Influence of Cross-Linkers on the Wash Resistance of Chitosan-Functionalized Polyester Fabrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Procedures
2.2.1. Alkali Hydrolysis
2.2.2. Functionalization of Polyester Fabrics with Chitosan
2.2.3. Washing Process
2.2.4. Staining Test
2.3. Methods
2.3.1. Gravimetric Analysis
2.3.2. Streaming Potential Method
2.3.3. Microscopic Observation
2.3.4. Remission Spectrophotometry
- DL*—difference in lightness (DL* = L*washed sample − L*standard),DC*—difference in chroma (DC* = C*washed sample − C*standard),DH*—difference in hue (DH* = H*washed sample − H*standard).
3. Results
3.1. Alkaline Hydrolysis
3.1.1. Weight Loss
3.1.2. Whiteness Quality
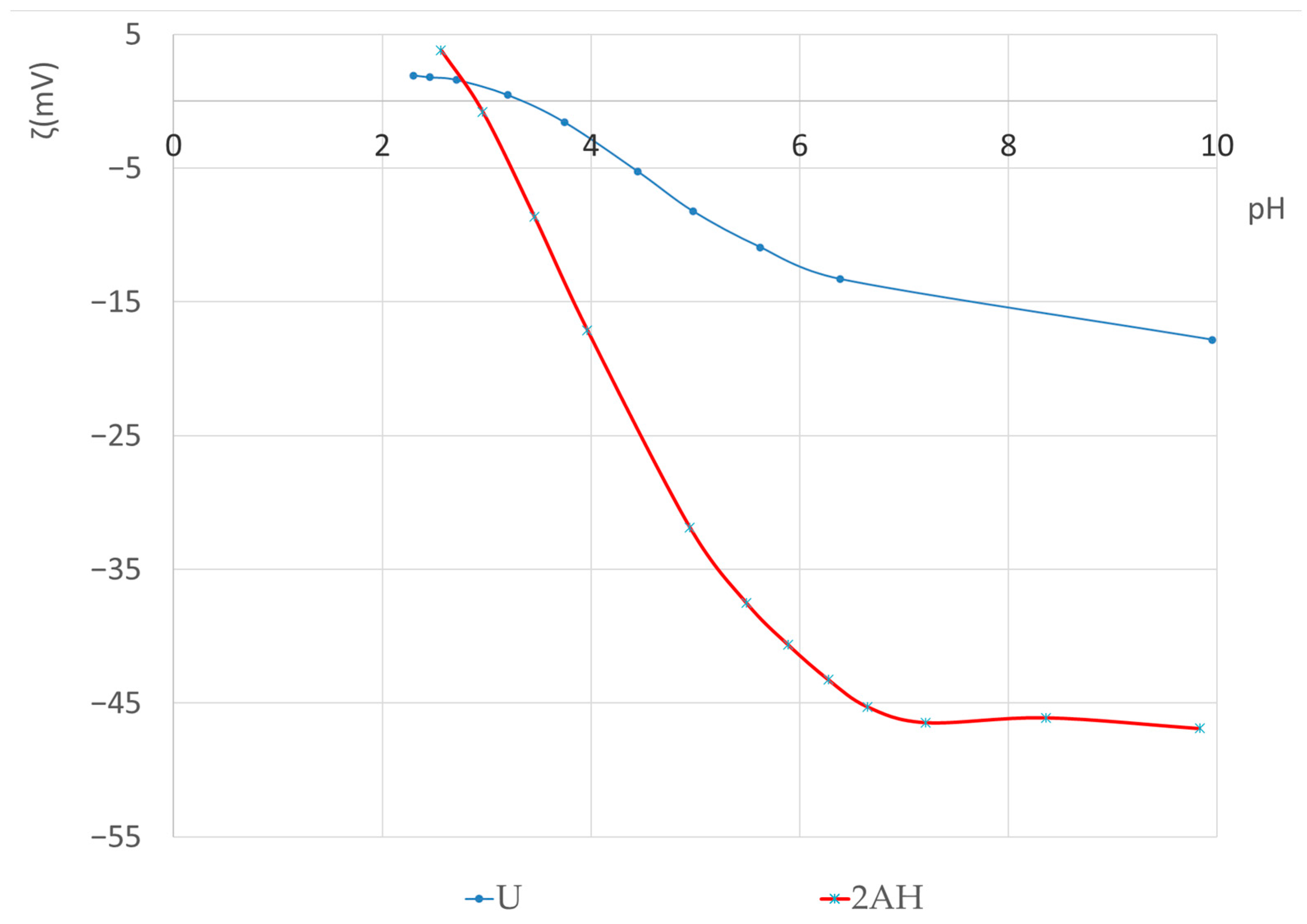
3.1.3. Streaming Potential
3.2. Stainability of Chitosan-Functionalized Polyester Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takara, E.A.; Marchese, J.; Ochoa, N.A. NaOH treatment of Chitosan films: Impact on macromolecular structure and film properties. Carbohydr. Polym. 2015, 132, 25–30. [Google Scholar] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar]
- Del Valle, L.; Diaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 217. [Google Scholar] [CrossRef]
- Enescu, D. Use of chitosan in surface modification of textile materials. Rom. Biotechnol. Lett. 2008, 13, 4037–4048. [Google Scholar]
- Saïed, N.; Aïder, M. Zeta Potential and Turbidimetry Analyzes for the Evaluation of Chitosan/Phytic Acid Complex Formation. J. Food Res. 2014, 3, 71–81. [Google Scholar]
- Flinčec Grgac, S.; Tarbuk, A.; Dekanić, T.; Sujka, W.; Draczynski, Z. The Chitosan Implementation into Cotton and Polyester/Cotton Blend Fabrics. Materials 2020, 13, 1616. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Ali, S.W.; Purwar, R. Ecofriendly antimicrobial finishing of textiles using bioactive agents based on natural products. Indian J. Fibre Text. Res. 2009, 34, 295–304. [Google Scholar]
- Bhavsar, S.P.; Dalla Fontana, G.; Zoccola, M. Sustainable Superheated Water Hydrolysis of Black Soldier Fly Exuviae for Chitin Extraction and Use of the Obtained Chitosan in the Textile Field. ACS Omega 2021, 13, 8884–8893. [Google Scholar]
- Luo, X.; Yao, M.Y.; Li, L. Application of chitosan in the form of textile: Production and sourcing. Text. Res. J. 2022, 92, 3522–3533. [Google Scholar]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar]
- Palacios-Mateo, C.; van der Meer, Y.; Seide, G. Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ. Sci. Eur. 2021, 33, 2. [Google Scholar] [PubMed]
- Vernaez, O.; Neubert, K.J.; Kopitzky, R.; Kabasci, S. Compatibility of Chitosan in Polymer Blends by Chemical Modification of Bio-based Polyesters. Polymers 2019, 25, 1939. [Google Scholar] [CrossRef]
- Mitić, J.; Amin, G.; Kodrić, M.; Šmelcerović, M.; Đorđević, D. Polyester fibres structure modification using some organic solutions. Tekstil 2016, 65, 196–200. [Google Scholar]
- Ferrero, F.; Periolatto, M. Chitosan Coating on Textile Fibers for Functional Properties. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017; Volume 4, pp. 165–197. [Google Scholar]
- Grancarić, A.M.; Pušić, T.; Kallay, N. Modifikacija poliesterskog vlakna alkalnom hidrolizom. Polimeri 1991, 12, 141–146. [Google Scholar]
- Čorak, I.; Tarbuk, A.; Đorđević, D.; Višić, K.; Botteri, L. Sustainable alkaline hydrolysis of polyester fabric at low temperature. Materials 2022, 15, 1530. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, A.P.; Tehrani-Bagha, A. A review on microplastic emission from textile materials and its reduction techniques. Polym.Degrad. Stab. 2022, 199, 109901. [Google Scholar]
- Raza, Z.A.; Anwar, F.; Abid, S. Multi-response optimization in impregnation of chitosan nanoparticles on polyester fabric. Polym. Bull. 2019, 76, 3039–3058. [Google Scholar]
- Hoque, M.-T.; Klinkhammer, K.; Mahltig, B. HT process for treatment of PET fabrics with chitosan containing recipes. Commun. Dev. Assem. Text. Prod. 2023, 4, 222–230. [Google Scholar]
- Klinkhammer, K.; Hohenbild, H.; Hoque, M.T.; Elze, L.; Teshay, H.; Mahltig, B. Functionalization of Technical Textiles with Chitosan. Textiles 2024, 4, 70–90. [Google Scholar] [CrossRef]
- EN ISO 6330:2021; Textiles—Domestic Washing and Drying Procedures for Textile Testing. European Committee for Standardization: Brussels, Belgium, 2021.
- Bellmann, C.; Klinger, C.; Opfermann, A.; Böhme, F.; Adler, H.J. Evaluation of surface modification by electrokinetic measurements. Prog. Org. Coat. 2002, 44, 93–98. [Google Scholar]
- Bišćan, J. Electrokinetic Data: Approaches, Interpretations and Applications. Croat. Chem. Acta 2007, 80, 357–365. [Google Scholar]
- AATCC Test Method 110: Whiteness of Textiles. Available online: https://members.aatcc.org/store/tm110/521/ (accessed on 5 June 2024).
- Parac-Osterman, Đ. Osnove o Boji i Sustav Vrednovanja II. Izdanje; Sveučilište u Zagrebu Tekstilno-tehnološki fakultet: Zagreb, Croatia, 2013. [Google Scholar]
- ISO 105-A03; Textiles—Tests for Colour Fastness Part A03: Grey Scale for Assessing Staining. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- AATCC Evaluation Procedure 7 Instrumental Assessment of the Change in Color of a Test Specimen. Available online: https://members.aatcc.org/store/ep7/464/ (accessed on 5 June 2024).
- Pušić, T.; Kaurin, T.; Liplin, M.; Budimir, A.; Čurlin, M.; Grgić, K.; Sutlović, A.; Valh, J.V. The Stability of the Chitosan Coating on Polyester Fabric in the Washing Process. Tekstilec 2023, 66, 85–104. [Google Scholar]
- De Smet, D.; Vanneste, M. Application of Biobased and Biodegradable Materials in Textile Coating. In Proceedings of the International Federation of Associations of Textile Chemists and Colorists, IFATCC 2018, Greenville, SC, USA, 6–8 March 2018; pp. 1–8. Available online: https://www.ifatcc.org/wp-content/uploads/2018/01/O30.pdf (accessed on 5 June 2024).
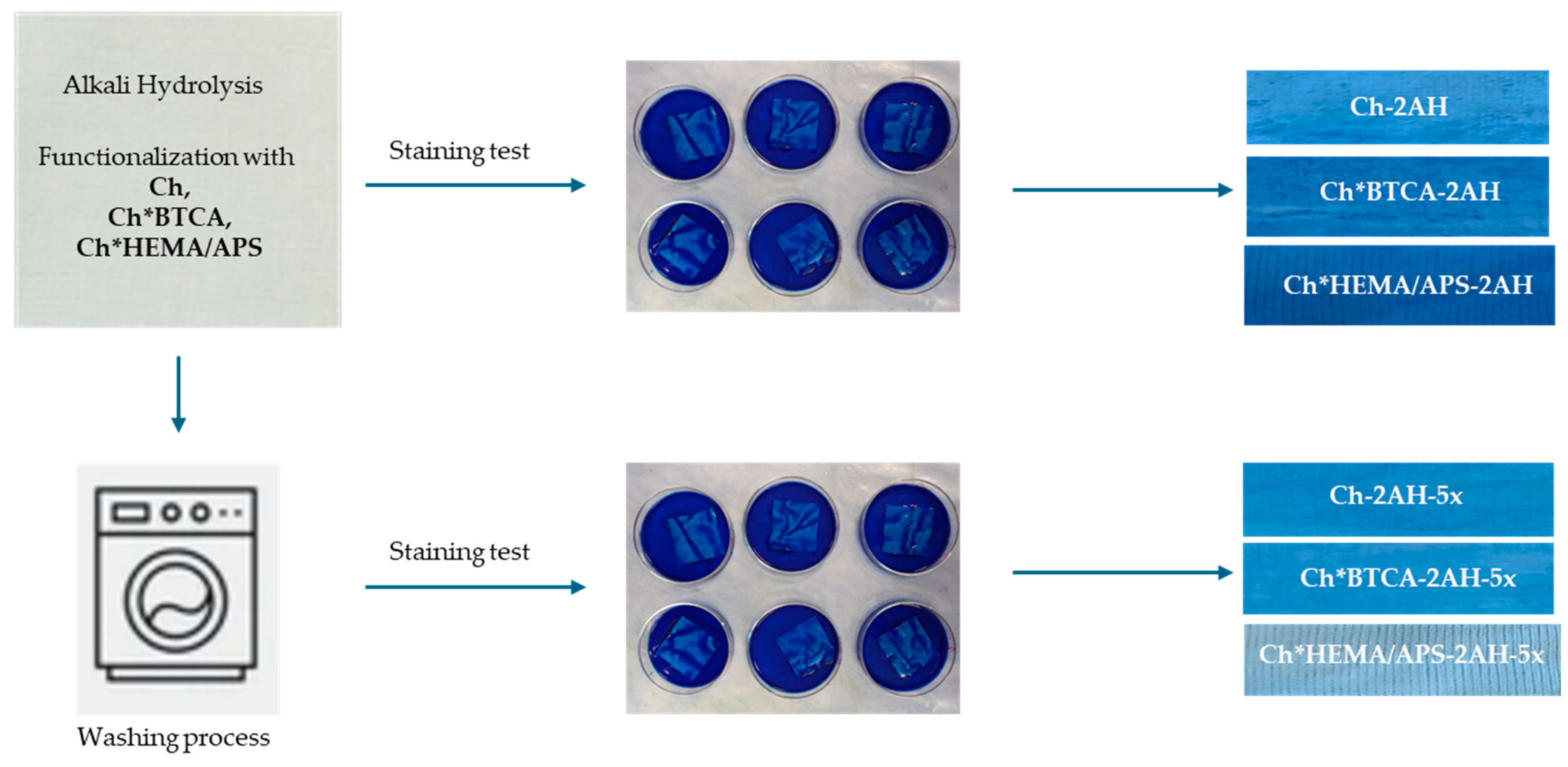
| Polyester Sample | Label |
|---|---|
| Untreated | U |
| Alkali treated (NaOH) 10 g/L, 20 g/L, 30 g/L | 1AH, 2AH, 3AH |
| Chitosan treated | Ch-U Ch-1AH, Ch-2AH, Ch-3AH |
| Chitosan treated with BTCA | Ch*BTCA-U Ch*BTCA-1AH, Ch*BTCA-2AH, Ch*BTCA-3AH |
| Chitosan treated with HEMA/APS | Ch*HEMA/APS-U Ch*HEMA/APS-1AH, Ch*HEMA/APS-2AH, Ch*HEMA/APS-3AH |
| Washed, cycles | −1×, −3×,−5× |
| Reference Polyester Fabric | ∆m (%) |
|---|---|
| 1AH | 1.5 ± 0.04 |
| 2AH | 4.2 ± 0.06 |
| 3AH | 7.1 ± 0.01 |
| Samples | W | TV | TD | Y |
|---|---|---|---|---|
| U | 68.1 | 0.1 | 81.8 | |
| 1AH | 56.7 | −0.5 | R1 | 80.3 |
| 2AH | 72.2 | 0.0 | 82.2 | |
| 3AH | 71.6 | 0.0 | 81.7 |
| Stained Sample | U | 1AH | 2AH | 3AH |
|---|---|---|---|---|
| Micrograph |  |  |  |  |
| Samples | Ch-U | Ch-1AH | Ch-2AH | Ch-3AH |
|---|---|---|---|---|
| Micrograph |  |  |  |  |
| Samples | Ch-U-1x | Ch-1AH-1x | Ch-2AH-1x | Ch-3AH-1x |
| Micrograph |  |  |  |  |
| Samples | Ch-U-3x | Ch-1AH-3x | Ch-2AH-3x | Ch-3AH-3x |
| Micrograph |  |  |  |  |
| Samples | Ch-U-5x | Ch-1AH-5x | Ch-2AH-5x | Ch-3AH-5x |
| Micrograph |  |  |  |  |
| Standard Ch-U | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 4 | 3-4 | 2.564 | 1.318 | 1.553 | −1.258 | |
| 3 | 3-4 | 3 | 3.813 | −0.041 | 3.461 | −0.251 | |
| 5 | 3-4 | 3 | 2.986 | 2.344 | 1.068 | −0.864 |
| Standard Ch-1AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 3-4 | 3 | 3.780 | 0.919 | 3.234 | −1.292 | |
| 3 | 3-4 | 3 | 3.778 | 0.738 | 3.503 | −0.829 | |
| 5 | 3-4 | 3 | 3.590 | 2.352 | 2.200 | −1.156 |
| Standard Ch-2AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 2-3 | 2 | 6.466 | 5.728 | −1.235 | −2.715 | |
| 3 | 3 | 3 | 3.979 | 2.988 | 1.982 | −1.130 | |
| 5 | 2 | 2 | 7.207 | 6.639 | −0.999 | −2.548 |
| Standard Ch-3AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 4 | 3 | 3.302 | –0.683 | 3.029 | 0.695 | |
| 3 | 4 | 4 | 2.118 | 1.359 | 1.311 | 0.280 | |
| 5 | 3-4 | 3-4 | 2.837 | 1.825 | 1.851 | 0.080 |
| Samples | Ch*BTCA-U | Ch*BTCA-1AH | Ch*BTCA-2AH | Ch*BTCA-3AH |
|---|---|---|---|---|
| Micrograph |  |  |  |  |
| Samples | Ch*BTCA-U-1x | Ch*BTCA-1AH-1x | Ch*BTCA-2AH-1x | Ch*BTCA-3AH-1x |
| Micrograph |  |  |  |  |
| Samples | Ch*BTCA-U-3x | Ch*BTCA-1AH-3x | Ch*BTCA-2AH-3x | Ch*BTCA-3AH-3x |
| Micrograph |  |  |  |  |
| Samples | Ch*BTCA-U-5x | Ch*BTCA-1AH-5x | Ch*BTCA-2AH-5x | Ch*BTCA-3AH-5x |
| Micrograph |  |  |  |  |
| Standard Ch*BTCA | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 2-3 | 2-3 | 4.665 | 3.973 | 0.077 | −2.274 | |
| 3 | 2 | 2 | 6.321 | 5.744 | −1.445 | −2.189 | |
| 5 | 2-3 | 2-3 | 4.938 | 4.588 | −0.648 | −1.686 |
| Standard Ch*BTCA-1AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 2 | 2 | 6.338 | 5.512 | 0.502 | −3.072 | |
| 3 | 2 | 2 | 7.217 | 6.581 | −0.633 | −2.764 | |
| 5 | 2 | 2 | 8.241 | 7.479 | −1.491 | −3.090 |
| Standard Ch*BTCA-2AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 3 | 3 | 3.923 | 1.958 | 2.665 | −1.722 | |
| 3 | 2-3 | 2-3 | 5.261 | 4.627 | 0.159 | −2.418 | |
| 5 | 2-3 | 2-3 | 4.673 | 3.715 | 1.541 | −2.262 |
| Standard Ch*BTCA-3AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 2 | 2 | 6.749 | 5.811 | 0.089 | −3.410 | |
| 3 | 2 | 1-2 | 8.526 | 7.465 | −1.447 | −3.840 | |
| 5 | 1-2 | 1-2 | 9.487 | 8.407 | −2.075 | −3.870 |
| Samples | Ch*HEMA/APS-U | Ch*HEMA/APS-1AH | Ch*HEMA/APS-2AH | Ch*HEMA/APS-3AH |
|---|---|---|---|---|
| Micrograph |  |  |  |  |
| Samples | Ch*HEMA/APS-U-1x | Ch*HEMA/APS-1AH-1x | Ch*HEMA/APS-2AH-1x | Ch*HEMA/APS-3AH-1x |
| Micrograph |  |  |  |  |
| Samples | Ch*HEMA/APS-U-3x | Ch*HEMA/APS-1AH-3x | Ch*HEMA/APS-2AH-3x | Ch*HEMA/APS-3AH-3x |
| Micrograph |  |  |  |  |
| Samples | Ch*HEMA/APS-U-5x | Ch*HEMA/APS-1AH-5x | Ch*HEMA/APS-2AH-5x | Ch*HEMA/APS-3AH-5x |
| Micrograph |  |  |  |  |
| Standard Ch*HEMA/APS-U | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 1 | 1 | 22.514 | 15.027 | −14.805 | −7.866 | |
| 3 | 1 | 1 | 22.023 | 14.794 | −14.536 | −7.379 | |
| 5 | 1 | 1 | 22.588 | 15.803 | −14.250 | −7.573 |
| Standard Ch*BTCA-1AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 1 | 1 | 17.446 | 11.939 | −11.246 | −5.672 | |
| 3 | 1 | 1 | 23.543 | 17.406 | −15.008 | −6.460 | |
| 5 | 1 | 1 | 25.545 | 18.144 | −16.470 | −7.187 |
| Standard Ch*BTCA-2AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 1 | 1 | 31.000 | 22.942 | −18.379 | −9.937 | |
| 3 | 1 | 1 | 33.907 | 25.103 | −20.304 | −10.360 | |
| 5 | 1 | 1 | 35.050 | 25.540 | −21.510 | −10.651 |
| Standard Ch*BTCA-3AH | Cycles | ISO A05 | AATCC | DE | DL* | DC* | DH* |
| 1 | 1 | 1 | 26.368 | 19.758 | −14.951 | −9.007 | |
| 3 | 1 | 1 | 30.535 | 22.440 | −18.457 | 9.387 | |
| 5 | 1 | 1 | 31.422 | 22.929 | −19.159 | −9.720 |
| Samples | K/S | K/S, Checksum | Micrographs |
|---|---|---|---|
| 2AH | 0.02 | 0.69 |  |
| Ch-2AH | 0.24 | 63.88 |  |
| Ch-2AH-5x | 0.13 | 42.34 |  |
| Ch*BTCA-2AH | 0.21 | 56.71 |  |
| Ch*BTCA-2AH-5x | 0.13 | 40.95 |  |
| Ch*HEMA/APS-2AH | 0.21 | 89.67 |  |
| Ch*HEMA/APS-2AH-5x | 0.12 | 32.49 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pušić, T.; Bušac, T.; Volmajer Valh, J. Influence of Cross-Linkers on the Wash Resistance of Chitosan-Functionalized Polyester Fabrics. Polymers 2024, 16, 2365. https://doi.org/10.3390/polym16162365
Pušić T, Bušac T, Volmajer Valh J. Influence of Cross-Linkers on the Wash Resistance of Chitosan-Functionalized Polyester Fabrics. Polymers. 2024; 16(16):2365. https://doi.org/10.3390/polym16162365
Chicago/Turabian StylePušić, Tanja, Tea Bušac, and Julija Volmajer Valh. 2024. "Influence of Cross-Linkers on the Wash Resistance of Chitosan-Functionalized Polyester Fabrics" Polymers 16, no. 16: 2365. https://doi.org/10.3390/polym16162365
APA StylePušić, T., Bušac, T., & Volmajer Valh, J. (2024). Influence of Cross-Linkers on the Wash Resistance of Chitosan-Functionalized Polyester Fabrics. Polymers, 16(16), 2365. https://doi.org/10.3390/polym16162365








