Generation of Microplastics from Biodegradable Packaging Films Based on PLA, PBS and Their Blend in Freshwater and Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Water Collection and Analysis
2.2. Blend and Films Preparation
2.3. Degradation Experiments
2.4. Characterizations
3. Results and Discussion
3.1. pH Changes of the Aquatic Media
3.2. Optical Microscopy Analysis of the Formed Microplastics
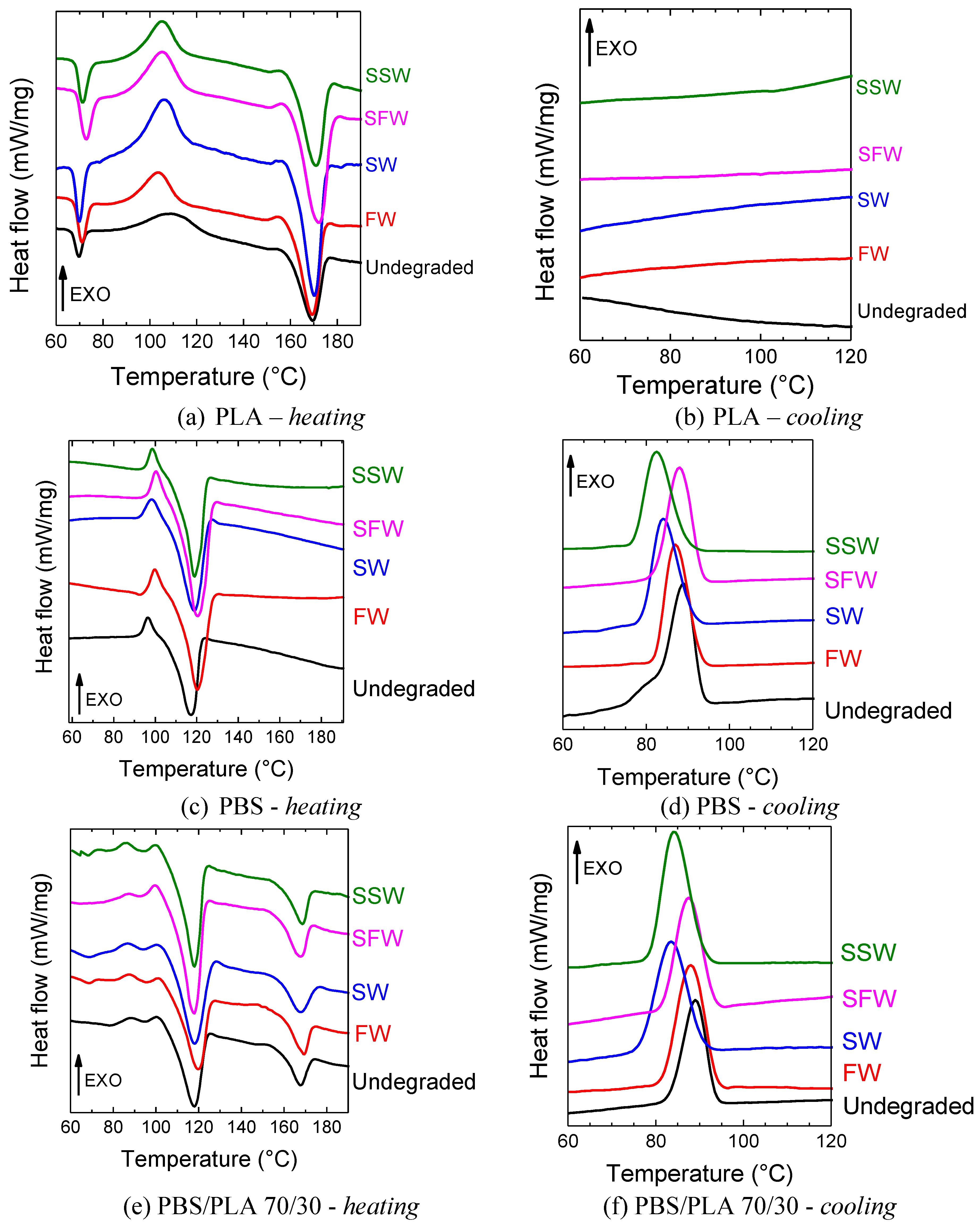
3.3. Melting Behavior, Crystallinity and Glass Transition Temperature
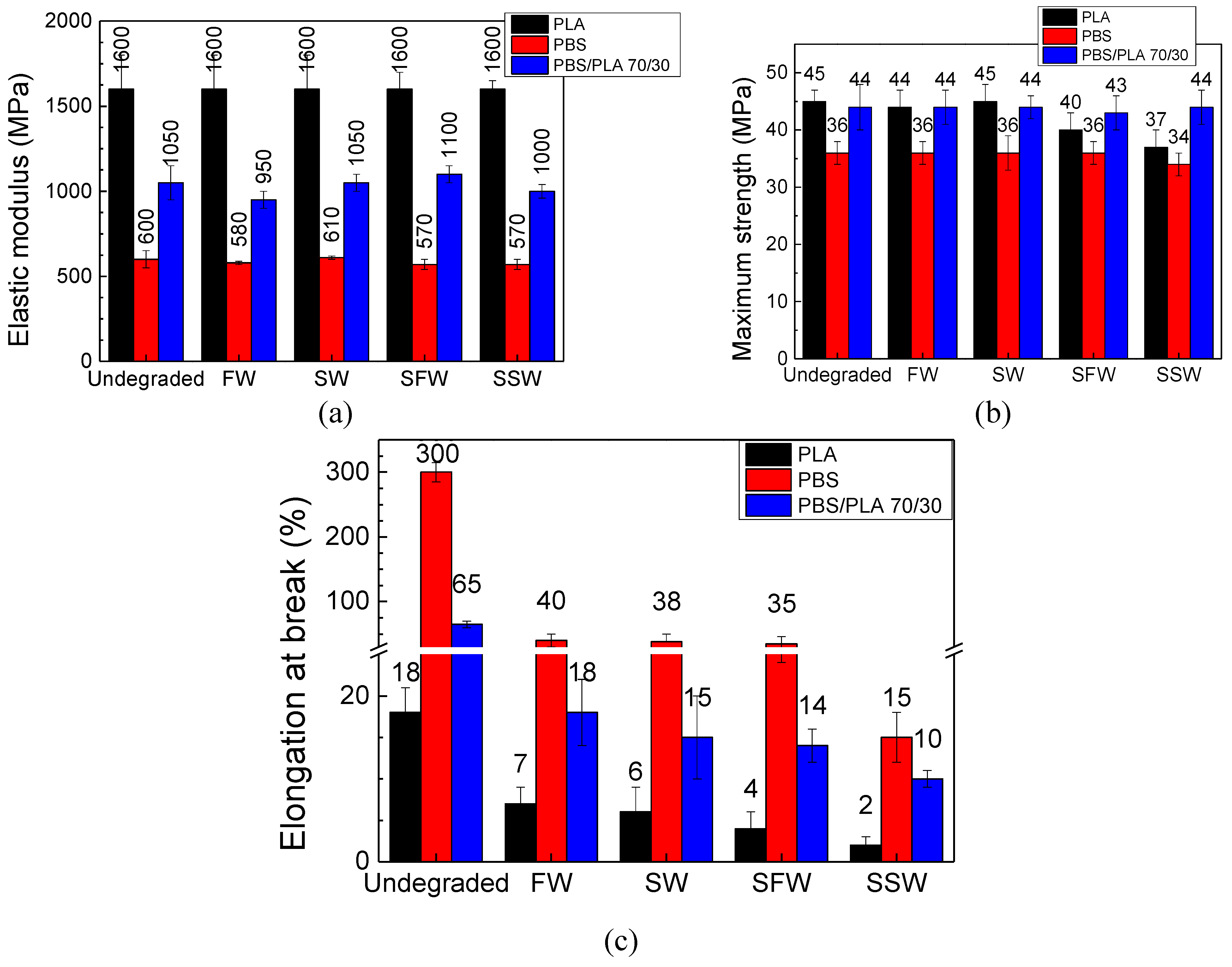
3.4. Mechanical Properties
3.5. Surface Wettability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.J. Understanding plastics pollution: The role of economic development and technological research. Environ. Pollut. 2019, 249, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Mong, G.R.; Tan, H.; Sheng, D.D.C.V.; Kek, H.Y.; Nyakuma, B.B.; Woon, K.S.; Othman, M.H.D.; Kang, H.S.; Goh, P.S.; Wong, K.Y. A review on plastic waste valorisation to advanced materials: Solutions and technologies to curb plastic waste pollution. J. Clean. Prod. 2024, 434, 140180. [Google Scholar] [CrossRef]
- Suzuki, G.; Uchida, N.; Tuyen, L.H.; Tanaka, K.; Matsukami, H.; Kunisue, T.; Takahashi, S.; Viet, P.H.; Kuralmochi, H.; Osako, M. Mechanical recycling of plastic waste as a point source of microplastic pollution. Environ. Pollut. 2022, 303, 119114. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- United Nations. Fast Facts—What is Plastic Pollution?—United Nations Sustainable Development. 2023. Available online: https://www.un.org/sustainabledevelopment/blog/2023/08/explainer-what-is-plastic-pollution/ (accessed on 18 July 2024).
- Resource Futures and Nextek. Eliminating Avoidable Plastic Waste by 2042: A Use-Based Approach to Decision and Policy Making. Resourcing the Future Conference. 2018. Available online: https://www.circularonline.co.uk/wp-content/uploads/2019/06/Eliminating-avoidable-plastic-waste-by-2042-a-use-based-approach-to-decision-and-policy-making.pdf (accessed on 18 July 2024).
- Bandaru, S.; Ravipati, M.; Busi, K.B.; Phukan, P.; Bag, S.; Chandu, B.; Dalapati, G.K.; Biring, S.; Chakrabortty, S. A Review on the Fate of Microplastics: Their Degradation and Advanced Analytical Characterization. J. Polym. Environ. 2023, 32, 2532–2550. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Dörnyei, K.R.; Uysal-Unalan, I.; Krauter, V.; Weinrich, R.; Incarnato, L.; Karlovits, I.; Colelli, G.; Chrysochou, P.; Fenech, M.C.; Pettersen, M.K.; et al. Sustainable food packaging: An updated definition following a holistic approach. Front. Sustain. Food Syst. 2023, 7, 1119052. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Li, H. A new journey of plastics: Towards a circular and low carbon future. Giant 2022, 11, 100115. [Google Scholar] [CrossRef]
- European Bioplastics, e.V. Bioplastics Market Development Update 2023. 2023. Available online: https://www.european-bioplastics.org/bioplastics-market-development-update-2023-2/ (accessed on 18 July 2024).
- Scarfato, P.; Di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132, 42597. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Carullo, D.; Casson, A.; Rovera, C.; Ghaani, M.; Bellesia, T.; Guidetti, R.; Farris, S. Testing a coated PE-based mono-material for food packaging applications: An in-depth performance comparison with conventional multi-layer configurations. Food Packag. Shelf Life 2023, 39, 101143. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Apicella, A.; Bradley, K.; Meile, J.; Chillet, M.; Scarfato, P.; Incarnato, L.; Remize, F. Effects of maturity level, steam treatment, or active packaging to maintain the quality of minimally processed mango (Mangifera indica cv. José). J. Food Process. Preserv. 2021, 45, e15600. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Oxygen absorption data of multilayer oxygen scavenger-polyester films with different layouts. Data Brief 2018, 19, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, G.; Bugatti, V.; Vittoria, V.; Gorrasi, G. Antimicrobial sorbate anchored to layered double hydroxide (LDH) nano-carrier employed as active coating on Polypropylene (PP) packaging: Application to bread stored at ambient temperature. Futur. Foods 2021, 4, 100063. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Garofalo, E.; Incarnato, L. Evaluation of performance of PET packaging films based on different copolyester O2-scavengers. AIP Conf. Proc. 2018, 1981, 020130. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; D’Arienzo, L.; Garofalo, E.; Di Maio, L.; Incarnato, L. Antimicrobial biodegradable coatings based on LAE for food packaging applications. AIP Conf. Proc. 2018, 1981, 020010. [Google Scholar] [CrossRef]
- Bugatti, V.; Brachi, P.; Viscusi, G.; Gorrasi, G. Valorization of tomato processing residues through the production of active bio-composites for packaging applications. Front. Mater. 2019, 6, 34. [Google Scholar] [CrossRef]
- Boccalon, E.; Viscusi, G.; Lamberti, E.; Fancello, F.; Zara, S.; Sassi, P.; Marinozzi, M.; Nocchetti, M.; Gorrasi, G. Composite films containing red onion skin extract as intelligent pH indicators for food packaging. Appl. Surf. Sci. 2022, 593, 153319. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Gorrasi, G. Formulation of a Bio-Packaging Based on Pure Cellulose Coupled with Cellulose Acetate Treated with Active Coating: Evaluation of Shelf Life of Pasta Ready to Eat. Foods 2020, 9, 1414. [Google Scholar] [CrossRef]
- Gorrasi, G.; Viscusi, G.; Gerardi, C.; Lamberti, E.; Giovinazzo, G. Physicochemical and Antioxidant Properties of White (Fiano cv) and Red (Negroamaro cv) Grape Pomace Skin Based Films. J. Polym. Environ. 2022, 30, 3609–3621. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Nilsson, F.; Yin, H.; Hedenqvist, M.S. Microplastics Originating from Polymer Blends: An Emerging Threat? Environ. Sci. Technol. 2021, 55, 4190–4193. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer packaging: Advances in preparation techniques and emerging food applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Incarnato, L. Tailor-made coextruded blown films based on biodegradable blends for hot filling and frozen food packaging. Food Packag. Shelf Life 2023, 37, 101096. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Pont, B.D.; Miketa, F.; Coltelli, M.-B.; Lazzeri, A. Tearing fracture of poly(lactic acid) (PLA)/poly(butylene succinate-co-adipate) (PBSA) cast extruded films: Effect of the PBSA content. Eng. Fract. Mech. 2023, 289, 109450. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Poly(lactic acid) (PLA)/Poly(butylene succinate-co-adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, A.; Tosin, M.; Degli-Innocenti, F. Biodegradation of plastics in soil: The effect of temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly(lactic acid). In Advances in Polymer Science; Springer: New York, NY, USA, 2018; pp. 119–151. [Google Scholar]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Chen, C.; Song, B.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Sifuentes-Nieves, I.; Flores-Silva, P.C.; Ledezma-Perez, A.S.; Hernandez-Gamez, J.F.; Gonzalez-Morones, P.; Saucedo-Salazar, E.; Hernandez-Hernandez, E. Sustainable and Ecological Poly (Vinyl Alcohol)/Agave Fiber-Based Films: Structural Features post Composting Process. J. Polym. Environ. 2024, 32, 2448–2456. [Google Scholar] [CrossRef]
- Kwon, S.; Zambrano, M.C.; Pawlak, J.J.; Ford, E.; Venditti, R.A. Aquatic Biodegradation of Poly(β-Hydroxybutyrate) and Polypropylene Blends with Compatibilizer and the Generation of Micro- and Nano-Plastics on Biodegradation. J. Polym. Environ. 2023, 31, 3619–3631. [Google Scholar] [CrossRef]
- Green, D.S. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut. 2016, 216, 95–103. [Google Scholar] [CrossRef]
- Malafeev, K.V.; Apicella, A.; Incarnato, L.; Scarfato, P. Understanding the Impact of Biodegradable Microplastics on Living Organisms Entering the Food Chain: A Review. Polymers 2023, 15, 3680. [Google Scholar] [CrossRef]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Impact of buried debris from agricultural biodegradable plastic mulches on two horticultural crop plants: Tomato and lettuce. Sci. Total Environ. 2023, 856, 159167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, M.; Su, X.; Yuan, P.; Li, X.; Zhou, C.; Wan, Z.; Zou, W. Photolytic degradation elevated the toxicity of polylactic acid microplastics to developing zebrafish by triggering mitochondrial dysfunction and apoptosis. J. Hazard. Mater. 2021, 413, 125321. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, Q.; Su, T.; Wang, Z.; Tong, H. Effect of Polycaprolactone Microplastics on Soil Microbial Communities and Plant Growth. J. Polym. Environ. 2023, 32, 1039–1045. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, L.; Li, C.; Zheng, H.; Luo, Y.; Pang, L.; Lin, Q.; Zhang, H.; Sun, C.; Chen, L.; et al. Photodegradation of biobased polymer blends in seawater: A major source of microplastics in the marine environment. Front. Mar. Sci. 2022, 9, 1046179. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Chaganti, S.R.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Padamati, R.B.; O’connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Wei, X.F.; Capezza, A.J.; Cui, Y.; Li, L.; Hakonen, A.; Liu, B.; Hedenqvist, M.S. Millions of microplastics released from a biodegradable polymer during biodegradation/enzymatic hydrolysis. Water Res. 2022, 211, 118068. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.E.; Dekle, J.L.; Leads, R.R.; Hunter, R.A. Degradation of bio-based and biodegradable plastics in a salt marsh habitat: Another potential source of microplastics in coastal waters. Mar. Pollut. Bull. 2020, 160, 111518. [Google Scholar] [CrossRef]
- Belioka, M.P.; Siddiqui, M.N.; Redhwi, H.H.; Achilias, D.S. Thermal degradation kinetics of recycled biodegradable and non-biodegradable polymer blends either neat or in the presence of nanoparticles using the random chain-scission model. Thermochim. Acta 2023, 726, 179542. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Belioka, M.P.; Achilias, D.S. Effect of nanoclay on the thermal degradation kinetics of recycled biodegradable/non-biodegradable polymer blends using the random chain-scission model. J. Anal. Appl. Pyrolysis 2024, 177, 106291. [Google Scholar] [CrossRef]
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Polyák, P.; Nagy, K.; Vértessy, B.; Pukánszky, B. Self-regulating degradation technology for the biodegradation of poly(lactic acid). Environ. Technol. Innov. 2023, 29, 103000. [Google Scholar] [CrossRef]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef]
- Radke, L. pH of Coastal Waterways—OzCoasts. 2002. Available online: https://ozcoasts.org.au/indicators/biophysical-indicators/ph_coastal_waterways/ (accessed on 18 July 2024).
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Lindström, A.; Albertsson, A.C.; Hakkarainen, M. Quantitative determination of degradation products an effective means to study early stages of degradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym. Degrad. Stab. 2004, 83, 487–493. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Huang, D.; Xu, P.-Y.; Lu, B.; Wang, G.-X.; Zhen, Z.-C.; Ji, J. Biobased Seawater-Degradable Poly(butylene succinate-l-lactide) Copolyesters: Exploration of Degradation Performance and Degradation Mechanism in Natural Seawater. ACS Sustain. Chem. Eng. 2022, 10, 3191–3202. [Google Scholar] [CrossRef]
- Hugenholtz, J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- Boskhomdzhiev, A.P.; Bonartsev, A.P.; Ivanov, E.A.; Makhina, T.; Myshkina, V.; Bagrov, D.; Filatova, E.; Bonartseva, G.; Iordanskii, A. Hydrolytic Degradation of Biopolymer Systems Based on Poly-3-hydroxybutyrate. Kinetic and Structural Aspects. Int. Polym. Sci. Technol. 2010, 37, 25–30. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Lucas, A.; Mallenco-Fornies, R.; Briones-Rizo, M.; Calvo, E.; Pelejero, C. Abiotic plastic leaching contributes to ocean acidification. Sci. Total Environ. 2023, 854, 158683. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Changing Ocean, Marine Ecosystems, and Dependent Communities. In The Ocean and Cryosphere in a Changing Climate: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022; pp. 447–588. [Google Scholar] [CrossRef]
- Von Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Hebner, T.S.; Maurer-Jones, M.A. Characterizing microplastic size and morphology of photodegraded polymers placed in simulated moving water conditions. Environ. Sci. Process Impacts 2020, 22, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crops Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Wang, Y.P.; Xiao, Y.J.; Duan, J.; Yang, J.H.; Wang, Y.; Zhang, C.L. Accelerated hydrolytic degradation of poly(lactic acid) achieved by adding poly(butylene succinate). Polym. Bull. 2016, 73, 1067–1083. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Influence of α′-/α-crystal polymorphism on properties of poly(l-lactic acid). Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]
- Tsuji, H.; Shimizu, K.; Sato, Y. Hydrolytic degradation of poly(L-lactic acid): Combined effects of UV treatment and crystallization. J. Appl. Polym. Sci. 2012, 125, 2394–2406. [Google Scholar] [CrossRef]
- Vu, T.; Nikaeen, P.; Chirdon, W.; Khattab, A.; Depan, D. Improved Weathering Performance of Poly(Lactic Acid) through Carbon Nanotubes Addition: Thermal, Microstructural, and Nanomechanical Analyses. Biomimetics 2020, 5, 61. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Hydrolytic degradation of biodegradable polyesters under simulated environmental conditions. J. Appl. Polym. Sci. 2015, 132, 42189. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Achilias, D.S. Effect of molecular weight on the cold-crystallization of biodegradable poly(ethylene succinate). Thermochim. Acta 2007, 457, 41–54. [Google Scholar] [CrossRef]
- Wang, X.S.; Yan, D.; Tian, G.H.; Li, X.G. Effect of molecular weight on crystallization and melting of poly(trimethylene terephthalate). 1: Isothermal and dynamic crystallization. Polym. Eng. Sci. 2001, 41, 1655–1664. [Google Scholar] [CrossRef]
- Oyama, H.T.; Kimura, M.; Nakamura, Y.; Ogawa, R. Environmentally safe bioadditive allows degradation of refractory poly(lactic acid) in seawater: Effect of poly(aspartic acid-co-l-lactide) on the hydrolytic degradation of PLLA at different salinity and pH conditions. Polym. Degrad. Stab. 2020, 178, 109216. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Giganate, V.; Cinelli, P. A Brief Review of Poly(Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Tsuji, H. In vitro hydrolysis of blends from enantiomeric poly(lactide)s. Part 4: Well-homo-crystallized blend and nonblended films. Biomaterials 2003, 24, 537–547. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Hua, K.; Duan, C.; Zhang, W.; Ji, J.; Yang, X. Enhanced mechanical properties and degradability of poly(butylene succinate) and poly(lactic acid) blends. Iran. Polym. J. 2013, 22, 267–275. [Google Scholar] [CrossRef]
- Arhant, M.; Le Gall, M.; Le Gac, P.Y.; Davies, P. Impact of hydrolytic degradation on mechanical properties of PET-towards an understanding of microplastics formation. Polym. Degrad. Stab. 2019, 161, 175–182. [Google Scholar] [CrossRef]
- Tham, C.Y.; Hamid, Z.A.A.; Ahmad, Z.; Ismail, H. Surface modification of poly(lactic acid) (PLA) via alkaline hydrolysis degradation. Adv. Mater. Res. 2014, 970, 324–327. [Google Scholar] [CrossRef]
- Taniguchi, I.; Nakano, S.; Nakamura, T.; El-Salmawy, A.; Miyamoto, M.; Kimura, Y. Mechanism of Enzymatic Hydrolysis of Poly(butylene succinate) and Poly(butylene succinate-co-L-lactate) with a Lipase from Pseudomonas cepacia. Macromol. Biosci. 2002, 2, 447–455. [Google Scholar] [CrossRef]


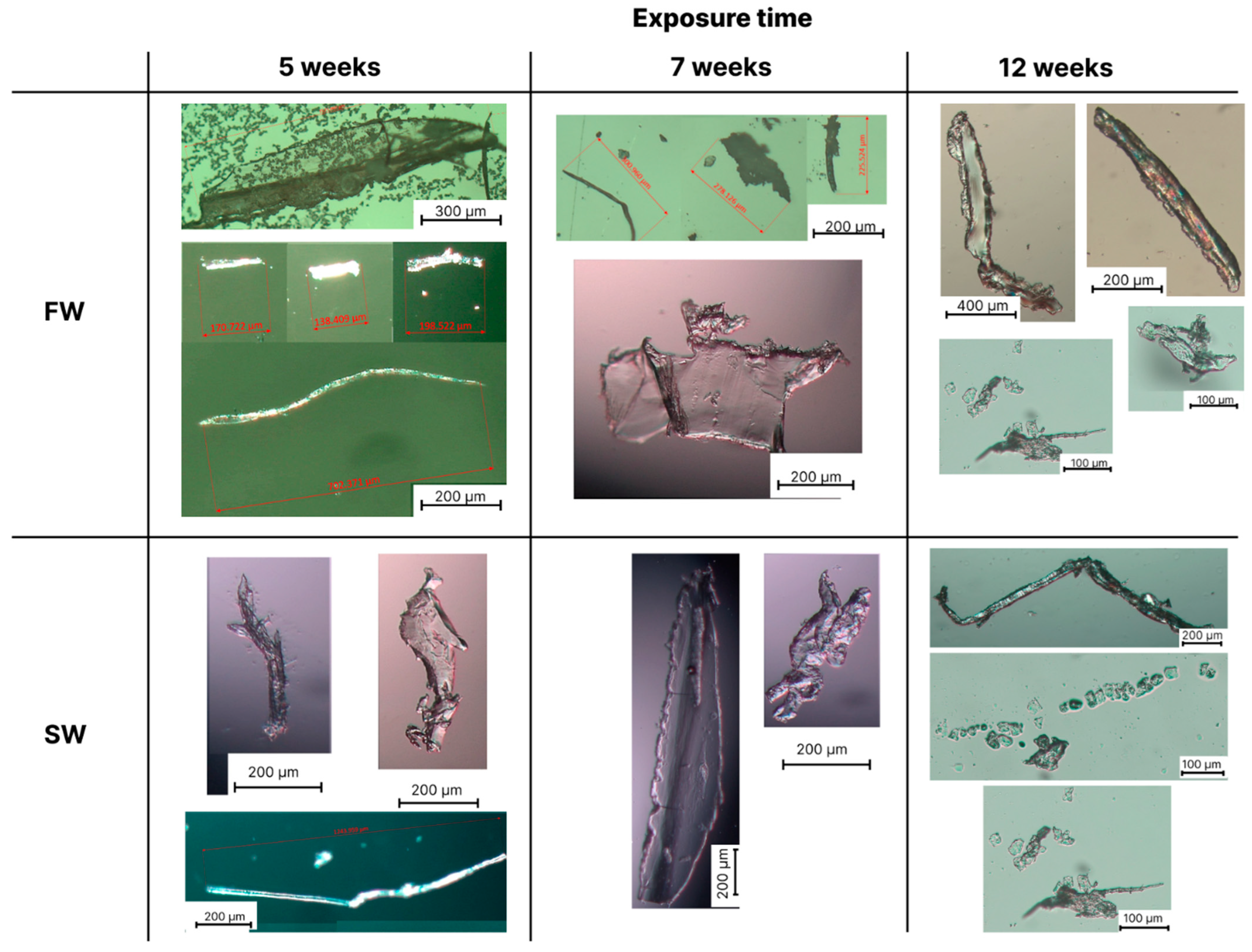
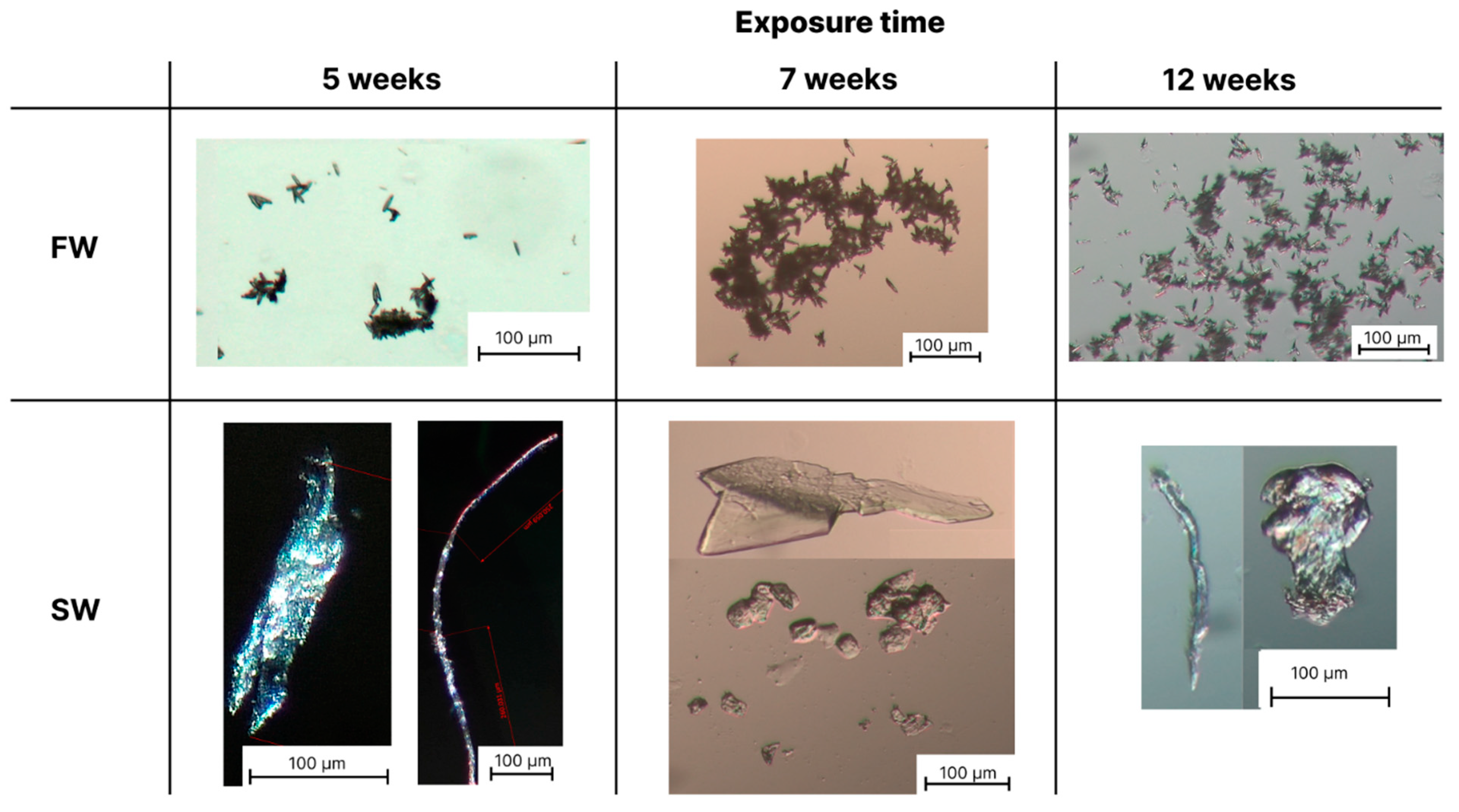
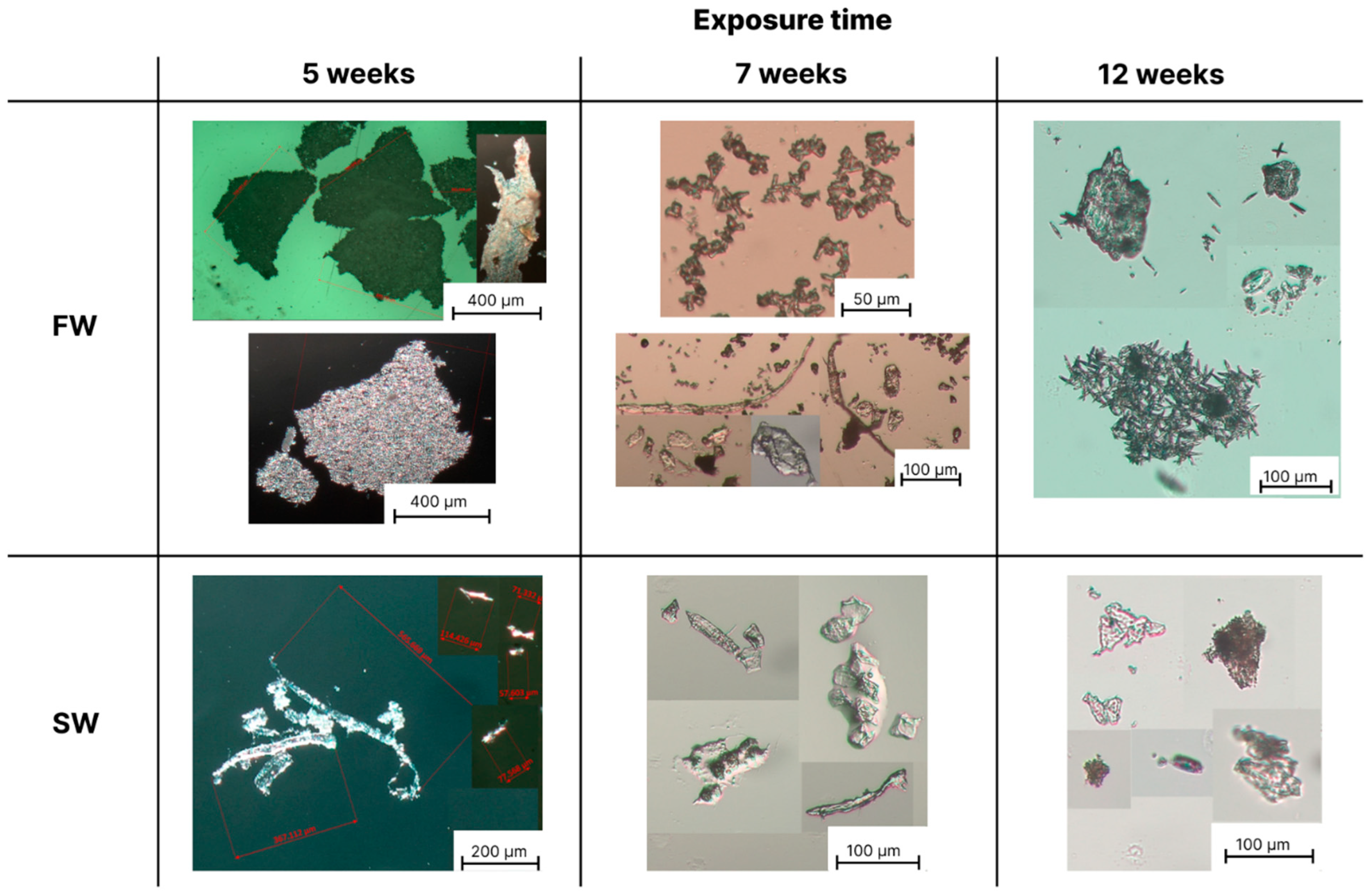

| Samples | Conditions | ΔpH (pHweek 0–pHweek 12) | |||
|---|---|---|---|---|---|
| FW | SFW | SW | SSW | ||
| Control | W&L | 0.03 | 0.01 | 0.04 | 0.02 |
| C&D | 0.02 | 0.01 | 0.03 | 0.02 | |
| PLA | W&L | 0.18 | 0.20 | 0.20 | 0.65 |
| C&D | 0.15 | 0.23 | 0.28 | 0.52 | |
| PBS | W&L | 0.40 | 0.20 | 0.30 | 0.70 |
| C&D | 0.05 | 0.08 | 0.22 | 0.48 | |
| PBS/PLA 70/30 | W&L | 0.45 | 0.30 | 0.28 | 0.70 |
| C&D | 0.20 | 0.15 | 0.33 | 0.51 | |
| Film Sample | Degradation Medium | Heating | Cooling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg, [°C] | Tcc PBS, [°C] | ΔHcc PBS, [mWs/mg] | Tcc PLA, [°C] | ΔHcc PLA, [mWs/mg] | Tm PBS, [°C] | ΔHm PBS, [mWs/mg] | XcPBS, [%] | Tm PLA, [°C] | ΔHm PLA, [mWs/mg] | XcPLA, [%] | Tcr, [°C] | ΔHcr, [mWs/mg] | ||
| PLA | Undegraded | 68 | 109 | 25.2 | 169 | 37.2 | 12.8 | |||||||
| FW | 69 | 104 | 20.4 | 169 | 37.9 | 18.7 | ||||||||
| SW | 69 | 106 | 19.6 | 170 | 36.4 | 17.9 | ||||||||
| SFW | 71 | 105 | 18.6 | 173 | 36.9 | 19.5 | ||||||||
| SSW | 70 | 106 | 21.7 | 172 | 37.8 | 17.2 | ||||||||
| PBS | Undegraded | 97 | 7.6 | 118 | 69.8 | 56.4 | 89 | 63.8 | ||||||
| FW | 100 | 7.4 | 120 | 66.4 | 56.6 | 88 | 55.1 | |||||||
| SW | 99 | 7 | 119 | 63.5 | 55.8 | 84 | 57.9 | |||||||
| SFW | 100 | 6.9 | 119 | 70.6 | 60.4 | 88 | 57.5 | |||||||
| SSW | 99 | 5.7 | 119 | 65.4 | 54.1 | 82 | 51.4 | |||||||
| PBS/PLA 70/30 | Undegraded | 88 | 4.1 | 100 | 118 | 49.7 | 59.1 | 167 | 14.7 | 52.3 | 89 | 38.2 | ||
| FW | 88 | 5.1 | 101 | 120 | 51.6 | 60.2 | 169 | 15.3 | 54.5 | 88 | 40.1 | |||
| SW | 87 | 5.6 | 100 | 118 | 48.4 | 55.4 | 168 | 12.8 | 45.6 | 84 | 37.1 | |||
| SFW | 87 | 3.4 | 100 | 118 | 51.5 | 62.3 | 168 | 14.4 | 51.2 | 88 | 39.5 | |||
| SSW | 87 | 4.5 | 100 | 118 | 51.4 | 60.7 | 168 | 14.3 | 51.0 | 84 | 37.8 | |||
| Sample | Water Contact Angle [°] | ||||
|---|---|---|---|---|---|
| Undegraded | FW | SW | SFW | SSW | |
| PLA | 63.7 ± 2.5 | 41.0 ± 6.1 | 45.8 ± 6.0 | 53.5 ± 6.4 | 56.7 ± 4.0 |
| PBS | 72.5 ± 2.1 | 60.6 ± 2.7 | 53.9 ± 2.9 | 60.1 ± 4.8 | 50.2 ± 2.7 |
| PBS/PLA 70/30 | 65.5 ± 3.1 | 50.4 ± 5.9 | 54.0 ± 3.9 | 57.5 ± 4.2 | 57.4 ± 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apicella, A.; Malafeev, K.V.; Scarfato, P.; Incarnato, L. Generation of Microplastics from Biodegradable Packaging Films Based on PLA, PBS and Their Blend in Freshwater and Seawater. Polymers 2024, 16, 2268. https://doi.org/10.3390/polym16162268
Apicella A, Malafeev KV, Scarfato P, Incarnato L. Generation of Microplastics from Biodegradable Packaging Films Based on PLA, PBS and Their Blend in Freshwater and Seawater. Polymers. 2024; 16(16):2268. https://doi.org/10.3390/polym16162268
Chicago/Turabian StyleApicella, Annalisa, Konstantin V. Malafeev, Paola Scarfato, and Loredana Incarnato. 2024. "Generation of Microplastics from Biodegradable Packaging Films Based on PLA, PBS and Their Blend in Freshwater and Seawater" Polymers 16, no. 16: 2268. https://doi.org/10.3390/polym16162268
APA StyleApicella, A., Malafeev, K. V., Scarfato, P., & Incarnato, L. (2024). Generation of Microplastics from Biodegradable Packaging Films Based on PLA, PBS and Their Blend in Freshwater and Seawater. Polymers, 16(16), 2268. https://doi.org/10.3390/polym16162268









