Fully Bio-Based Blends of Poly (Pentamethylene Furanoate) and Poly (Hexamethylene Furanoate) for Sustainable and Flexible Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Poly (Hexamethylene 2,5-Furanoate) and Poly (Pentamethylene 2,5-Furanoate) Synthesis
2.3. Molecular Characterization
2.4. Blend Preparation and Processing
2.5. Morphological Characterization of the Homopolymers and of the Blends
2.6. Thermal and Structural Characterization of the Homopolymers and of the Blends
2.7. Mechanical Characterization of the Homopolymers and of the Blends
2.8. Gas Permeability Measurements of the Homopolymers and of the Blends
2.9. Lab-Scale Composting Studies of the Homopolymers and of the Blends
3. Results and Discussion
3.1. Synthesis and Molecular Characterization
3.2. Morphological Characterization
3.3. Thermal and Structural Characterization
3.4. Mechanical Characterization
3.5. Evaluation of Gas Barrier Properties
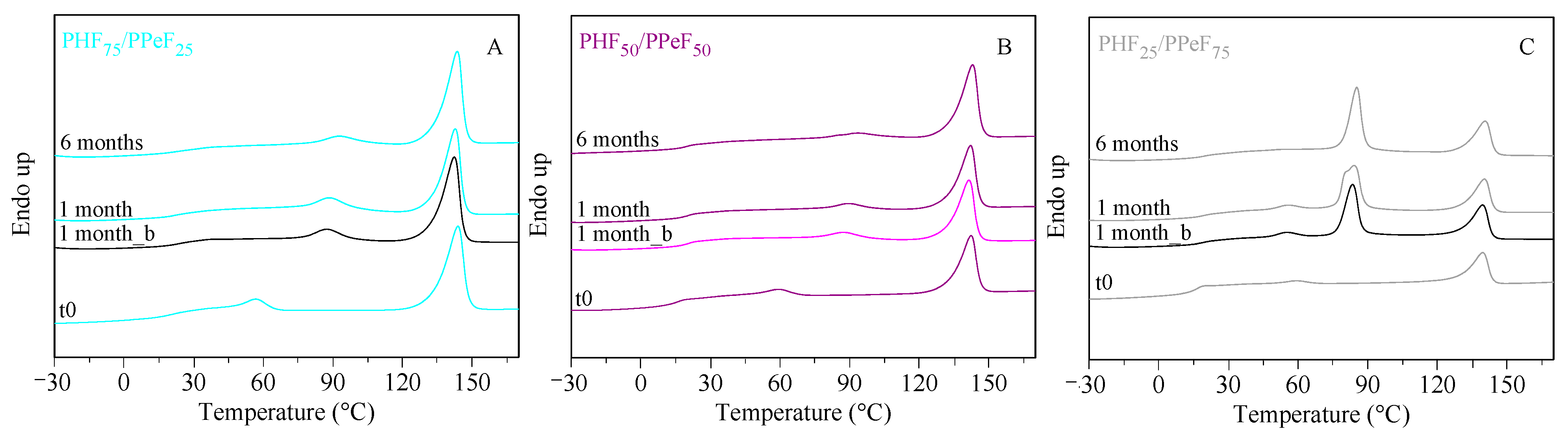
3.6. Lab-Scale Composting Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total. Environ. 2018, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Plastics—The Fast Facts 2023, Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 18 July 2024).
- Filho, W.L.; Salvia, A.L.; Bonoli, A.; Saari, U.A.; Voronova, V.; Klõga, M.; Kumbhar, S.S.; Olszewski, K.; De Quevedo, D.M.; Barbir, J. An assessment of attitudes towards plastics and bioplastics in Europe. Sci. Total. Environ. 2020, 755, 142732. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.-W.; Huber, G.W. A Review of Biodegradable Plastics: Chemistry, Applications, Properties, and Future Research Needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- European Environment Agency. Available online: https://www.eea.europa.eu/publications/microplastics-from-textiles-towards-a (accessed on 9 August 2024).
- Environment Action Programme to 2030, European Commission. Available online: https://environment.ec.europa.eu/strategy/environment-action-programme-2030_en#:~:text=The%208th%20EAP%20entered,conditions%20needed%20to%20achieve%20these (accessed on 18 July 2024).
- Sustainable Development Goals (SDG), the United Nations. Available online: Department of Economic and Social Affairs Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/news/communications-material/ (accessed on 24 October 2021).
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Flexible Packaging Europe. Available online: https://www.flexpack-europe.org/ (accessed on 18 July 2024).
- Ahamed, A.; Veksha, A.; Giannis, A.; Lisak, G. Flexible packaging plastic waste—Environmental implications, management solutions, and the way forward. Curr. Opin. Chem. Eng. 2021, 32, 100684. [Google Scholar] [CrossRef]
- Cabrera, G.; Li, J.; Maazouz, A.; Lamnawar, K. A Journey from Processing to Recycling of Multilayer Waste Films: A Review of Main Challenges and Prospects. Polymers 2022, 14, 2319. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Curtzwiler, G.W.; Vorst, K.L. The challenges in recycling post-consumer polyolefins for food contact applications: A review. Resour. Conserv. Recycl. 2021, 167, 105422. [Google Scholar] [CrossRef]
- Agostinho, B.; Silvestre, A.J.D.; Coutinho, J.A.P.; Sousa, A.F. Synthetic (bio)degradable polymers—When does recycling fail? Green. Chem. 2023, 25, 13–31. [Google Scholar] [CrossRef]
- Bello, A.S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Sustainable and long-term management of municipal solid waste: A review. Bioresour. Technol. Rep. 2022, 18, 101067. [Google Scholar] [CrossRef]
- Lavagnolo, M.C.; Grossule, V.; Cossu, R. Landfill Disposal in Developing Countries. In Waste Management in Developing Countries; Waste as a Resource; El Bari, H., Trois, C., Eds.; Springer: Cham, Switzerland, 2023; pp. 23–38. [Google Scholar]
- Vaverková, M.D. Landfill Impacts on the Environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]
- European-Bioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 18 July 2024).
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Barbir, J.; Abubakar, I.R.; Paço, A.; Stasiskiene, Z.; Hornbogen, M.; Fendt, M.T.C.; Voronova, V.; Klõga, M. Consumer attitudes and concerns with bioplastics use: An international study. PLoS ONE 2022, 17, e0266918. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. Economic Evaluation and Environmental Impacts. In Plastics Design Library, Bi-Opolymers Reuse, Recycling, and Disposal; Niaounakis, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 275–290. [Google Scholar]
- Lee, T.-H.; Yu, H.; Forrester, M.; Wang, T.-P.; Shen, L.; Liu, H.; Li, J.; Li, W.; Kraus, G.; Cochran, E. Next-Generation High-Performance Bio-Based Naphthalate Polymers Derived from Malic Acid for Sustainable Food Packaging. ACS Sustain. Chem. Eng. 2022, 10, 2624–2633. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohy-drates—The US department of energy’s “Top 10” revisited. Green. Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Fei, X.; Wang, J.; Zhang, X.; Jia, Z.; Jiang, Y.; Liu, X. Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA). Polymers 2022, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Furandicarboxylic Acid Market Size, Share & Trend Analysis Report by Application (PET, Polyamides, Polycarbonates, Plasticizers, Polyester Polyols), by Region, and Segment Forecasts, 2023–2030, Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/fdca-industry (accessed on 24 July 2024).
- Gandini, A.; Lacerda, T.M. Furan Polymers: State of the Art and Perspectives. Macromol. Mater. Eng. 2022, 307, 2100902. [Google Scholar] [CrossRef]
- Singhal, S.; Agarwal, S.; Mudoi, M.P.; Singhal, N.; Singh, R. Chemical conversion of furan dicarboxylic acid to environ-mentally benign polyesters: An overview. Biomass Convers. Biorefin. 2023, 13, 15619–15636. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2011, 50, 1026–1036. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A facile method to synthesize high-molecular-weight biobased polyesters from 2,5-furandicarboxylic acid and long-chain diols. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Karlinskii, B.Y.; Ananikov, V.P. Recent advances in the development of green furan ring-containing polymeric materials based on renewable plant biomass. Chem. Soc. Rev. 2022, 52, 836–862. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Dannecker, P.-K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.G.; Dubois, P. Modification of Poly(ethylene 2,5-furandicarboxylate) with Biobased 1,5-Pentanediol: Significantly Toughened Copolyesters Retaining High Tensile Strength and O2 Barrier Property. Biomacromolecules 2019, 20, 353–364. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. New Random Aromatic/Aliphatic Copolymers of 2,5-Furandicarboxylic and Camphoric Acids with Tunable Mechanical Properties and Exceptional Gas Barrier Capability for Sustainable Mono-Layered Food Packaging. Molecules 2023, 28, 4056. [Google Scholar] [CrossRef]
- Guidotti, G.; Genovese, L.; Soccio, M.; Gigli, M.; Munari, A.; Siracusa, V.; Lotti, N. Block Copolyesters Containing 2,5-Furan and trans-1,4-Cyclohexane Subunits with Outstanding Gas Barrier Properties. Int. J. Mol. Sci. 2019, 20, 2187. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Sablong, R.J.; Soccio, M.; Guidotti, G.; Siracusa, V.; Lotti, N. Bio-based aliphatic/aromatic poly(trimethylene furanoate/sebacate) random copolymers: Correlation between mechanical, gas barrier performances and compostability and copolymer composition. Polym. Degrad. Stab. 2021, 195, 109800. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Shi, L.; Bin Ying, W.; Wang, J.; Zhu, J. Modification of Poly(butylene 2,5-furandicarboxylate) with Lactic Acid for Biodegradable Copolyesters with Good Mechanical and Barrier Properties. Ind. Eng. Chem. Res. 2018, 57, 11020–11030. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Papadopoulos, L.; Grigora, M.E.; Tsongas, K.; Tzetzis, D.; Bikiaris, D.N.; Papageorgiou, G.Z. Blending PLA with polyesters based on 2,5-furan dicarboxylic acid: Evaluation of physicochemical and nanome-chanical properties. Polymers 2022, 14, 4725. [Google Scholar] [CrossRef]
- Rigotti, D.; Soccio, M.; Dorigato, A.; Gazzano, M.; Siracusa, V.; Fredi, G.; Lotti, N. Novel biobased polylactic ac-id/poly(pentamethylene 2,5-furanoate) blends for sustainable food packaging. ACS Sustain. Chem. Eng. 2021, 9, 13742–13750. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Sustainable Polymers from Renewable Resources: Polymer Blends of Furan-Based Polyesters. Macromol. Mater. Eng. 2018, 303. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Zhang, J.; Rayand, N.; Hu, G.-H.; Yang, J.; Zhu, J. Retroreflection in binary bio-based PLA/PBF blends. Polymer 2017, 125, 138–143. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Jiang, Y.; Hu, G.; Yang, J.; Zhu, J. Tensile Property Balanced and Gas Barrier Im-proved Poly(lactic acid) by Blending with Biobased Poly(butylene 2,5-furan dicarboxylate). ACS Sustain. Chem. Eng. 2017, 5, 9244–9253. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Piesowicz, E. Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials 2020, 13, 2673. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Wang, J.; Shao, N.; Xiong, Z.; Zhang, R.; Zhu, J. Nucleation and crystallization of poly(propylene 2,5-furan dicarboxylate) by direct blending of microcrystalline cellulose: Improved tensile and barrier properties. Cellulose 2020, 27, 9423–9436. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Smyrnioti, D.; Nikolaidis, G.N.; Tsitsimaka, I.; Christodoulou, E.; Bikiaris, D.N.; Charitopoulou, M.A.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Sustainable Plastics from Biomass: Blends of Polyesters Based on 2,5-Furandicarboxylic Acid. Polymers 2020, 12, 225. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New poly(pentylene furanoate) and poly(heptylene furanoate) sustainable polyesters from diols with odd methylene groups. Mater. Lett. 2016, 178, 64–67. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.A.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.-C.; Gutiérrez-Fernández, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-Ordered Structure in Biobased Poly(pentamethylene furanoate) Responsible for Its Outstanding Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Q.; Xie, W.; Peng, L.; He, L.; He, Z.; Chowdhury, S.P.; Christensen, R.; Ni, Y. An eco-friendly method to get a bio-based dicarboxylic acid monomer 2,5-furandicarboxylic acid and its application in the synthesis of poly(-hexylene 2,5-furandicarboxylate) (PHF). Polymers 2019, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tomishige, K. Production of 1,5-pentanediol from biomass via furfural and tetrahydrofurfuryl alcohol. Catal. Today 2012, 195, 136–143. [Google Scholar] [CrossRef]
- Guidotti, G.; Massari, D.; Gigli, M.; Soccio, M.; Siracusa, V.; Crestini, C.; Lotti, N. High-performance sustainable active packaging from poly(hexamethylene furanoate) and bark extracts. ACS Sustain. Chem. Eng. 2023, 11, 16585–16593. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-based poly-esters from renewable resources: Crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Martínez-Tong, D.E.; Soccio, M.; Robles-Hernández, B.; Guidotti, G.; Gazzano, M.; Lotti, N.; Alegria, A. Evidence of Nanostructure Development from the Molecular Dynamics of Poly(pentamethylene 2,5-furanoate). Macromolecules 2020, 53, 10526–10537. [Google Scholar] [CrossRef]
- Gao, X.-R.; Li, Y.; Huang, H.-D.; Xu, J.-Z.; Xu, L.; Ji, X.; Zhong, G.-J.; Li, Z.-M. Extensional Stress-Induced Orientation and Crystallization can Regulate the Balance of Toughness and Stiffness of Polylactide Films: Interplay of Oriented Amorphous Chains and Crystallites. Macromolecules 2019, 52, 5278–5288. [Google Scholar] [CrossRef]
- Xie, X.L.; Li, Y.; Xu, J.Z.; Yan, Z.; Zhong, G.J.; Li, Z.M. Largely enhanced mechanical performance of poly(butylene suc-cinate) multiple system via shear stress-induced orientation of the hierarchical structure. J. Mater. Chem. A 2018, 6, 13373–13385. [Google Scholar] [CrossRef]
- Aharoni, S.M. Correlations between chain parameters and failure characteristics of polymers below their glass transition temperature. Macromolecules 1985, 18, 2624–2630. [Google Scholar] [CrossRef]
- Hedenqvist, M.S. Barrier Packaging Materials. In Handbook of Environmental Degradation of Materials, 2nd ed.; Kutz, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 840–842. [Google Scholar]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Ordered structures of poly(butylene 2,5-thiophenedicarboxylate) and their impact on material functional properties. Eur. Polym. J. 2018, 106, 284–290. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. Poly(Alkylene 2,5-Thiophenedicarboxylate) Polyesters: A New Class of Bio-Based High-Performance Polymers for Sustainable Packaging. Polymers 2021, 13, 2460. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Sousa, A.F. Inside PEF: Chain Conformation and Dynamics in Crystalline and Amorphous Domains. Macromolecules 2018, 51, 3515–3526. [Google Scholar] [CrossRef]
- Di, Y.; Kang, M.; Zhao, Y.; Yan, S.; Wang, X. Morphology and mechanical properties of blends of thermoplastic polyurethane and polyolefins. J. Appl. Polym. Sci. 2005, 99, 875–883. [Google Scholar] [CrossRef]
- Posch, W. 3—Polyolefins. In Plastics Design Library, Applied Plastics Engineering Handbook; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 43–48. [Google Scholar]
- Robeson, L.M.; Liu, Q.; Freeman, B.D.; Paul, D.R. Comparison of transport properties of rubbery and glassy polymers and the relevance to the upper bound relationship. J. Membr. Sci. 2015, 476, 421–431. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Catalá, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2004, 20, 1–7. [Google Scholar] [CrossRef]
- Koros, W.J. Barrier Polymers and Structures: Overview. In Barrier Polymers and Structures; Koros, W.J., Ed.; Chapter 1; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Bianchi, E.; Soccio, M.; Siracusa, V.; Gazzano, M.; Thiyagarajan, S.; Lotti, N. Poly(butylene 2,4-furanoate), an Added Member to the Class of Smart Furan-Based Polyesters for Sustainable Packaging: Structural Isomerism as a Key to Tune the Final Properties. ACS Sustain. Chem. Eng. 2021, 9, 11937–11949. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Azzurri, F.; Floris, R.; Alfonso, G.C.; Balzano, L.; Peters, G.W. Continuous cooling curves diagrams of pro-pene/ethylene random copolymers. The role of ethylene counits in mesophase development. Macromolecules 2010, 43, 2890–2896. [Google Scholar] [CrossRef]
- Mensitieri, G.; Di Maio, E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Iannace, S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]
- Hu, Y.S.; Prattipati, V.; Mehta, S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Improving gas barrier of PET by blending with ar-omatic polyamides. Polymer 2005, 46, 2685–2698. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Lotti, N.; Siracusa, V.; Gazzano, M.; Munari, A. New multi-block copolyester of 2,5-furandicarboxylic acid containing PEG-like sequences to form flexible and degradable films for sustainable packaging. Polym. Degrad. Stab. 2019, 169, 108963. [Google Scholar] [CrossRef]
- Bianchi, E.; Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Lotti, N. Biobased and Compostable Multiblock Copolymer of Poly(l-lactic acid) Containing 2,5-Furandicarboxylic Acid for Sustainable Food Packaging: The Role of Parent Homopolymers in the Composting Kinetics and Mechanism. Biomacromolecules 2023, 24, 2356–2368. [Google Scholar] [CrossRef]
- Quattrosoldi, S.; Guidotti, G.; Soccio, M.; Siracusa, V.; Lotti, N. Bio-based and one-day compostable poly(diethylene 2,5-furanoate) for sustainable flexible food packaging: Effect of ether-oxygen atom insertion on the final properties. Chemosphere 2022, 291, 132996. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Steck, J.; Yang, X.; Zhang, G.; Yin, J.; Suo, Z. Cracks outrun erosion in degradable polymers. Extreme Mech. Lett. 2020, 40, 100978. [Google Scholar] [CrossRef]
- Klimczuk, B.; Rudnicka, A.; Owczarek, O.; Puszkarz, A.K.; Szparaga, G.; Puchalski, M. Investigation of the Hydrolytic Degradation Kinetics of 3D-Printed PLA Structures under a Thermally Accelerated Regime. Materials 2024, 17, 1043. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Pereira, T.d.S.; Freire, V.d.A.; Santiago, M.; Oliveira, H.M.L.; Conrado, L.d.S.; de Gusmão, R.P. Influence of enzymatic hydrolysis on the properties of red rice starch. Int. J. Biol. Macromol. 2019, 141, 1210–1219. [Google Scholar] [CrossRef]
- Yan, F.; Wei, R.; Cui, Q.; Bornscheuer, U.T.; Liu, Y.-J. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum. Microb. Biotechnol. 2021, 14, 374–385. [Google Scholar] [CrossRef]


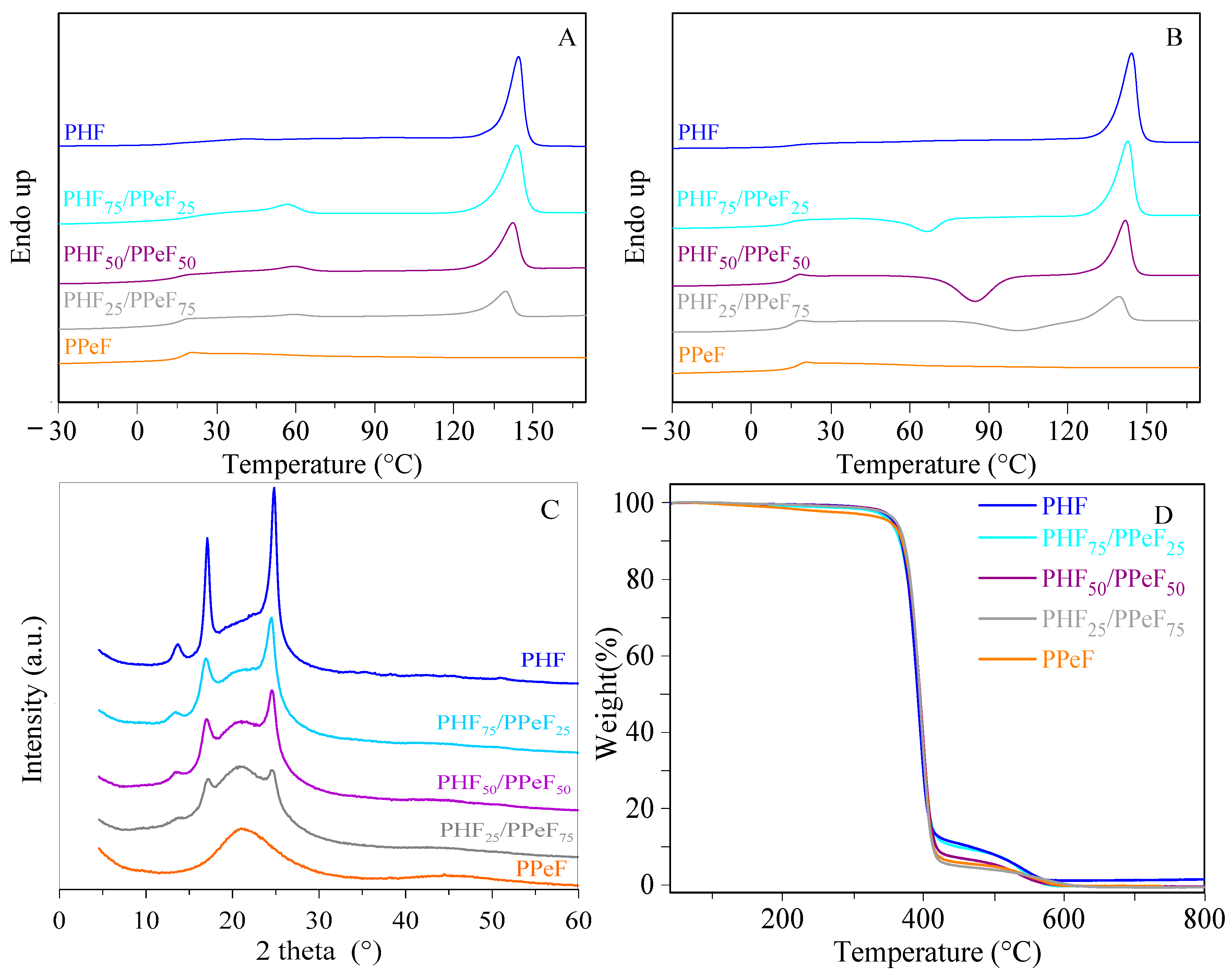


| Tonset [°C] | Tmax [°C] | I Scan | II Scan | Xc [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg [°C] | ∆cp [J/g°C] | Tm [°C] | ∆Hm [J/g] | Tg [°C] | ∆cp [J/g°C] | Tcc [°C] | ∆Hcc [J/g] | Tm [°C] | ∆Hm [J/g] | ||||
| PHF | 374 | 394 | 14 | 0.146 | 144 | 39 | 16 | 0.156 | - | - | 144 | 39 | 35 |
| PHF75/PPeF25 | 373 | 396 | 18 | 0.176 | 57–144 | 5.3–37 | 14 | 0.373 | 66 | 10 | 143 | 34 | 24 |
| PHF50/PPeF50 | 375 | 398 | 14 | 0.247 | 59–142 | 3.1–27 | 14 | 0.425 | 85 | 23 | 142 | 23 | 16 |
| PHF25/PPeF75 | 376 | 396 | 14 | 0.234 | 59–140 | 1.3–13 | 14 | 0.366 | 101 | 13 | 139 | 13 | 12 |
| PPeF | 377 | 399 | 16 | 0.319 | - | - | 16 | 0.277 | - | - | - | - | 0 |
| E (MPa) | σB (MPa) | εB (%) | O2-TR (cm3 cm m−2 d−1 atm−1) | CO2-TR (cm3 cm m−2 d−1 atm−1) | |
|---|---|---|---|---|---|
| PHF | 906 ± 34 | 22 ± 1 | 42 ± 6 | 0.19 | 0.5 |
| PHF75/PPeF25 | 205 ± 21 | 16 ± 2 | 368 ± 12 | 0.852 | 1.29 |
| PHF50/PPeF50 | 85 ± 4 | 14 ± 1 | 657 ± 39 | 0.997 | 1.55 |
| PHF25/PPeF75 | 32 ± 3 | 5 ± 1 | 803 ± 43 | 0.505 | 0.969 |
| PPeF | 9 ± 1 | 6 ± 1 | 1050 ± 200 | 0.0016 | 0.0014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidotti, G.; Palumbo, A.; Soccio, M.; Gazzano, M.; Salatelli, E.; Siracusa, V.M.; Lotti, N. Fully Bio-Based Blends of Poly (Pentamethylene Furanoate) and Poly (Hexamethylene Furanoate) for Sustainable and Flexible Packaging. Polymers 2024, 16, 2342. https://doi.org/10.3390/polym16162342
Guidotti G, Palumbo A, Soccio M, Gazzano M, Salatelli E, Siracusa VM, Lotti N. Fully Bio-Based Blends of Poly (Pentamethylene Furanoate) and Poly (Hexamethylene Furanoate) for Sustainable and Flexible Packaging. Polymers. 2024; 16(16):2342. https://doi.org/10.3390/polym16162342
Chicago/Turabian StyleGuidotti, Giulia, Arianna Palumbo, Michelina Soccio, Massimo Gazzano, Elisabetta Salatelli, Valentina M. Siracusa, and Nadia Lotti. 2024. "Fully Bio-Based Blends of Poly (Pentamethylene Furanoate) and Poly (Hexamethylene Furanoate) for Sustainable and Flexible Packaging" Polymers 16, no. 16: 2342. https://doi.org/10.3390/polym16162342
APA StyleGuidotti, G., Palumbo, A., Soccio, M., Gazzano, M., Salatelli, E., Siracusa, V. M., & Lotti, N. (2024). Fully Bio-Based Blends of Poly (Pentamethylene Furanoate) and Poly (Hexamethylene Furanoate) for Sustainable and Flexible Packaging. Polymers, 16(16), 2342. https://doi.org/10.3390/polym16162342











