 11.2 | 8.09 | 89.41 |
| 10 | 70.0 | 2500.0 | 20.0 | 7.56 | 83.63 |
| 11 | 70.0 | 1600.0 | 11.6 | 8.14 | 90.02 |
| 12 | 2428.0 | 1600.0 | 20.0 | 8.05 | 89.05 |
| 13 | 4000.0 | 1037.5 | 6.0 | 7.80 | 86.24 |
| 14 | 4000.0 | 2500.0 | 15.1 | 8.17 | 90.38 |
| 15 | 463.0 | 475.0 | 13.0 | 7.88 | 87.13 |
| 16 | 1484.8 | 2500.0 | 6.0 | 8.73 | 96.51 |
| 17 | 2428.0 | 1600.0 | 20.0 | 8.07 | 89.27 |
| 18 | 463.0 | 1375.0 | 18.6 | 8.22 | 90.87 |
| 19 | 70.0 | 1600.0 | 11.6 | 8.10 | 89.54 |
| 20 | 70.0 | 250.0 | 20.0 | 8.69 | 96.09 |
| Viability (Log10 (CFU/mL)) | Encapsulation Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| Sum of Squares | Degree Freedom | p-Value | Sum of Squares | Degree Freedom | p-Value | |
| Model | 1.09 | 9 | 0.025 | 147.56 | 9 | 0.014 |
| AB | 0.125 | 1 | 0.077 | 20.58 | 1 | 0.038 |
| AC | 0.001 | 1 | 0.668 | 2.17 | 1 | 0.457 |
| BC | 0.739 | 1 | 0.001 | 96.48 | 1 | 0.001 |
| Residuals | 0.322 | 10 | 36.35 | 10 | ||
| Lack of fit | 0.215 | 5 | 0.228 | 22.93 | 5 | 0.285 |
| Pure Error | 0.106 | 5 | 13.42 | 5 | ||
| R2 | 0.813 | 0.802 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Muñoz, Y.V.; de Jesús Perea-Flores, M.; Quintanilla-Carvajal, M.X. Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion (Part 2: Operational Conditions of Vibrational Technology). Polymers 2024, 16, 2492. https://doi.org/10.3390/polym16172492
Rojas-Muñoz YV, de Jesús Perea-Flores M, Quintanilla-Carvajal MX. Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion (Part 2: Operational Conditions of Vibrational Technology). Polymers. 2024; 16(17):2492. https://doi.org/10.3390/polym16172492
Chicago/Turabian StyleRojas-Muñoz, Yesica Vanesa, María de Jesús Perea-Flores, and María Ximena Quintanilla-Carvajal. 2024. "Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion (Part 2: Operational Conditions of Vibrational Technology)" Polymers 16, no. 17: 2492. https://doi.org/10.3390/polym16172492
APA StyleRojas-Muñoz, Y. V., de Jesús Perea-Flores, M., & Quintanilla-Carvajal, M. X. (2024). Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion (Part 2: Operational Conditions of Vibrational Technology). Polymers, 16(17), 2492. https://doi.org/10.3390/polym16172492








