Properties of EPDM Nanocomposites Reinforced with Modified Montmorillonite
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of DMMT Gel
2.3. Preparation of EPDM/DMMT Nanocomposites
2.4. Characterization and Measurements
3. Results and Discussion
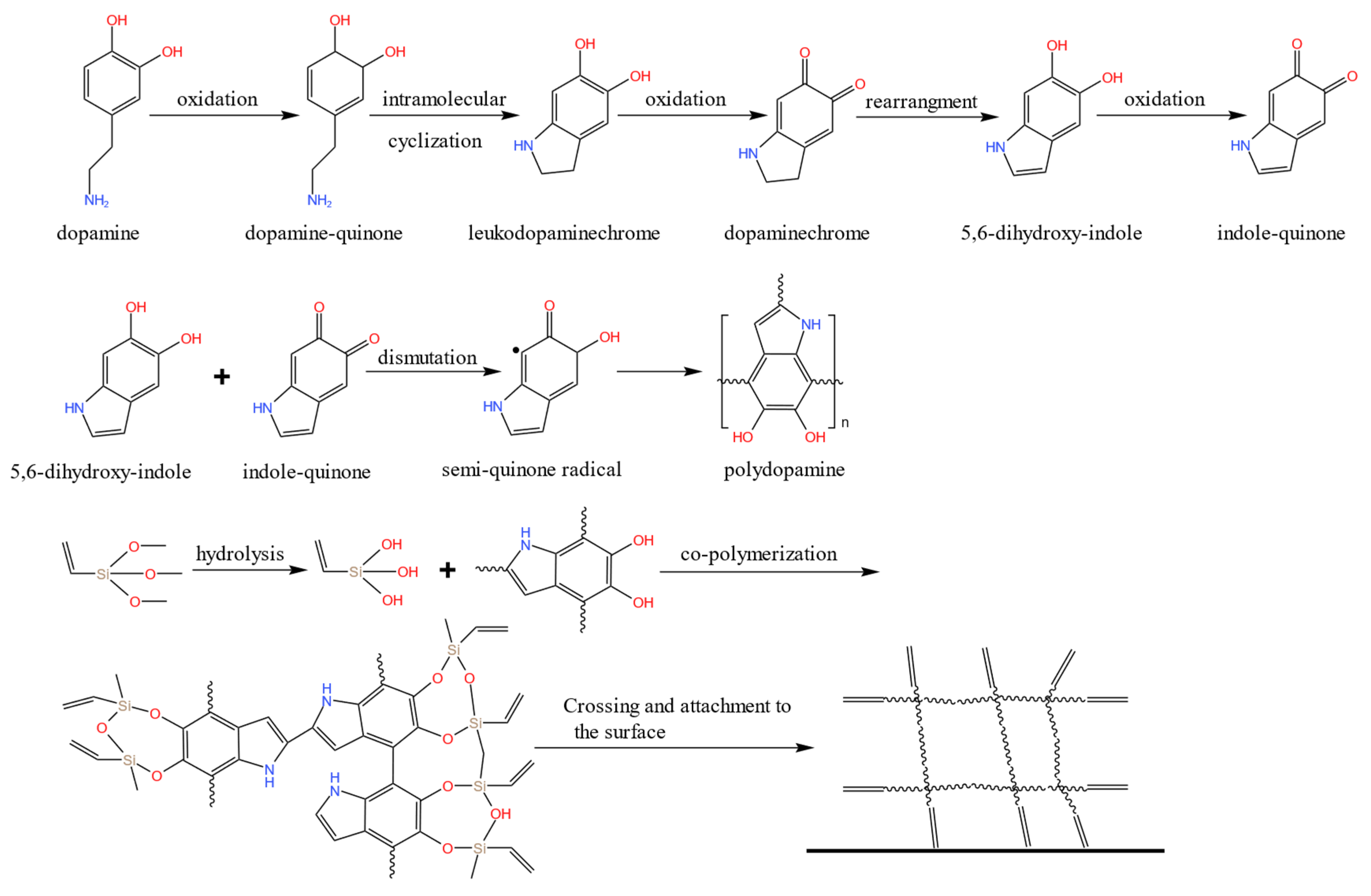
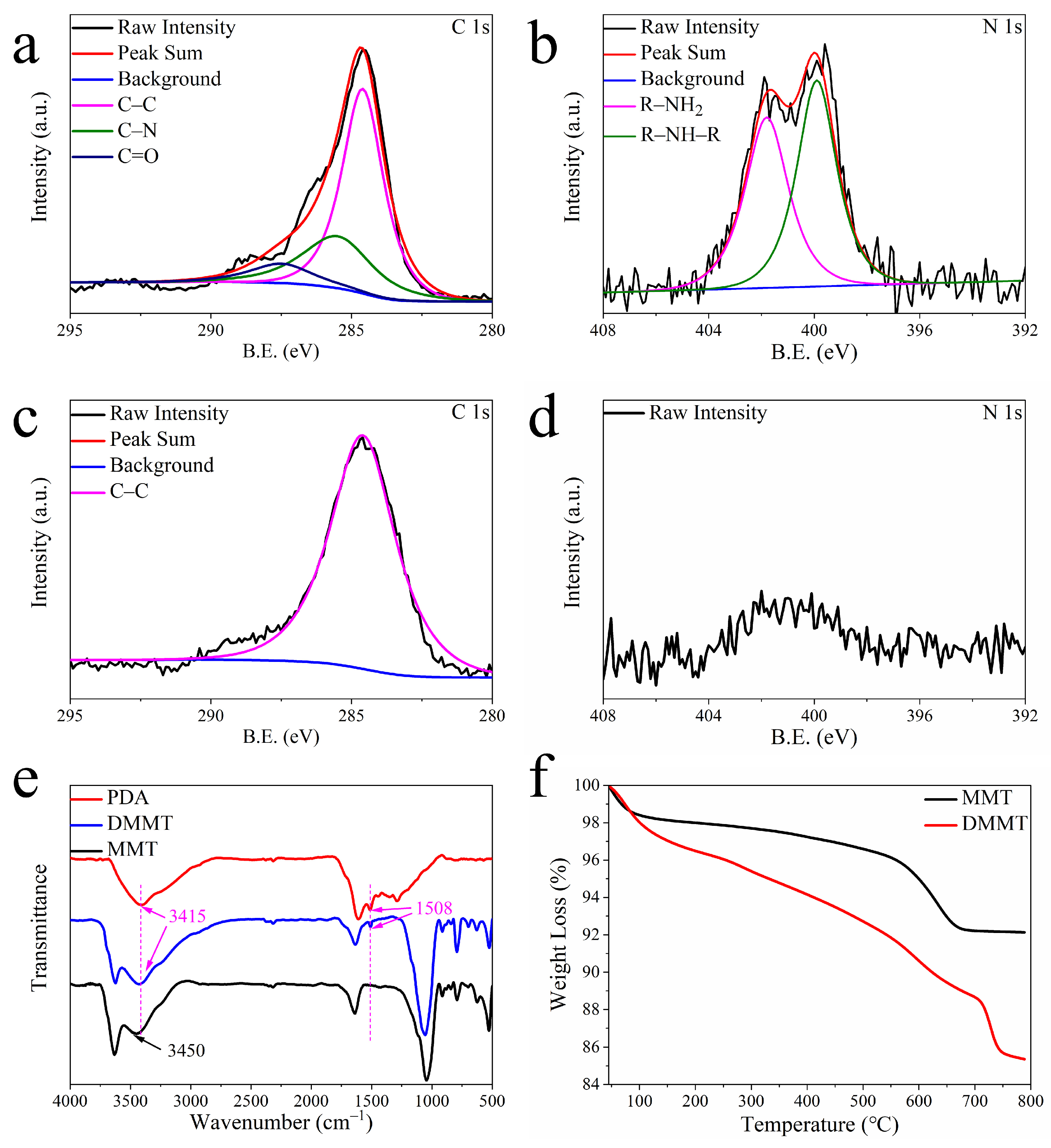
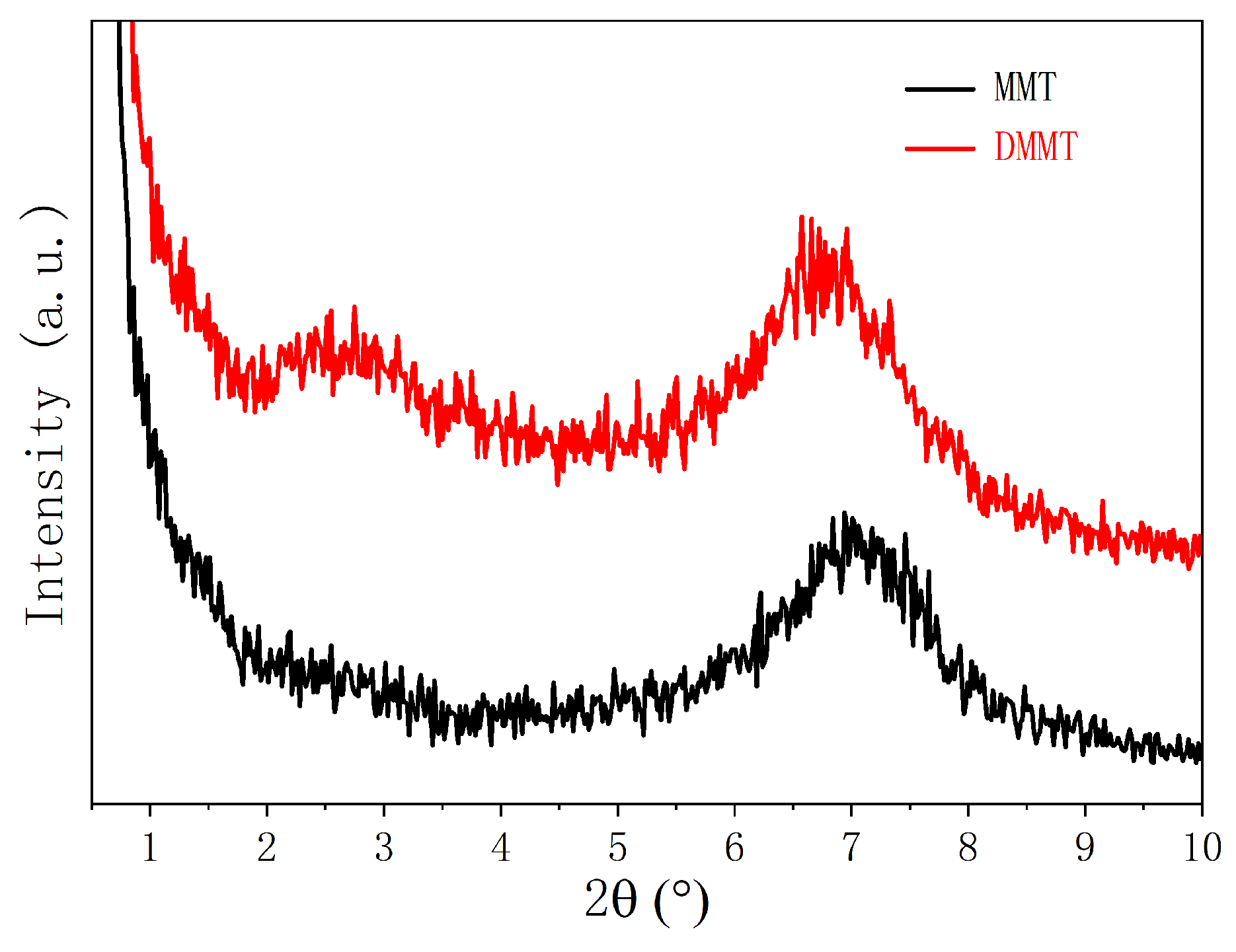
3.1. DMMT Characterization
3.2. Structure and Morphology of EPDM Composites
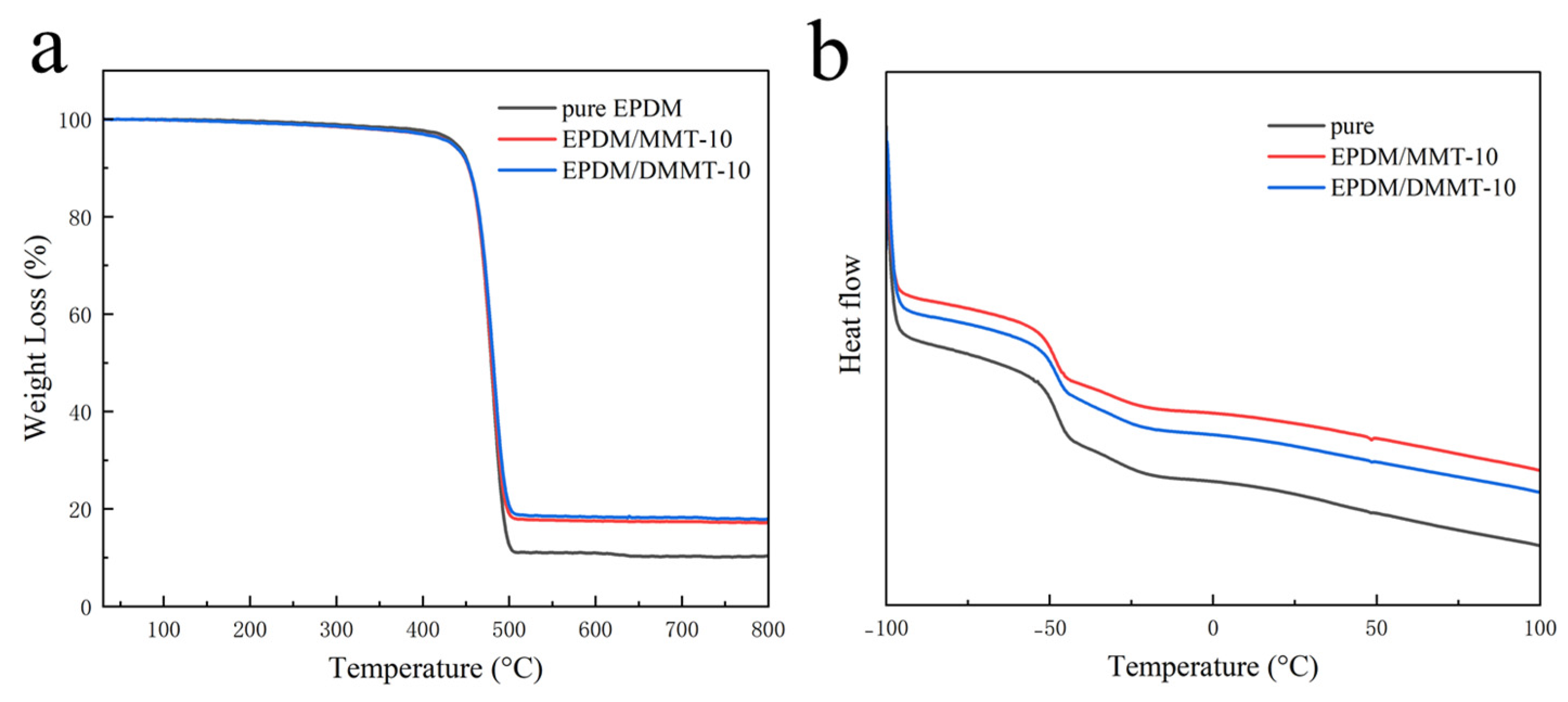
3.3. Properties of EPDM Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, S.J.; Huang, Y.D.; Li, W. Morphology and Characterization of Clay-reinforced EPDM Nanocomposites. J. Compos. Mater. 2016, 39, 745–754. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, T.K.; Wang, Y.C.; Alamani, B.G.; Lin, J.J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Fei, B.; Qian, B.; Yang, Z.; Wang, R.; Liu, W.C.; Mak, C.L.; Xin, J.H. Coating carbon nanotubes by spontaneous oxidative polymerization of dopamine. Carbon 2008, 46, 1795–1797. [Google Scholar] [CrossRef]
- Feng, J.; Fan, H.; Zha, D.A.; Wang, L.; Jin, Z. Characterizations of the Formation of Polydopamine-Coated Halloysite Nanotubes in Various pH Environments. Langmuir 2016, 32, 10377–10386. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Compos. Part A-Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- He, S.J.; Wang, J.Q.; Hu, J.B.; Zhou, H.F.; Nguyen, H.; Luo, C.M.; Lin, J. Silicone rubber composites incorporating graphitic carbon nitride and modified by vinyl tri-methoxysilane. Polym. Test. 2019, 79, 106005–106010. [Google Scholar] [CrossRef]
- Jaber, M.; Lambert, J.-F. A New Nanocomposite: L-DOPA/Laponite. J. Phys. Chem. Lett. 2009, 1, 85–88. [Google Scholar] [CrossRef]
- He, S.J.; Bai, F.J.; Liu, S.X.; Ma, H.F.; Hu, J.B.; Chen, L.; Lin, J.; Wei, G.S.; Du, X.Z. Aging properties of styrene-butadiene rubber nanocomposites filled with carbon black and rectorite. Polym. Test. 2017, 64, 92–100. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Zhang, C.; Yang, W.; Qiao, B.; Yin, L. The Effect of Various Fillers on the Properties of Methyl Vinyl Silicone Rubber. Polymers 2023, 15, 1584. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Potiyaraj, P. A critical review on the utilization of various reinforcement modifiers in filled rubber composites. J. Elastom. Plast. 2020, 52, 167–193. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Wang, W.C.; Li, J.; Lin, J.; Li, F.Z.; Zhang, L.Q.; He, S.J. Bioinspired design of nitrile-butadiene rubber/montmorillonite nanocomposites with hydrogen bond interactions leading to highly effective reinforcement. Polymer 2023, 277, 125968. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Feng, Y.; Yin, J. Recent progress in polymer/two-dimensional nanosheets composites with novel performances. Prog. Polym. Sci. 2022, 126, 101505. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Y.P.; Zhang, L.Q.; Li, Q.F. The role of rubber characteristics in preparing rubber/clay nanocomposites by melt compounding. J. Appl. Polym. Sci. 2008, 109, 1925–1934. [Google Scholar] [CrossRef]
- Mohammadpour, Y.; Katbab, A.A. Effects of the ethylene-propylene-diene monomer microstructural parameters and interfacial compatibilizer upon the EPDM/montmorillonite nanocomposites microstructure: Rheology/permeability correlation. J. Appl. Polym. Sci. 2007, 106, 4209–4218. [Google Scholar] [CrossRef]
- Meng, Z.; Li, J.; Zou, Y.; Li, N.; Fu, X.; Zhang, R.; Hu, S.; Liu, Q. Advanced montmorillonite modification by using corrosive microorganisms as an alternative filler to reinforce natural rubber. Appl. Clay Sci. 2022, 225, 106534. [Google Scholar] [CrossRef]
- Phua, S.L.; Yang, L.; Toh, C.L.; Huang, S.; Tsakadze, Z.; Lau, S.K.; Mai, Y.W.; Lu, X. Reinforcement of polyether polyurethane with dopamine-modified clay: The role of interfacial hydrogen bonding. ACS Appl. Mater. Interfaces 2012, 4, 4571–4578. [Google Scholar] [CrossRef]
- Li, P.; Yin, L.; Song, G.; Sun, J.; Wang, L.; Wang, H. High-performance EPDM/organoclay nanocomposites by melt extrusion. Appl. Clay Sci. 2008, 40, 38–44. [Google Scholar] [CrossRef]
- Donchak, V.; Stetsyshyn, Y.; Bratychak, M.; Broza, G.; Harhay, K.; Stepina, N.; Kostenko, M.; Voronov, S. Nanoarchitectonics at surfaces using multifunctional initiators of surface-initiated radical polymerization for fabrication of the nanocomposites. Appl. Surf. Sci. Adv. 2021, 5, 100104. [Google Scholar] [CrossRef]
- Kostenko, M.; Stetsyshyn, Y.; Harhay, K.; Melnyk, Y.; Donchak, V.; Gubriy, Z.; Kracalik, M. Impact of the functionalized clay nanofillers on the properties of the recycled polyethylene terephthalate nanocomposites. J. Appl. Polym. Sci. 2024, 141, e55543. [Google Scholar] [CrossRef]
- Qu, C.; Li, S.; Zhang, Y.; Wang, T.; Wang, Q.; Chen, S. Surface modification of Ti3C2-MXene with polydopamine and amino silane for high performance nitrile butadiene rubber composites. Tribol. Int. 2021, 163, 107150. [Google Scholar] [CrossRef]
- Rana, A.S.; Vamshi, M.K.; Naresh, K.; Velmurugan, R.; Sarathi, R. Mechanical, thermal, electrical and crystallographic behaviour of EPDM rubber/clay nanocomposites for out-door insulation applications. Adv. Mater. Process. Technol. 2019, 6, 54–74. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.R.; Yuan, X.C.; Jia, X.Y.; Lin, J.; He, S.J. High-performance proton exchange membrane employing water-insoluble hybrid formed by chemically bonding phosphotungstic acid with polydopamine. Clean Energy Sci. Technol. 2024, 2, 138. [Google Scholar] [CrossRef]
- Surya, I.; Muniyadi, M.; Ismail, H. A review on clay-reinforced ethylene propylene diene terpolymer composites. Polym. Compos. 2021, 42, 1698–1711. [Google Scholar] [CrossRef]
- Liao, Y.F.; Weng, Y.X.; Wang, J.Q.; Zhou, H.F.; Lin, J.; He, S.J. Silicone rubber composites with high breakdown strength and low dielectric loss based on polydopamine coated mica. Polymers 2019, 11, 2030. [Google Scholar] [CrossRef]
- Plagge, J.; Lang, A. Filler-polymer interaction investigated using graphitized carbon blacks: Another attempt to explain reinforcement. Polymer 2021, 218, 123513. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Gao, S.; Zhao, D.; Zhang, L.; Wang, W. Bio-inspired polydopamine-coated clay and its thermo-oxidative stabilization mechanism for styrene butadiene rubber. RSC Adv. 2015, 5, 9314–9324. [Google Scholar] [CrossRef]
- Chen, Z.L.; Li, J.; Li, Z.X.; Wang, J.; Li, Q.; Lin, J.; Zhang, L.Q.; He, S.J. Rubber/clay nanocomposites prepared by compounding clay gel with hydrophilically treated styrene-butadiene rubber. Eur. Polym. J. 2024, 213, 113137. [Google Scholar] [CrossRef]
- Chen, Z.L.; Li, J.; Li, Z.X.; Lin, J.; Zhang, L.Q.; He, S.J. A novel strategy to prepare rubber/clay nanocomposites via compounding clay gel into cocoamidopropyl betaine modified styrene butadiene rubber. Compos. Sci. Technol. 2024, 252, 110602. [Google Scholar] [CrossRef]
- He, S.J.; He, T.F.; Wang, J.Q.; Wu, X.H.; Xue, Y.; Zhang, L.Q.; Lin, J. A novel method to prepare acrylonitrile-butadiene rubber/clay nanocomposites by compounding with clay gel. Compos. Part B-Eng. 2019, 167, 356–361. [Google Scholar] [CrossRef]
- Wang, X.; Sinha, T.K.; Sun, J.; Wang, C.; Kim, J.K.; Zong, C. Facile preparation of hydrogenated nitrile butadiene rubber/reduced graphene oxide nanocomposite with one-pot reduction approach via the latex way. Colloid Polym. Sci. 2021, 299, 1703–1715. [Google Scholar] [CrossRef]
- ISO 37-2011; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2011.
- Yang, L.; Phua, S.L.; Teo, J.K.; Toh, C.L.; Lau, S.K.; Ma, J.; Lu, X. A biomimetic approach to enhancing interfacial interactions: Polydopamine-coated clay as reinforcement for epoxy resin. ACS Appl. Mater. Interfaces 2011, 3, 3026–3032. [Google Scholar] [CrossRef]
- Ye, N.; Zheng, J.; Ye, X.; Xue, J.; Han, D.; Xu, H.; Wang, Z.; Zhang, L. Performance enhancement of rubber composites using VOC-Free interfacial silica coupling agent. Compos. Part B-Eng. 2020, 202, 108301. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Q.; Wu, S.; Gou, Z.; Wu, X.; Xu, D. Starch/chitosan films reinforced with polydopamine modified MMT: Effects of dopamine concentration. Food Hydrocolloid. 2016, 61, 678–684. [Google Scholar] [CrossRef]
- Yang, M.-C.; Kao, B.-J.; Tsou, C.-H.; Suen, M.-C.; Wu, C.-S.; Tsou, C.-Y.; Chu, C.-K.; Yao, W.-H.; Wu, W.-Y.; Hong, W.-S.; et al. The Properties and a New Preparation of Ethylene Propylene Diene Monomer/Montmorillonite Nanocomposites. Polym. Polym. Compos. 2015, 23, 181–190. [Google Scholar] [CrossRef]
- Xu, Z.J.; Song, Y.H.; Zheng, Q. Payne effect of carbon black filled natural rubber compounds and their carbon black gels. Polymer 2019, 185, 121953. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Shao, M.; Yang, J.; Wang, Q.; Wang, T.; Zhang, X. Regulating interfacial compatibility with amino silane and bio-inspired polydopamine for high-performance epoxy composites. Tribol. Int. 2019, 140, 105861. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Peng, Z.; Zhang, Y. Influence of clay modification on the structure and mechanical properties of EPDM/montmorillonite nanocomposites. Polym. Test. 2004, 23, 217–223. [Google Scholar] [CrossRef]
- Yazici, N.; Kodal, M.; Ozkoc, G. Lab-Scale Twin-Screw Micro-Compounders as a New Rubber-Mixing Tool: ‘A Comparison on EPDM/Carbon Black and EPDM/Silica Composites’. Polymers 2021, 13, 4391. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.Y.; Wang, H.T.; Guo, W.J. Synergistic effect of aluminum hydroxide and nanoclay on flame retardancy and mechanical properties of EPDM composites. J. Appl. Polym. Sci. 2013, 130, 2042–2048. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhao, Y. Effect of dendrimer modified montmorillonite on structure and properties of EPDM nanocomposites. Polym. Test. 2017, 62, 41–50. [Google Scholar] [CrossRef]
- Chang, Y.W.; Yang, Y.; Ryu, S.; Nah, C. Preparation and properties of EPDM/organomontmorillonite hybrid nanocomposites. Polym. Int. 2002, 51, 319–324. [Google Scholar] [CrossRef]


| Ingredient | Pure EPDM | EPDM/MMT-10 | EPDM/DMMT-10 |
|---|---|---|---|
| EPDM | 100 | 100 | 100 |
| VTMS | 0 | 0 | 2 |
| MMT | 0 | 10 | 0 |
| DMMT | 0 | 0 | 10 |
| ZnO | 8 | 8 | 8 |
| MgO | 4 | 4 | 4 |
| TAIC | 2 | 2 | 2 |
| DCP | 4.5 | 4.5 | 4.5 |
| 4010NA | 2 | 2 | 2 |
| Filler Type | Filler Loading (phr) | Tensile Strength (MPa) | Elongation at Break (%) | References |
|---|---|---|---|---|
| Carbon black | 100 | 7.0 | 410 | [42] |
| Silica | 25 | 1.8 | 290 | [42] |
| Aluminium hydroxide | 50 | 7.2 | 315 | [43] |
| Dendrimer-modified MMT | 10 | 4.3 | 135 | [44] |
| Octadecyl ammonium ion-modified MMT | 10 | 4.2 | 320 | [45] |
| DMMT | 10 | 6.5 | 720 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, Z.; Sun, W.; Liu, Y.; Wang, X.; Lin, J.; Wang, J.; He, S. Properties of EPDM Nanocomposites Reinforced with Modified Montmorillonite. Polymers 2024, 16, 2381. https://doi.org/10.3390/polym16162381
Li Z, Chen Z, Sun W, Liu Y, Wang X, Lin J, Wang J, He S. Properties of EPDM Nanocomposites Reinforced with Modified Montmorillonite. Polymers. 2024; 16(16):2381. https://doi.org/10.3390/polym16162381
Chicago/Turabian StyleLi, Zhanxu, Zilong Chen, Weichong Sun, Yangling Liu, Xiong Wang, Jun Lin, Jian Wang, and Shaojian He. 2024. "Properties of EPDM Nanocomposites Reinforced with Modified Montmorillonite" Polymers 16, no. 16: 2381. https://doi.org/10.3390/polym16162381
APA StyleLi, Z., Chen, Z., Sun, W., Liu, Y., Wang, X., Lin, J., Wang, J., & He, S. (2024). Properties of EPDM Nanocomposites Reinforced with Modified Montmorillonite. Polymers, 16(16), 2381. https://doi.org/10.3390/polym16162381








