Bio-Based Polyurethane–Urea with Self-Healing and Closed-Loop Recyclability Synthesized from Renewable Carbon Dioxide and Vanillin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amino-Terminal Polyurea Oligomers (TTD*)
2.3. Synthesis of Vanillin-Based Epoxy Monomeracetal Monomer (VL-H)
2.4. Synthesis of Vanillin-Based 5-Membered Dicyclic Carbonates (VL-C)
2.5. Preparation of the VL-TTD* Networks
2.6. Characterization Methods
3. Results and Discussion
3.1. Synthesis and Characterization of VL-C and TTD*
3.2. Synthesis and Characterization of VL-TTD*s
3.3. Surface Properties and Rheological Properties of VL-TTD*s
3.4. Thermal Properties of VL-TTD*s
3.5. Mechanical Properties of VL-TTD*s
3.6. Reprocessing Recyclability of VL-TTD*s
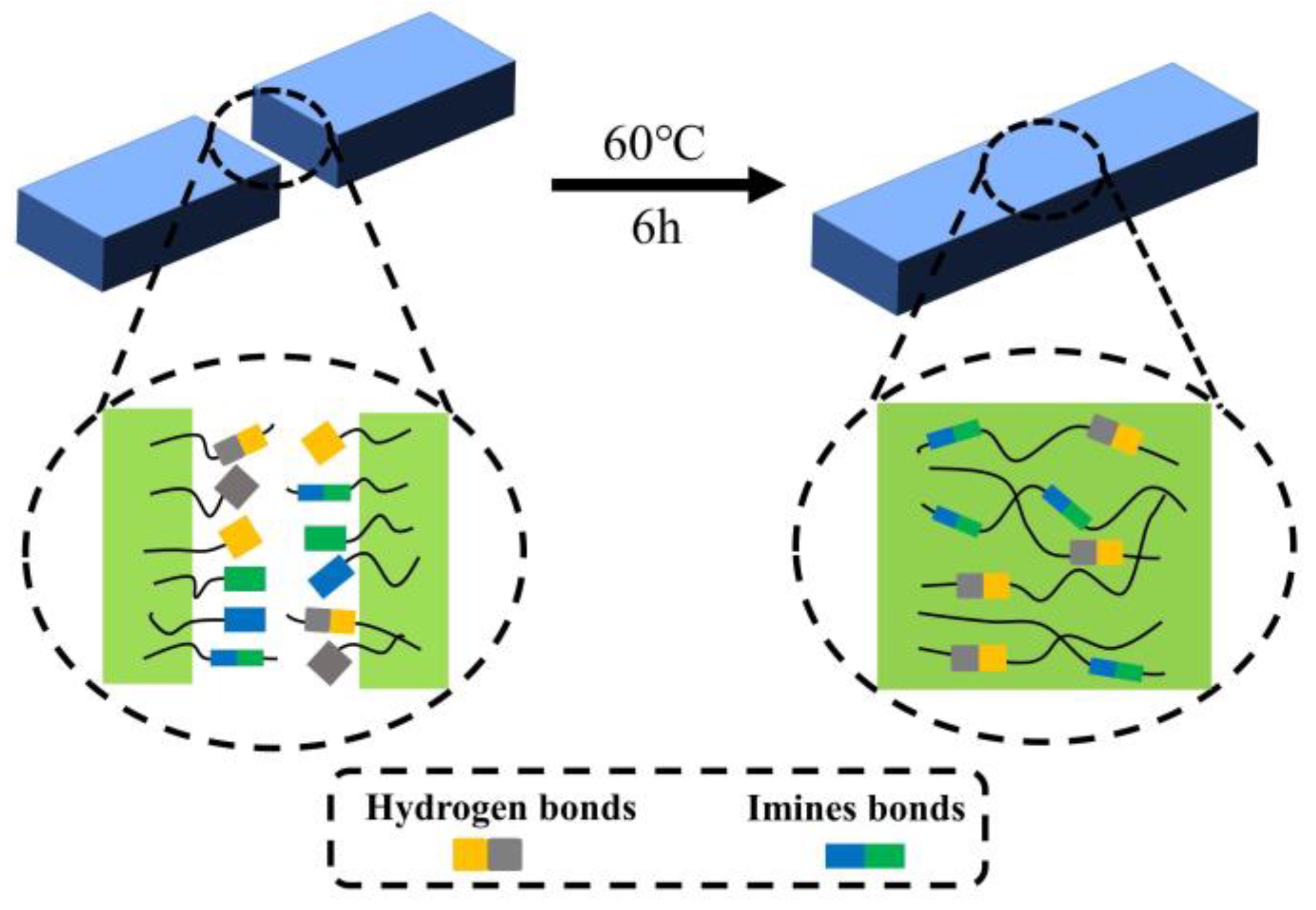
3.7. Self-Healing of VL-TTD*s
3.8. Closed-Loop Recyclability of VL-TTD*s
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Farshchi, N.; Gedan-Smolka, M. Polyurethane Powder Coatings: A Review of Composition and Characterization. Ind. Eng. Chem. Res. 2020, 59, 15121–15132. [Google Scholar] [CrossRef]
- Habets, T.; Siragusa, F.; Grignard, B.; Detrembleur, C. Advancing the Synthesis of Isocyanate-Free Poly(oxazolidones)s: Scope and Limitations. Macromolecules 2020, 53, 6396–6408. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, S.; Lu, Z.; Li, J.; Yang, X.; Qiao, Y.; Men, Y.; Sun, J. Healable, Recyclable, and Mechanically Tough Polyurethane Elastomers with Exceptional Damage Tolerance. Adv. Mater. 2020, 32, 2005759. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A review on the production, properties and applications of non-isocyanate polyurethane: A greener perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Alferov, K.A.; Fu, Z.; Ye, S.; Han, D.; Wang, S.; Xiao, M.; Meng, Y. One-Pot Synthesis of Dimethyl Hexane-1,6-diyldicarbamate from CO2, Methanol, and Diamine over CeO2 Catalysts: A Route to an Isocyanate-Free Feedstock for Polyurethanes. ACS Sustain. Chem. Eng. 2019, 7, 10708–10715. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Reworkable Polyhydroxyurethane Films with Reversible Acetal Networks Obtained from Multifunctional Six-Membered Cyclic Carbonates. J. Am. Chem. Soc. 2018, 140, 884–887. [Google Scholar] [CrossRef]
- Bourguignon, M.; Grignard, B.; Detrembleur, C. Water-Induced Self-Blown Non-Isocyanate Polyurethane Foams. Angew. Chem. Int. Ed. 2022, 61, e202213422. [Google Scholar] [CrossRef]
- Gennen, S.; Grignard, B.; Tassaing, T.; Jérôme, C.; Detrembleur, C. CO2-Sourced α-Alkylidene Cyclic Carbonates: A Step Forward in the Quest for Functional Regioregular Poly(urethane)s and Poly(carbonate)s. Angew. Chem. Int. Ed. 2017, 56, 10394–10398. [Google Scholar] [CrossRef]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Dhers, S.; Vantomme, G.; Avérous, L. A fully bio-based polyimine vitrimer derived from fructose. Green Chem. 2019, 21, 1596–1601. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Liu, J.; He, M.; Leng, X.; Li, Y. Bio-based non-isocyanate polyurethane with closed-loop recyclability and its potential application. Chem. Eng. J. 2023, 475, 146398. [Google Scholar] [CrossRef]
- Fanjul-Mosteirín, N.; Fonseca, L.P.; Dove, A.P.; Sardon, H. Bio-based non-isocyanate poly(hydroxy urethane)s (PHU) derived from vanillin and CO2. Mater. Adv. 2023, 4, 2437–2448. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Wang, S.; Wang, S.; Wang, Z.; Liu, S.; Xu, X.; Liu, H.; Song, Z. Fully Bio-Based Polyhydroxyurethanes with a Dynamic Network from a Terpene Derivative and Cyclic Carbonate Functional Soybean Oil. ACS Sustain. Chem. Eng. 2021, 9, 4175–4184. [Google Scholar] [CrossRef]
- Yang, X.; Ren, C.; Liu, X.; Sun, P.; Xu, X.; Liu, H.; Shen, M.; Shang, S.; Song, Z. Recyclable non-isocyanate polyurethanes containing a dynamic covalent network derived from epoxy soybean oil and CO2. Mater. Chem. Front. 2021, 5, 6160–6170. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, H.; Wang, X.; Shi, R.; Zhang, C.; Arai, M.; Zhao, F. A self-healing and recyclable polyurethane-urea Diels-Alder adduct synthesized from carbon dioxide and furfuryl amine. Green Chem. 2021, 23, 552–560. [Google Scholar] [CrossRef]
- Shrestha, M.L.; Ionescu, M. Aliphatic-Aromatic Polyols by Thiol-Ene Reactions. J. Polym. Environ. 2017, 26, 2257–2267. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Torkelson, J.M. Biobased Reprocessable Polyhydroxyurethane Networks: Full Recovery of Crosslink Density with Three Concurrent Dynamic Chemistries. ACS Sustain. Chem. Eng. 2019, 7, 10025–10034. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Behera, P.K.; Raut, S.K.; Mondal, P.; Sarkar, S.; Singha, N.K. Self-Healable Polyurethane Elastomer Based on Dual Dynamic Covalent Chemistry Using Diels-Alder “Click” and Disulfide Metathesis Reactions. ACS Appl. Polym. Mater. 2021, 3, 847–856. [Google Scholar] [CrossRef]
- Jiang, H.; Pang, W.; Cheng, M.; Yan, T.; Wang, Z.; Zhao, Z.; Li, C.; Sun, S.; Hu, S. Construction and mechanism of phase-locked structured PU elastomers with tunable mechanical and self-healing properties. Appl. Surf. Sci. 2024, 649, 159136. [Google Scholar] [CrossRef]
- Wang, X.Z.; Lu, M.S.; Zeng, J.B.; Weng, Y.; Li, Y.D. Malleable and thermally recyclable polyurethane foam. Green Chem. 2021, 23, 307–313. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, Y.J.; Zhang, H.; Lu, X.; Sun, J. Room-Temperature Self-Healing and Recyclable Tough Polymer Composites Using Nitrogen-Coordinated Boroxines. Adv. Funct. Mater. 2018, 28, 1800560. [Google Scholar] [CrossRef]
- Ding, H.; Wang, J.; Yu, P.; He, H.; Wang, H.; Zhang, W.; Wang, L.; Lei, Y.; Yu, B. Rapidly recyclable, monomer recovery and flame-retardant bio-based polyimine networks. Chem. Eng. J. 2024, 481, 148024. [Google Scholar] [CrossRef]
- Yang, C.; Xia, X.; Xiao, Y.; Wei, G.; Li, W.; Lu, Y. Degradable, intrinsically flame-retardant, low-water-absorbing vanillin-derived epoxy thermoset with a Schiff base structure. Polym. Degrad. Stab. 2024, 221, 110666. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X.; Shi, R.; Cheng, H.; Zhao, F. A self-healing and recyclable poly(urea-imine) thermoset synthesized from CO2. Green Chem. 2022, 24, 1561–1569. [Google Scholar] [CrossRef]
- Becker, G.; Marquetant, T.A.; Wagner, M.; Wurm, F.R. Multifunctional Poly(phosphoester)s for Reversible Diels-Alder Postmodification To Tune the LCST in Water. Macromolecules 2017, 50, 7852–7862. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Liu, X.; Huang, Z.; Huang, X.; Xu, X.; Liu, H.; Wang, D.; Shang, S. Preparation of non-isocyanate polyurethanes from epoxy soybean oil: Dual dynamic networks to realize self-healing and reprocessing under mild conditions. Green Chem. 2021, 23, 6349–6355. [Google Scholar] [CrossRef]
- Ciaccia, M.; Cacciapaglia, R.; Mencarelli, P.; Mandolini, L.; Di Stefano, S. Fast transimination in organic solvents in the absence of proton and metal catalysts. A key to imine metathesis catalyzed by primary amines under mild conditions. Chem. Sci. 2013, 4, 2253–2261. [Google Scholar] [CrossRef]
- Cordes, E.H.; Jencks, W.P. On the Mechanism of Schiff Base Formation and Hydrolysis. J. Am. Chem. Soc. 1962, 84, 832–837. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, D.; Zhang, C.; Wang, Z.; Jin, Y.; Zhang, W.; Liu, X.; Sun, J. Strong and Tough Supramolecular Covalent Adaptable Networks with Room-Temperature Closed-Loop Recyclability. Adv. Mater. 2022, 35, 2208619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, L. Novel carbon dioxide based poly(hydroxyurethane-urea)s: Synthesis and properties. Polymer 2022, 244, 124652. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, H.; Wang, Y.; Shi, R.; Wu, Z.; Arai, M.; Zhao, F. New Kind of Thermoplastic Polyurea Elastomers Synthesized from CO2 and with Self-Healing Properties. ACS Sustain. Chem. Eng. 2020, 8, 12677–12685. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Jiang, Z.; Wang, S.; Liu, H.; Xu, X.; Wang, D.; Miao, Y.; Shang, S.; Song, Z. Mechanical reinforcement of room-temperature-vulcanized silicone rubber using modified cellulose nanocrystals as cross-linker and nanofiller. Carbohydr. Polym. 2020, 229, 115509. [Google Scholar] [CrossRef]
- Naik, A.D.; Fontaine, G.; Bellayer, S.; Bourbigot, S. Crossing the Traditional Boundaries: Salen-Based Schiff Bases for Thermal Protective Applications. ACS Appl. Mater. Interfaces 2015, 7, 21208–21217. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.N.; Chen, L.; Fu, T.; Zhao, H.B.; Guo, D.M.; Wang, X.L.; Wang, Y.Z. New application for aromatic Schiff base: High efficient flame-retardant and anti-dripping action for polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- GB/T 1040-2006; Plastics-Determination of Tensile Properties. China, GB: Beijing, China, 2006.










| Samples | T5% (°C) | Tmax (°C) | CR (wt%) | Tg (°C) |
|---|---|---|---|---|
| VL-TTD*-30 | 255 | 332 | 14 | −11 |
| VL-TTD*-40 | 243 | 326 | 16 | −4 |
| VL-TTD*-50 | 235 | 327 | 16 | −1 |
| VL-TTD*-60 | 216 | 336 | 18 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.; Tian, T.; Jiang, S.; Lu, B. Bio-Based Polyurethane–Urea with Self-Healing and Closed-Loop Recyclability Synthesized from Renewable Carbon Dioxide and Vanillin. Polymers 2024, 16, 2277. https://doi.org/10.3390/polym16162277
Han T, Tian T, Jiang S, Lu B. Bio-Based Polyurethane–Urea with Self-Healing and Closed-Loop Recyclability Synthesized from Renewable Carbon Dioxide and Vanillin. Polymers. 2024; 16(16):2277. https://doi.org/10.3390/polym16162277
Chicago/Turabian StyleHan, Tianyi, Tongshuai Tian, Shan Jiang, and Bo Lu. 2024. "Bio-Based Polyurethane–Urea with Self-Healing and Closed-Loop Recyclability Synthesized from Renewable Carbon Dioxide and Vanillin" Polymers 16, no. 16: 2277. https://doi.org/10.3390/polym16162277
APA StyleHan, T., Tian, T., Jiang, S., & Lu, B. (2024). Bio-Based Polyurethane–Urea with Self-Healing and Closed-Loop Recyclability Synthesized from Renewable Carbon Dioxide and Vanillin. Polymers, 16(16), 2277. https://doi.org/10.3390/polym16162277





