Constructing Multifunctional Composite Single Crystals via Polymer Gel Incorporation
Abstract
:1. Introduction
2. Mechanisms and Controlling Factors of Incorporation
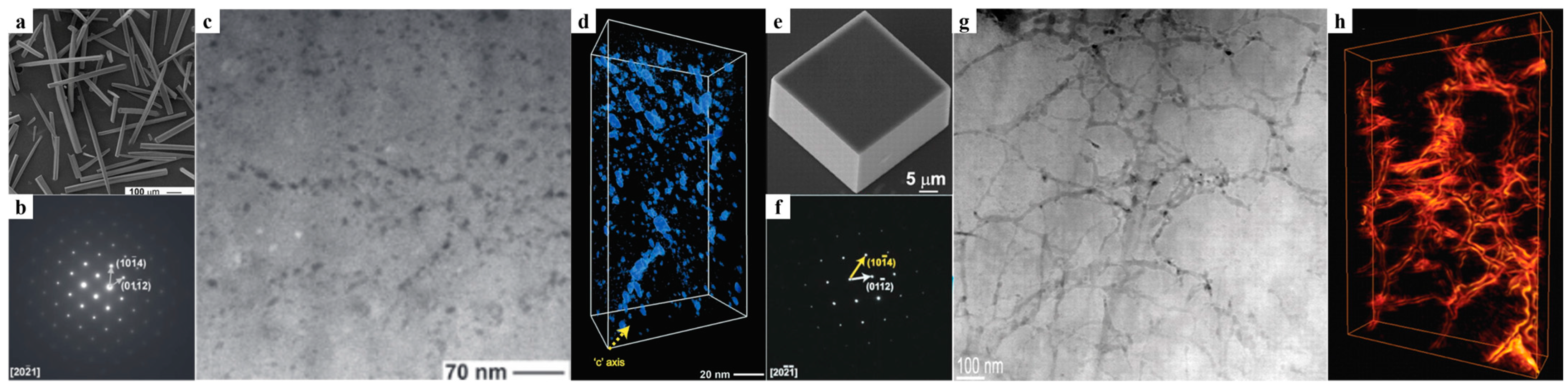
2.1. Structural Features of the Incorporation
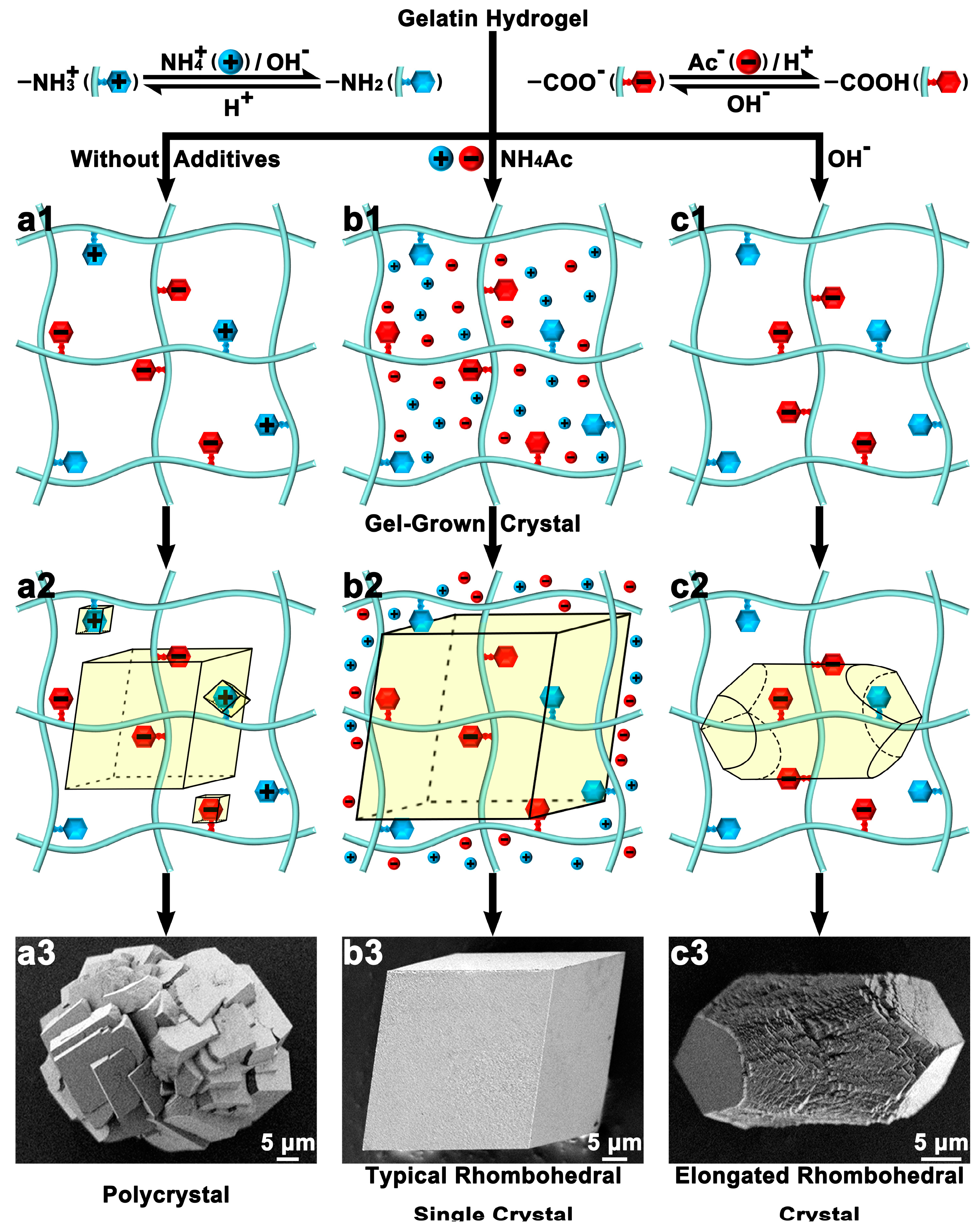
2.2. The Mechanisms of Gel-Incorporation
2.3. The Mechanism of Gel-Mediated Incorporation of Three-Component Materials
3. Multiple Functions
3.1. Macroscopic Morphology Control
3.2. Mechanical Reinforcement
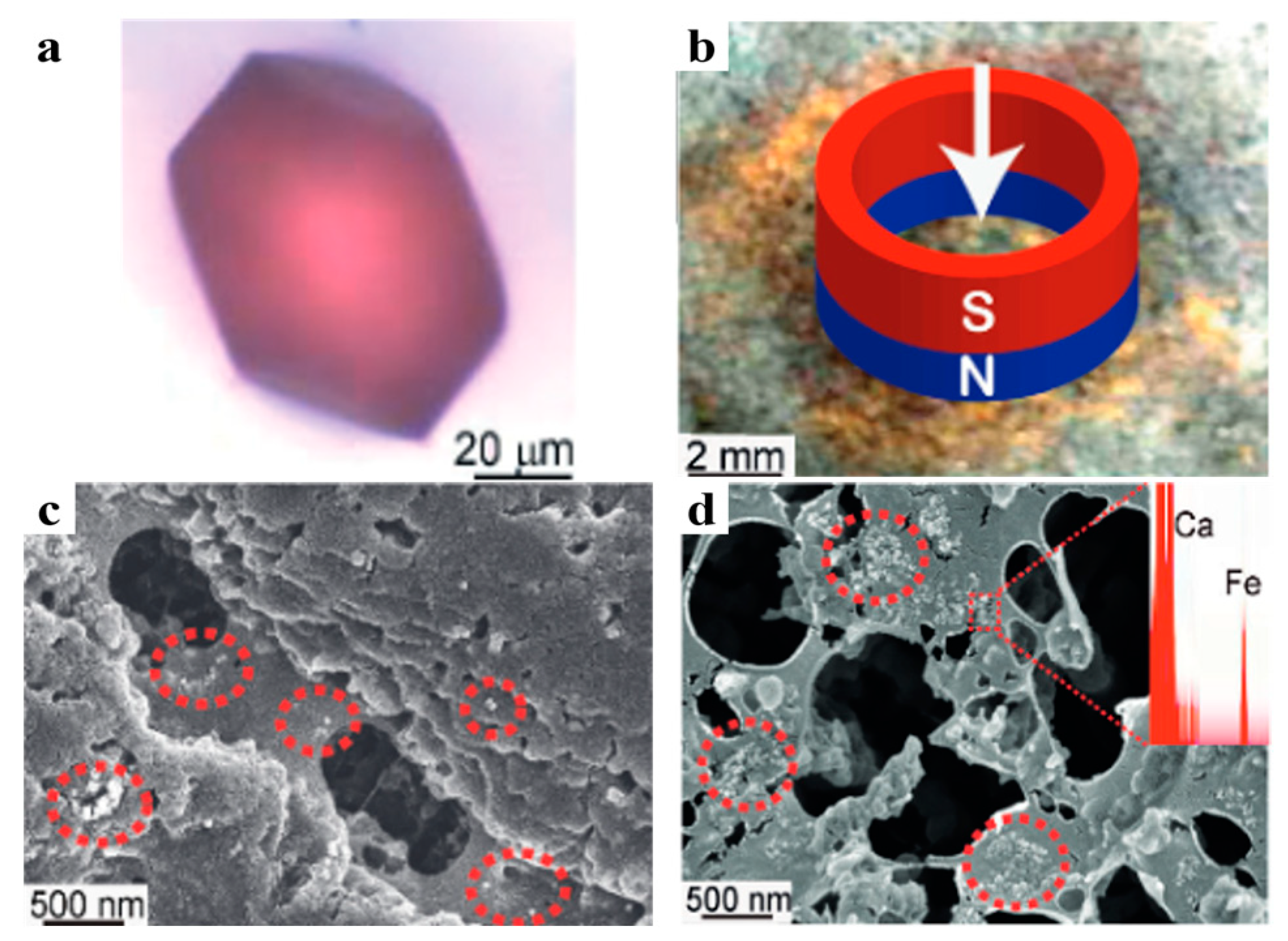
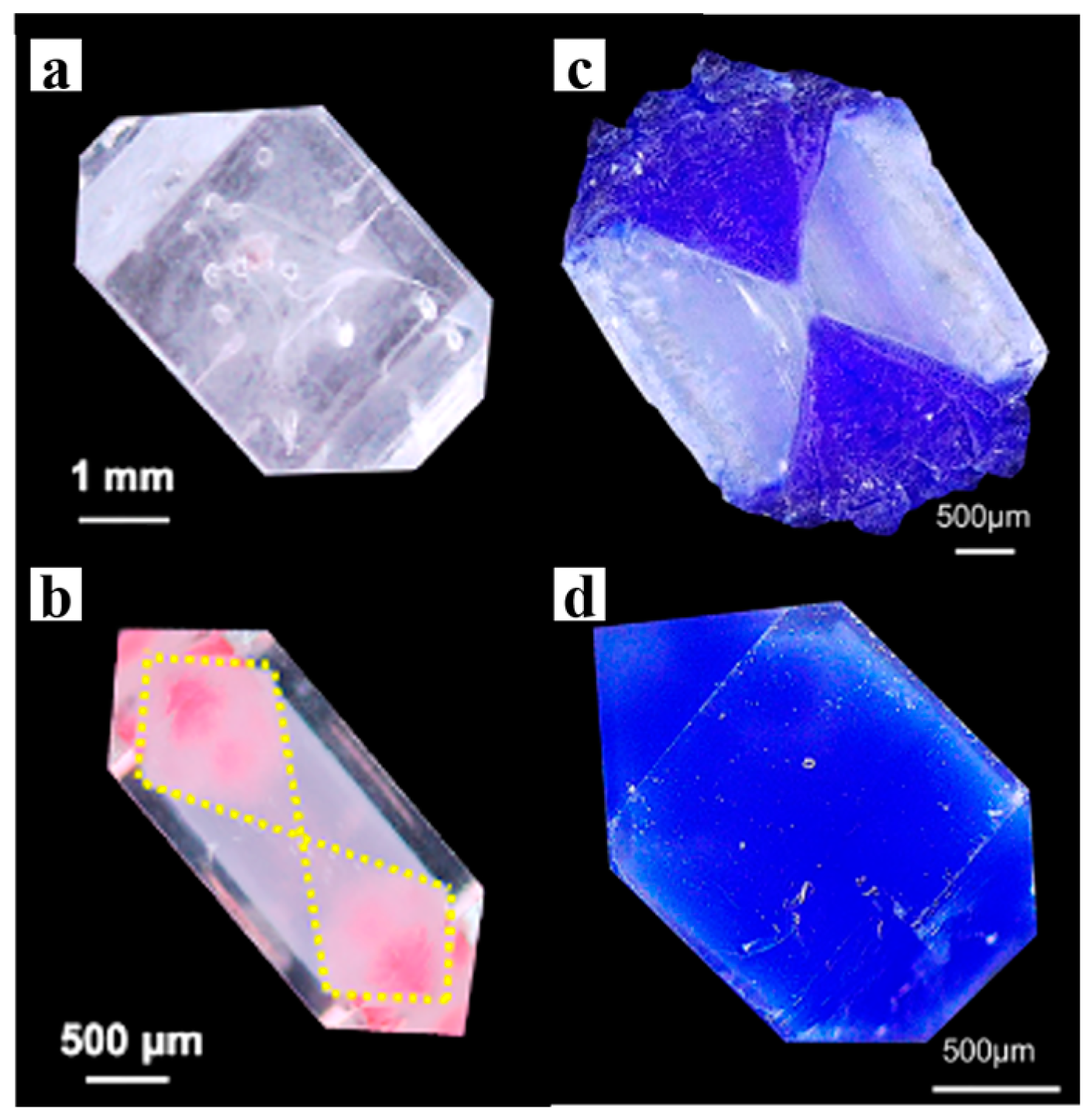
3.3. Color and Paramagnetism
3.4. Bandgap Engineering
3.5. Self-Healing
4. Optoelectronic Applications
4.1. Photoluminescence
4.2. Photodetection
4.3. X-ray Detection
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henisch, H.K. Crystals in Gels and Liesegang Rings; Cambridge University Press: Cambridge, UK, 1988; ISBN 978-0-521-34503-3. [Google Scholar]
- Nickl, H.J.; Henisch, H.K. Growth of Calcite Crystals in Gels. J. Electrochem. Soc. 1969, 116, 1258. [Google Scholar] [CrossRef]
- Yang, T.; Chen, H.; Jia, Z.; Deng, Z.; Chen, L.; Peterman, E.M.; Weaver, J.C.; Li, L. A Damage-Tolerant, Dual-Scale, Single-Crystalline Microlattice in the Knobby Starfish, Protoreaster Nodosus. Science 2022, 375, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A. Introduction: Biomineralization. Chem. Rev. 2008, 108, 4329–4331. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Nudelman, F.; Sone, E.; Zaslansky, P.; Addadi, L. Mineralized Biological Materials: A Perspective on Interfaces and Interphases Designed over Millions of Years. Biointerphases 2006, 1, P12–P14. [Google Scholar] [CrossRef] [PubMed]
- Robach, J.S.; Stock, S.R.; Veis, A. Mapping of Magnesium and of Different Protein Fragments in Sea Urchin Teeth via Secondary Ion Mass Spectroscopy. J. Struct. Biol. 2006, 155, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gries, K.; Kröger, R.; Kübel, C.; Fritz, M.; Rosenauer, A. Investigations of Voids in the Aragonite Platelets of Nacre. Acta Biomater. 2009, 5, 3038–3044. [Google Scholar] [CrossRef]
- Berman, A.; Hanson, J.; Leiserowitz, L.; Koetzle, T.F.; Weiner, S.; Addadi, L. Biological Control of Crystal Texture: A Widespread Strategy for Adapting Crystal Properties to Function. Science 1993, 259, 776–779. [Google Scholar] [CrossRef]
- Seknazi, E.; Pokroy, B. Residual Strain and Stress in Biocrystals. Adv. Mater. 2018, 30, 1707263. [Google Scholar] [CrossRef]
- Polishchuk, I.; Bracha, A.A.; Bloch, L.; Levy, D.; Kozachkevich, S.; Etinger-Geller, Y.; Kauffmann, Y.; Burghammer, M.; Giacobbe, C.; Villanova, J.; et al. Coherently Aligned Nanoparticles within a Biogenic Single Crystal: A Biological Prestressing Strategy. Science 2017, 358, 1294–1298. [Google Scholar] [CrossRef]
- Ma, Y.; Aichmayer, B.; Paris, O.; Fratzl, P.; Meibom, A.; Metzler, R.A.; Politi, Y.; Addadi, L.; Gilbert, P.U.P.A.; Weiner, S. The Grinding Tip of the Sea Urchin Tooth Exhibits Exquisite Control over Calcite Crystal Orientation and Mg Distribution. Proc. Natl. Acad. Sci. USA 2009, 106, 6048–6053. [Google Scholar] [CrossRef]
- Ma, Y.; Cohen, S.R.; Addadi, L.; Weiner, S. Sea Urchin Tooth Design: An “All-Calcite” Polycrystalline Reinforced Fiber Composite for Grinding Rocks. Adv. Mater. 2008, 20, 1555–1559. [Google Scholar] [CrossRef]
- Berman, A.; Addadi, L.; Kvick, Å.; Leiserowitz, L.; Nelson, M.; Weiner, S. Intercalation of Sea Urchin Proteins in Calcite: Study of a Crystalline Composite Material. Science 1990, 250, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, K.; Yuan, W.; Jin, X.; Liang, T.; Wang, Y.; Xin, H.L.; Chen, H.; Gao, C.; Li, H. Visualizing the Toughening Origins of Gel-Grown Calcite Single-Crystal Composites. Chin. Chem. Lett. 2018, 29, 1666–1670. [Google Scholar] [CrossRef]
- Li, H.; Estroff, L.A. Calcite Growth in Hydrogels: Assessing the Mechanism of Polymer-Network Incorporation into Single Crystals. Adv. Mater. 2009, 21, 470–473. [Google Scholar] [CrossRef]
- Deng, X.; Chen, M.; Ren, J.; Li, H. Spontaneous Formation of a Periodically Striped Structure in a Gel-Incorporated KDP Single Crystal. J. Cryst. Growth 2023, 623, 127418. [Google Scholar] [CrossRef]
- Chen, L.; Ye, T.; Jin, X.; Ren, J.; Huang, B.; Xu, Z.-K.; Chen, H.; Li, H. Gel Network Incorporation into Single Crystals Grown by Decomplexation Method. CrystEngComm 2015, 17, 8113–8118. [Google Scholar] [CrossRef]
- Hu, C.; Ye, T.; Liu, Y.; Ren, J.; Jin, X.; Chen, H.; Li, H. PbI2 Band Gap Engineering by Gel Incorporation. Mater. Chem. Front. 2018, 2, 362–368. [Google Scholar] [CrossRef]
- Chen, L.; Ye, T.; Liu, Y.; Liu, W.; Wu, G.; Chen, H.; Li, H. Gel Network Incorporation into Single-Crystals: Effects of Gel Structures and Crystal–Gel Interaction. CrystEngComm 2014, 16, 6901. [Google Scholar] [CrossRef]
- Aleshin, A.N.; Lee, J.Y.; Chu, S.W.; Kim, J.S.; Park, Y.W. Mobility Studies of Field-Effect Transistor Structures Basedon Anthracene Single Crystals. Appl. Phys. Lett. 2004, 84, 5383–5385. [Google Scholar] [CrossRef]
- Ren, J.; Huang, B.; Chen, L.; Liu, Y.; Ye, T.; Liu, W.; Jin, X.; Xu, Z.-K.; Chen, H.; Li, H. Constructing Bulk-Contact inside Single Crystals of Organic Semiconductors through Gel Incorporation. CrystEngComm 2016, 18, 800–806. [Google Scholar] [CrossRef]
- Yu, G.; Ren, J.; Yan, S.; Yuan, W.; Li, H. Long-Range Ordered Organic Bulk-Heterojunction: C60 and O-IDTBR Single Crystals Penetrated by Crystalline P3HT Fibrous Networks. Small 2023, 19, 2302046. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Niu, M.; Guo, X.; Liu, Y.; Yang, X.; Chen, M.; Hao, X.; Zhu, Y.; Chen, H.; Li, H. Bulk-Heterojunction with Long-Range Ordering: C60 Single-Crystal with Incorporated Conjugated Polymer Networks. J. Am. Chem. Soc. 2020, 142, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.A.; García-Ruiz, J.M. Agarose as Crystallisation Media for Proteins II: Trapping of Gel Fibres into the Crystals. Acta Crystallogr. D 2002, 58, 1653–1656. [Google Scholar] [CrossRef]
- Yu, G.; Xu, C.; Ju, H.; Ren, J.; Wu, G.; Zhang, C.; Zhang, X.; Xu, Z.; Zhu, W.; Yang, H.; et al. Key Progresses of MOE Key Laboratory of Macromolecular Synthesis and Functionalization in 2023. Chin. Chem. Lett. 2024, 35, 109893. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Li, H. Incorporating Polymers within a Single-crystal: From Heterogeneous Structure to Multiple Functions. J. Polym. Sci. 2022, 60, 1151–1173. [Google Scholar] [CrossRef]
- Yao, Y.; Ye, T.; Ren, J.; Li, H. Morphological Evolution of Calcite Grown in Zwitterionic Hydrogels: Charge Effects Enhanced by Gel-Incorporation. Chem. Eur. J. 2023, 29, e202300169. [Google Scholar] [CrossRef]
- Yin, X.; Griesshaber, E.; Fernández-Díaz, L.; Ziegler, A.; García-García, F.J.; Schmahl, W.W. Influence of Gelatin–Agarose Composites and Mg on Hydrogel-Carbonate Aggregate Formation and Architecture. Cryst. Growth Des. 2019, 19, 5696–5715. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Bhagat, S.D.; Hirashima, H.; Pajonk, G.M. Synthesis of Flexible Silica Aerogels Using Methyltrimethoxysilane (MTMS) Precursor. J. Colloid Interface Sci. 2006, 300, 279–285. [Google Scholar] [CrossRef]
- Wen, Q.; Ma, W.; Liu, Y.; Jin, X.; Ren, J.; Lin, C.; Hu, C.; Yang, Y.M.; Li, H. PbI2–TiO2 Bulk Heterojunctions with Long-Range Ordering for X-Ray Detectors. J. Phys. Chem. Lett. 2021, 12, 11176–11181. [Google Scholar] [CrossRef]
- Liu, Y.; Zang, H.; Wang, L.; Fu, W.; Yuan, W.; Wu, J.; Jin, X.; Han, J.; Wu, C.; Wang, Y.; et al. Nanoparticles Incorporated inside Single-Crystals: Enhanced Fluorescent Properties. Chem. Mater. 2016, 28, 7537–7543. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, W.; Shi, Y.; Chen, X.; Wang, Y.; Chen, H.; Li, H. Functionalizing Single Crystals: Incorporation of Nanoparticles Inside Gel-Grown Calcite Crystals. Angew. Chem. Int. Ed. 2014, 53, 4127–4131. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Schenk, A.S.; Walsh, D.; Kulak, A.N.; Cespedes, O.; Meldrum, F.C. Bio-Inspired Formation of Functional Calcite/Metal Oxide Nanoparticle Composites. Nanoscale 2013, 6, 852–859. [Google Scholar] [CrossRef]
- Gao, F.; Ren, J.; Jin, X.; Deng, X.; Li, H. Isotropically Dyed Single Crystals Produced via Gel-Incorporation. ACS Mater. Lett. 2022, 4, 1207–1213. [Google Scholar] [CrossRef]
- Deng, X.; Gao, F.; Ren, J.; Li, H. A Synergetic Mechanism of Gel-Incorporation Induced Single Crystal Dyeing. Cryst. Growth Des. 2023, 23, 5397–5402. [Google Scholar] [CrossRef]
- Jin, X.; Xue, J.; Kang, D.H.; Liu, Y.; Ren, J.; Wen, Q.; Hu, D.; Yu, Y.; Yang, W.; Du, B.; et al. Incorporation of Fluorescent Microgels inside Calcite Single Crystals. Giant 2020, 3, 100023. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Shi, C.; Qi, R.; Hu, M. Single-Crystal Lattice Filling in Connected Spaces inside 3D Networks. J. Am. Chem. Soc. 2021, 143, 6447–6459. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Sommerdijk, N.A.J.M. Biomineralization as an Inspiration for Materials Chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.K.; Charnock, J.M.; Lennie, A.; Meldrum, F.C. Synthesis-Dependant Structural Variations in Amorp. CrystEngComm 2007, 9, 1226. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.L.; Muller, D.A.; Estroff, L.A. Visualizing the 3D Internal Structure of Calcite Single Crystals Grown in Agarose Hydrogels. Science 2009, 326, 1244–1247. [Google Scholar] [CrossRef]
- Jin, X.; Chen, L.; Liu, Y.; Ye, T.; Hu, C.; Ren, J.; Chen, H.; Li, H. Patterning the Internal Structure of Single Crystals by Gel Incorporation. J. Phys. Chem. C 2019, 123, 13147–13153. [Google Scholar] [CrossRef]
- Nindiyasari, F.; Fernández-Díaz, L.; Griesshaber, E.; Astilleros, J.M.; Sánchez-Pastor, N.; Schmahl, W.W. Influence of Gelatin Hydrogel Porosity on the Crystallization of CaCO3. Cryst. Growth Des. 2014, 14, 1531–1542. [Google Scholar] [CrossRef]
- Pokroy, B.; Fitch, A.N.; Marin, F.; Kapon, M.; Adir, N.; Zolotoyabko, E. Anisotropic Lattice Distortions in Biogenic Calcite Induced by Intra-Crystalline Organic Molecules. J. Struct. 2006, 155, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Weiner, S. Control and Design Principles in Biological Mineralization. Angew. Chem. Int. Ed. 1992, 31, 153–169. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Buder, J.; Cardoso-Gil, R.; Prots, Y.; Carrillo-Cabrera, W.; Simon, P.; Kniep, R. Shape Development and Structure of a Complex (Otoconia-Like?) Calcite–Gelatine Composite. Angew. Chem. Int. Ed. 2008, 47, 8280–8284. [Google Scholar] [CrossRef]
- Yin, X.; Weitzel, F.; Jiménez-López, C.; Griesshaber, E.; Fernández-Díaz, L.; Rodríguez-Navarro, A.; Ziegler, A.; Schmahl, W.W. Directing Effect of Bacterial Extracellular Polymeric Substances (EPS) on Calcite Organization and EPS–Carbonate Composite Aggregate Formation. Cryst. Growth Des. 2020, 20, 1467–1484. [Google Scholar] [CrossRef]
- Yin, X.; Weitzel, F.; Griesshaber, E.; Fernández-Díaz, L.; Jimenez-Lopez, C.; Ziegler, A.; Rodriguez-Navarro, A.B.; Schmahl, W.W. Bacterial EPS in Agarose Hydrogels Directs Mineral Organization in Calcite Precipitates: Species-Specific Biosignatures of Bacillus Subtilis, Mycobacterium Phley, Mycobacterium Smagmatis, and Pseudomonas Putida EPS. Cryst. Growth Des. 2020, 20, 4402–4417. [Google Scholar] [CrossRef]
- Yao, S.; Jin, B.; Liu, Z.; Shao, C.; Zhao, R.; Wang, X.; Tang, R. Biomineralization: From Material Tactics to Biological Strategy. Adv. Mater. 2017, 29, 1605903. [Google Scholar] [CrossRef]
- Veis, A. A Window on Biomineralization. Science 2005, 307, 1419–1420. [Google Scholar] [CrossRef]
- Kabalah-Amitai, L.; Mayzel, B.; Kauffmann, Y.; Fitch, A.N.; Bloch, L.; Gilbert, P.U.P.A.; Pokroy, B. Vaterite Crystals Contain Two Interspersed Crystal Structures. Science 2013, 340, 454–457. [Google Scholar] [CrossRef]
- Gordon, L.M.; Joester, D. Nanoscale Chemical Tomography of Buried Organic–Inorganic Interfaces in the Chiton Tooth. Nature 2011, 469, 194–197. [Google Scholar] [CrossRef]
- Aizenberg, J.; Hanson, J.; Koetzle, T.F.; Weiner, S.; Addadi, L. Control of Macromolecule Distribution within Synthetic and Biogenic Single Calcite Crystals. J. Am. Chem. Soc. 1997, 119, 881–886. [Google Scholar] [CrossRef]
- Aizenberg, J.; Tkachenko, A.; Weiner, S.; Addadi, L.; Hendler, G. Calcitic Microlenses as Part of the Photoreceptor System in Brittlestars. Nature 2001, 412, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea Urchin Spine Calcite Forms via a Transient Amorphous Calcium Carbonate Phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.L.; Kunitake, M.E.; Keene, E.C.; Muller, D.A.; Estroff, L.A. Calcite Prisms from Mollusk Shells (Atrina Rigida): Swiss-cheese-like Organic–Inorganic Single-crystal Composites. Adv. Funct. 2011, 21, 2028–2034. [Google Scholar] [CrossRef]
- Li, H.; Estroff, L.A. Hydrogels Coupled with Self-Assembled Monolayers: An in Vitro Matrix To Study Calcite Biomineralization. J. Am. Chem. Soc. 2007, 129, 5480–5483. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Heinrich, M.; Siegrist, T.; Pflaum, J. Growth and Electronic Transport in 9,10-Diphenylanthracene Single Crystals—An Organic Semiconductor of High Electron and Hole Mobility. Adv. Mater. 2007, 19, 2097–2101. [Google Scholar] [CrossRef]
- Pannella, G. Tidal Growth Patterns in Recent and Fossil Mollusc Bivalve Shells: A Tool for the Reconstruction of Paleotides. Sci. Nat. 1976, 63, 539–543. [Google Scholar] [CrossRef]
- Rhoads, D.C.; Pannella, G. The Use of Molluscan Shell Growth Patterns in Ecology and Paleoecology. Lethaia 1970, 3, 143–161. [Google Scholar] [CrossRef]
- Dauphin, Y.; Cuif, J.; Doucet, J.; Salomé, M.; Susini, J.; Williams, C. In Situ Mapping of Growth Lines in the Calcitic Prismatic Layers of Mollusc Shells Using X-ray Absorption near-Edge Structure (XANES) Spectroscopy at the Sulphur K-Edge. Mar. Biol. 2003, 142, 299–304. [Google Scholar] [CrossRef]
- Younis, S.; Kauffmann, Y.; Bloch, L.; Zolotoyabko, E. Inhomogeneity of Nacre Lamellae on the Nanometer Length Scale. Cryst. Growth Des. 2012, 12, 4574–4579. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Ganesan, K.; Yang, P.; Kulak, A.N.; Borukhin, S.; Pechook, S.; Ribeiro, L.; Kröger, R.; Eichhorn, S.J.; Armes, S.P.; et al. An Artificial Biomineral Formed by Incorporation of Copolymer Micelles in Calcite Crystals. Nat. Mater. 2011, 10, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Gorna, K.; Muñoz-Espí, R.; Gröhn, F.; Wegner, G. Bioinspired Mineralization of Inorganics from Aqueous Media Controlled by Synthetic Polymers. Macromol. Biosci. 2007, 7, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Subramony, J.A.; Jang, S.-H.; Kahr, B. Dyeing KDP. Ferroelectrics 1997, 191, 293–300. [Google Scholar] [CrossRef]
- Kahr, B.; Bullard, T.; Kurimoto, M.; De Yoreo, J.J. Complex Organic Guests in Simple Crystals: Potassium Hydrogen Phthalate as Case Study. Cryst. Res. Technol. 2013, 48, 849–863. [Google Scholar] [CrossRef]
- Asakuma, Y.; Nishimura, M.; Li, Q.; Ang, H.M.; Tade, M.; Maeda, K.; Fukui, K. Colouring Mechanism of Dyed KDP Crystal by Quantum Chemistry. J. Mol. Struct. Theochem 2007, 810, 7–13. [Google Scholar] [CrossRef]
- Gavira, J.A.; Van Driessche, A.E.S.; Garcia-Ruiz, J.-M. Growth of Ultrastable Protein–Silica Composite Crystals. Cryst. Growth Des. 2013, 13, 2522–2529. [Google Scholar] [CrossRef]
- Ye, T.; Jin, X.-Y.; Chen, L.; Hu, C.; Ren, J.; Liu, Y.-J.; Wu, G.; Chen, L.-J.; Chen, H.-Z.; Li, H.-Y. Shape Change of Calcite Single Crystals to Accommodate Interfacial Curvature: Crystallization in Presence of Mg2+ Ions and Agarose Gel-Networks. Chin. Chem. Lett. 2017, 28, 857–862. [Google Scholar] [CrossRef]
- Risan, J.; Jain, G.; Pendola, M.; Evans, J.S. Intracrystalline Incorporation of Nacre Protein Hydrogels Modifies the Mechanical Properties of Calcite Crystals: A Microcompression Study. J. Mater. Chem. B 2018, 6, 4191–4196. [Google Scholar] [CrossRef]
- Smith, B.L.; Schäffer, T.E.; Viani, M.; Thompson, J.B.; Frederick, N.A.; Kindt, J.; Belcher, A.; Stucky, G.D.; Morse, D.E.; Hansma, P.K. Molecular Mechanistic Origin of the Toughness of Natural Adhesives, Fibres and Composites. Nature 1999, 399, 761–763. [Google Scholar] [CrossRef]
- Kunitake, M.E.; Mangano, L.M.; Peloquin, J.M.; Baker, S.P.; Estroff, L.A. Evaluation of Strengthening Mechanisms in Calcite Single Crystals from Mollusk Shells. Acta Biomater. 2013, 9, 5353–5359. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Cao, W.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.; Holloway, P.H.; Qian, L. High-Efficiency Light-Emitting Devices Based on Quantum Dots with Tailored Nanostructures. Nat. Photon. 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P. Stable and Efficient Quantum-Dot Light-Emitting Diodes Based on Solution-Processed Multilayer Structures. Nat. Photon. 2011, 5, 543–548. [Google Scholar] [CrossRef]
- Zhang, L.; Bailey, J.B.; Subramanian, R.H.; Groisman, A.; Tezcan, F.A. Hyperexpandable, Self-Healing Macromolecular Crystals with Integrated Polymer Networks. Nature 2018, 557, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bull, B.; Szymanski, C.; Christensen, K.; McNeill, J. Multicolor Conjugated Polymer Dots for Biological Fluorescence Imaging. ACS Nano 2008, 2, 2415–2423. [Google Scholar] [CrossRef]
- Kim, K.; Park, Y.-G.; Hyun, B.G.; Choi, M.; Park, J.-U. Recent Advances in Transparent Electronics with Stretchable Forms. Adv. Mater. 2019, 31, 1804690. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, K.; Pang, K.; Liu, X.; Gao, M.; Ren, J.; Yang, G.; Wu, G.; Zhang, C.; Ni, X.; et al. Key Progresses of MOE Key Laboratory of Macromolecular Synthesis and Functionalization in 2022. Chin. Chem. Lett. 2024, 35, 108861. [Google Scholar] [CrossRef]
- Gong, X.; Tong, M.; Xia, Y.; Cai, W.; Moon, J.S.; Cao, Y.; Yu, G.; Shieh, C.-L.; Nilsson, B.; Heeger, A.J. High-Detectivity Polymer Photodetectors with Spectral Response from 300 Nm to 1450 Nm. Science 2009, 325, 1665–1667. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.; Sariciftci, N.S. Morphology of Polymer/Fullerene Bulk Heterojunction Solar Cells. J. Mater. Chem. 2006, 16, 45–61. [Google Scholar] [CrossRef]
- Li, H.; Xue, G.; Wu, J.; Zhang, W.; Huang, Z.; Xie, Z.; Xin, H.L.; Wu, G.; Chen, H.; Li, H. Long-Range Ordering of Composites for Organic Electronics: TIPS-Pentacene Single Crystals with Incorporated Nano-Fibers. Chin. Chem. Lett. 2017, 28, 2121–2124. [Google Scholar] [CrossRef]

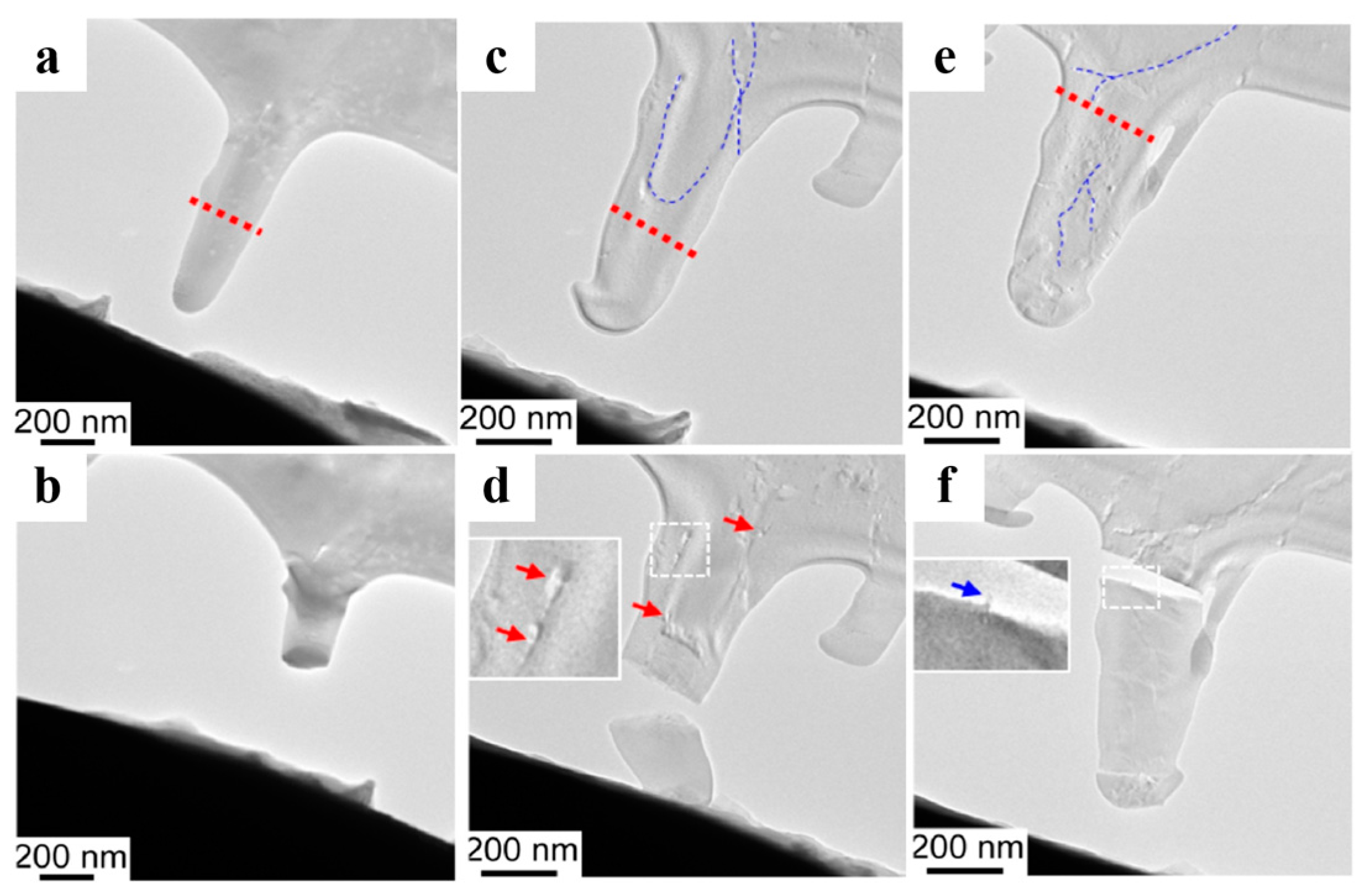
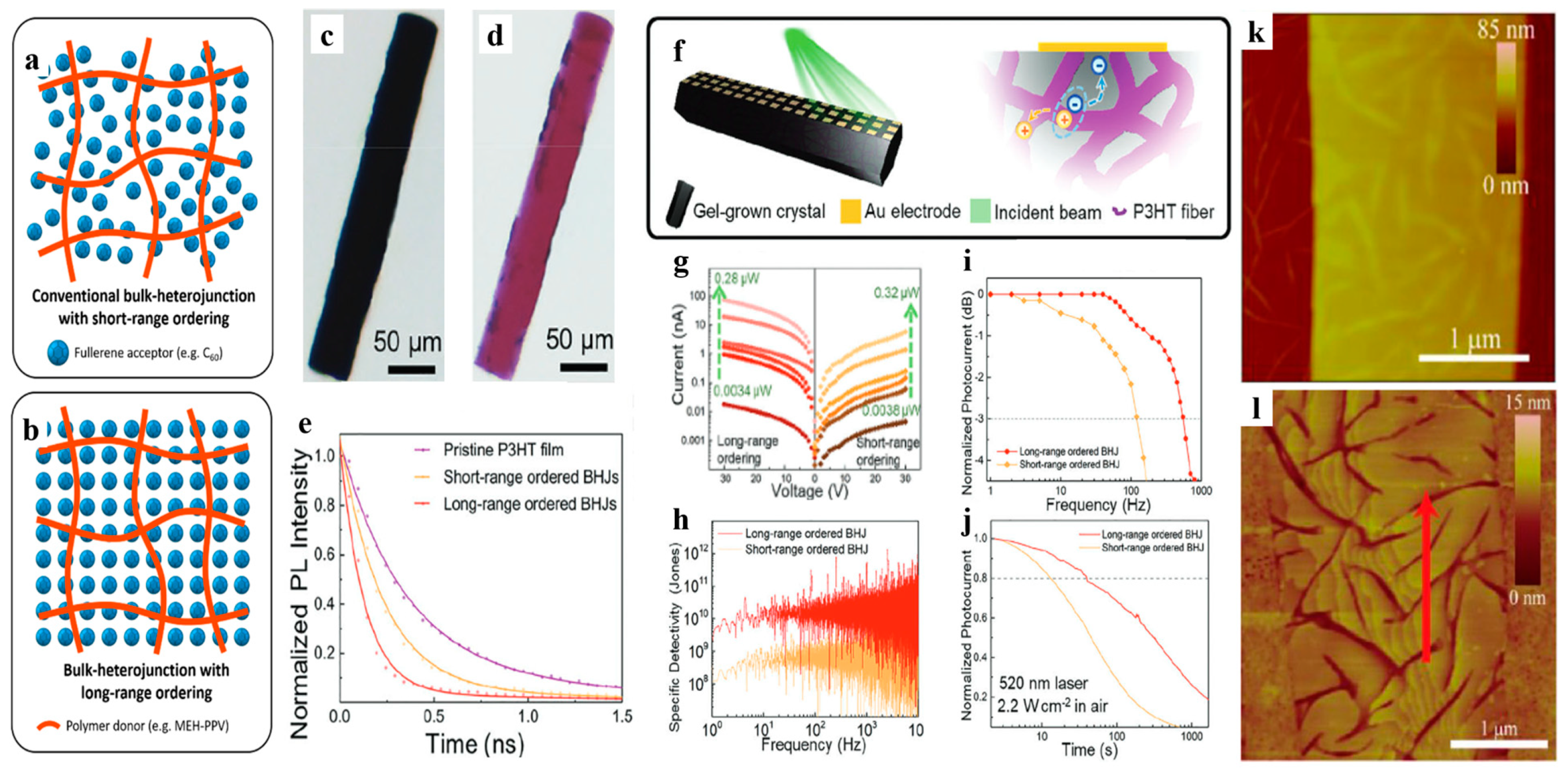
| Gel | Third-Component Materials | Crystals | Functions | References |
|---|---|---|---|---|
| Agarose | - | Calcite | Mechanical properties | [14,40] |
| Fe3O4 nanoparticles | Calcite | Paramagnetism | [32] | |
| Au nanoparticles | Calcite | Color | [32] | |
| microgels | Calcite | Fluorescence | [36] | |
| Quantum dots | Calcite | Fluorescence | [31] | |
| MWCNTs | Calcite | Mechanical reinforcement | [14] | |
| Graphene oxide | Calcite | Mechanical reinforcement | [14] | |
| Gelatin | - | Calcite | Morphological control | [27] |
| Silica | - | PbI2 | Bandgap engineering | [18] |
| Dye | KDP | Fluorescence | [34] | |
| P3HT | - | C60 | Photodetection | [22] |
| - | O-IDTBR | Photodetection | [22] | |
| MEH-PPV | - | C60 | Photodetection | [23] |
| TiO2 | - | PbI2 | X-ray detection | [30] |
| Poly (acrylic acid-acrylamide) hydrogel | - | Ferritin | Self-healing | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Ren, J.; Li, H. Constructing Multifunctional Composite Single Crystals via Polymer Gel Incorporation. Polymers 2024, 16, 2379. https://doi.org/10.3390/polym16162379
Mao Z, Ren J, Li H. Constructing Multifunctional Composite Single Crystals via Polymer Gel Incorporation. Polymers. 2024; 16(16):2379. https://doi.org/10.3390/polym16162379
Chicago/Turabian StyleMao, Zhiwen, Jie Ren, and Hanying Li. 2024. "Constructing Multifunctional Composite Single Crystals via Polymer Gel Incorporation" Polymers 16, no. 16: 2379. https://doi.org/10.3390/polym16162379
APA StyleMao, Z., Ren, J., & Li, H. (2024). Constructing Multifunctional Composite Single Crystals via Polymer Gel Incorporation. Polymers, 16(16), 2379. https://doi.org/10.3390/polym16162379







