The Buffer Capacity of Polyelectrolyte Microcapsules Depends on the Type of Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyelectrolyte Microcapsules
2.3. Temperature Treatment
2.4. Measurement of Buffer Capacity
2.5. Statistical Processing
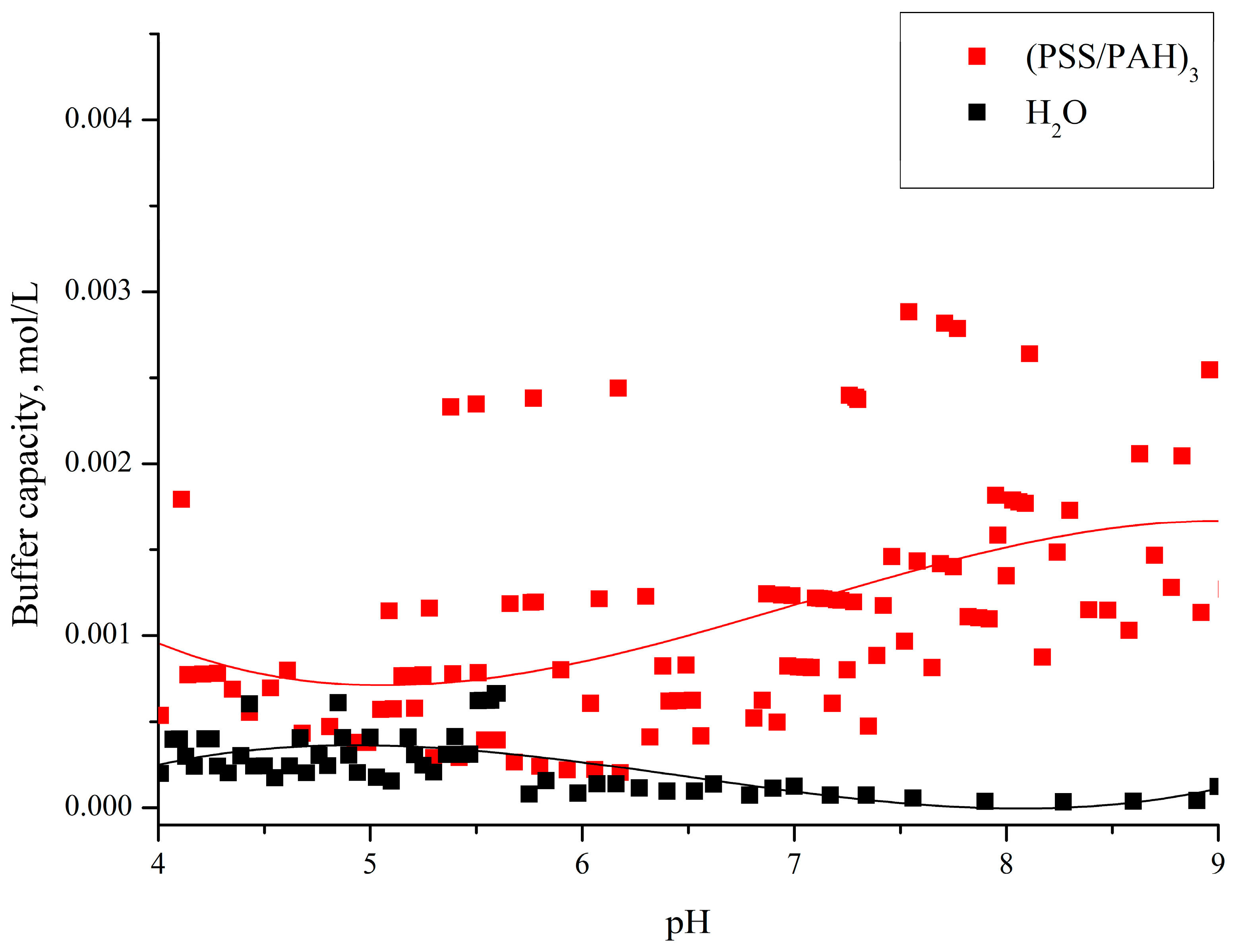
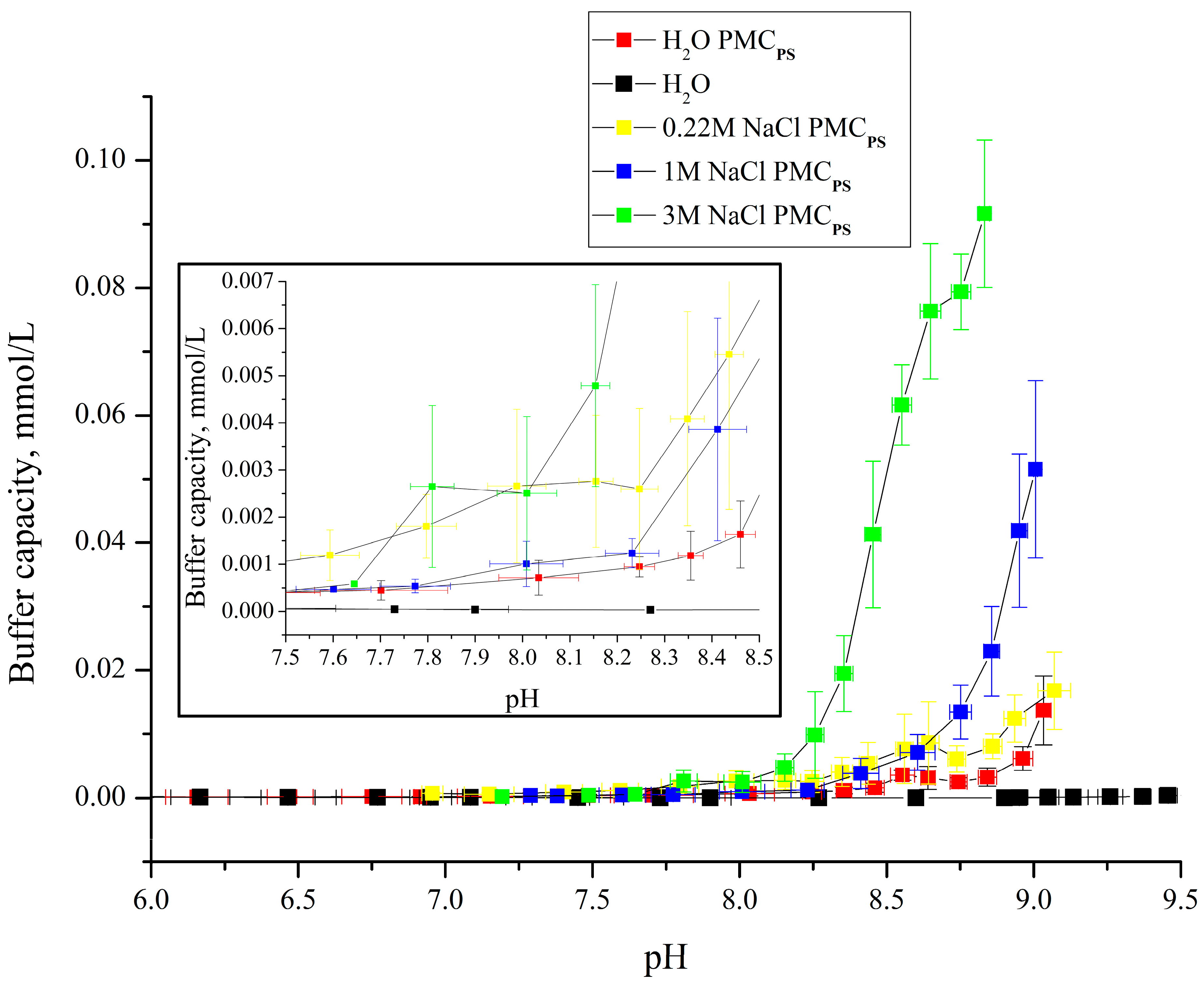
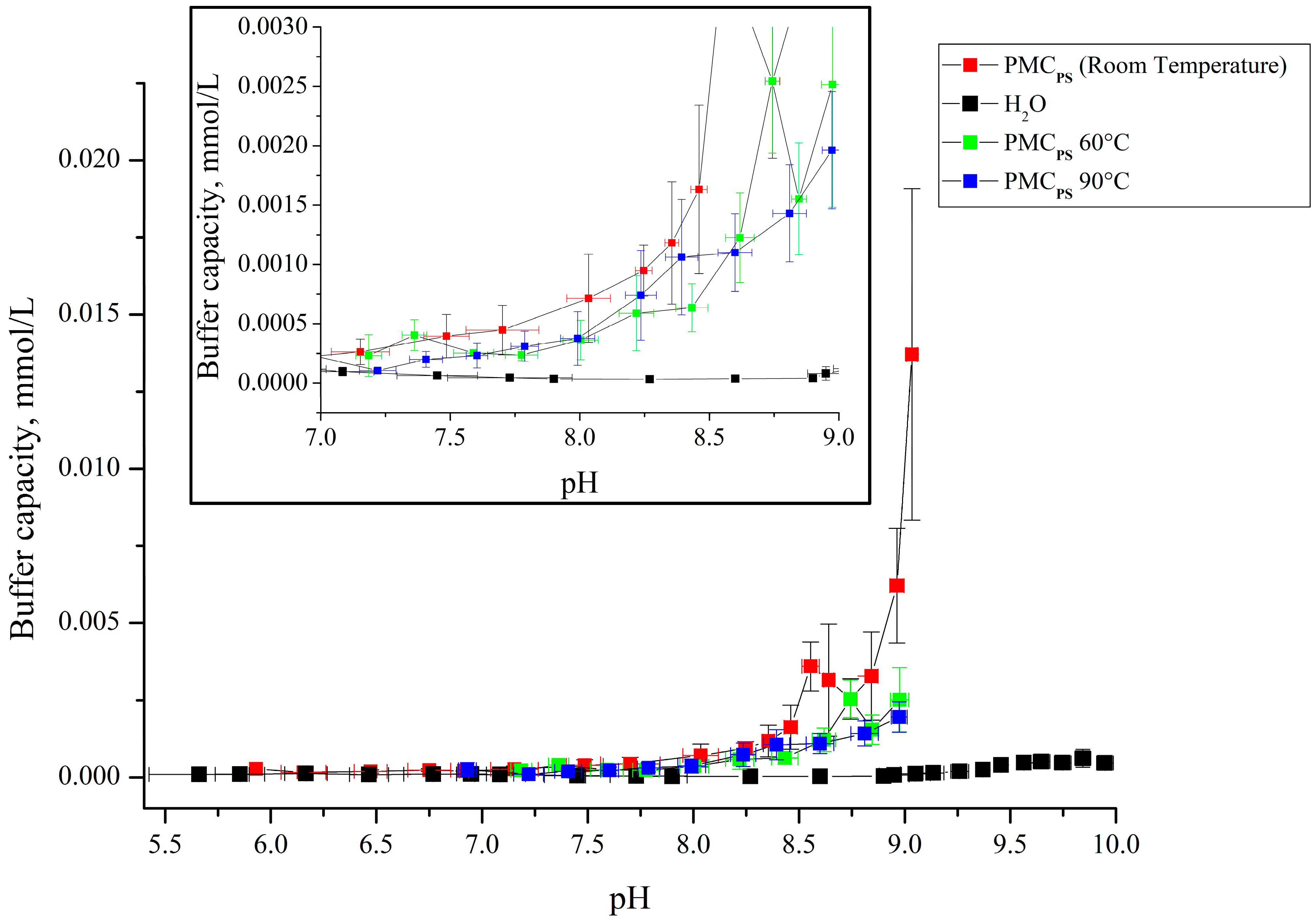
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sukhorukov, G.B.; Donath, E.; Davis, S.; Lichtenfeld, H.; Caruso, F.; Popov, V.I.; Möhwald, H. Stepwise polyelectrolyte assembly on particle surfaces: A novel approach to colloid design. Polym. Adv. Technol. 1998, 9, 759–767. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Lichtenfeld, H.; Knippel, E.; Knippel, M.; Budde, A.; Möhwald, H. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 137, 253–266. [Google Scholar] [CrossRef]
- Köhler, K.; Shchukin, D.G.; Sukhorukov, G.B.; Möhwald, H. Drastic Morphological Modification of Polyelectrolyte Microcapsules Induced by High Temperature. Macromolecules 2004, 37, 9546–9550. [Google Scholar] [CrossRef]
- Gao, C.; Donath, E.; Möhwald, H.; Shen, J. Spontaneous Deposition of Water-Soluble Substances into Microcapsules: Phenomenon, Mechanism, and Application. Angew. Chem. Int. Ed. 2002, 41, 3789–3793. [Google Scholar] [CrossRef]
- Dubreuil, F.; Elsner, N.; Fery, A. Elastic properties of polyelectrolyte capsules studied by atomic-force microscopy and RICM. Eur. Phys. J. E 2003, 12, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, G.B.; Shchukin, D.G.; Dong, W.; Möhwald, H.; Lulevich, V.V.; Vinogradova, O.I. Comparative Analysis of Hollow and Filled Polyelectrolyte Microcapsules Templated on Melamine Formaldehyde and Carbonate Cores. Macromol. Chem. Phys. 2004, 205, 530–535. [Google Scholar] [CrossRef]
- Köhler, K.; Shchukin, D.G.; Möhwald, H.; Sukhorukov, G.B. Thermal Behavior of Polyelectrolyte Multilayer Microcapsules. 1. The Effect of Odd and Even Layer Number. J. Phys. Chem. B 2005, 109, 18250–18259. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Yashchenok, A.M.; Möhwald, H. Encapsulation, release and applications of LbL polyelectrolyte multilayer capsules. Chem. Commun. 2011, 47, 12736. [Google Scholar] [CrossRef]
- De Geest, B.G.; Skirtach, A.G.; Mamedov, A.A.; Antipov, A.A.; Kotov, N.A.; De Smedt, S.C.; Sukhorukov, G.B. Ultrasound-Triggered Release from Multilayered Capsules. Small 2007, 3, 804–808. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Ermakov, A.; Sindeeva, O.; Prikhozhdenko, E.; Kozlova, A.; Grishin, O.; Makarkin, M.; Gorin, D.; Bratashov, D. Effect of Size on Magnetic Polyelectrolyte Microcapsules Behavior: Biodistribution, Circulation Time, Interactions with Blood Cells and Immune System. Pharmaceutics 2021, 13, 2147. [Google Scholar] [CrossRef]
- Palamarchuk, K.V.; Borodina, T.N.; Kostenko, A.V.; Chesnokov, Y.M.; Kamyshinsky, R.A.; Palamarchuk, N.P.; Yudina, E.B.; Nikolskaya, E.D.; Yabbarov, N.G.; Mollaeva, M.R.; et al. Development of Submicrocapsules Based on Co-Assembled Like-Charged Silica Nanoparticles and Detonation Nanodiamonds and Polyelectrolyte Layers. Pharmaceutics 2022, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Gileva, A.; Trushina, D.; Yagolovich, A.; Gasparian, M.; Kurbanova, L.; Smirnov, I.; Burov, S.; Markvicheva, E. Doxorubicin-Loaded Polyelectrolyte Multilayer Capsules Modified with Antitumor DR5-Specific TRAIL Variant for Targeted Drug Delivery to Tumor Cells. Nanomaterials 2023, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.M.; Subramanian, A.; Ochs, C.J.; Dewavrin, J.-Y.; Beyer, S.; Trau, D.W. Edible polyelectrolyte microcapsules with water-soluble cargo assembled in organic phase. RSC Adv. 2014, 4, 35163–35166. [Google Scholar] [CrossRef]
- Tan, C.; Selig, M.J.; Lee, M.C.; Abbaspourrad, A. Encapsulation of copigmented anthocyanins within polysaccharide microcapsules built upon removable CaCO3 templates. Food Hydrocoll. 2018, 84, 200–209. [Google Scholar] [CrossRef]
- Khan, A.; Sliem, M.H.; Arif, A.; Salih, M.A.; Shakoor, R.A.; Montemor, M.F.; Kahraman, R.; Mansour, S.; Abdullah, A.M.; Hasan, A. Designing and performance evaluation of polyelectrolyte multilayered composite smart coatings. Prog. Org. Coat. 2019, 137, 105319. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.; Gould, D.J.; Shcherbakov, A.B.; Sukhorukov, G.B.; Ivanov, V.K. Ceria Nanoparticles-Decorated Microcapsules as a Smart Drug Delivery/Protective System: Protection of Encapsulated P. pyralis Luciferase. ACS Appl. Mater. Interfaces 2018, 10, 14367–14377. [Google Scholar] [CrossRef] [PubMed]
- Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Destruction of polyelectrolyte microcapsules formed on CaCO3 microparticles and the release of a protein included by the adsorption method. Polymers 2020, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Lulevich, V.V.; Vinogradova, O.I. Effect of pH and Salt on the Stiffness of Polyelectrolyte Multilayer Microcapsules. Langmuir 2004, 20, 2874–2878. [Google Scholar] [CrossRef]
- Kim, B.; Vinogradova, O.I. pH-Controlled Swelling of Polyelectrolyte Multilayer Microcapsules. J. Phys. Chem. B 2004, 8161–8165. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Sukhorukov, G.B. Co-encapsulation of enzyme and sensitive dye as a tool for fabrication of microcapsule based sensor for urea measuring. Phys. Chem. Chem. Phys. 2011, 13, 11110–11117. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and biosensors based on polyelectrolyte microcapsules containing fluorescent dyes and enzymes. Anal. Bioanal. Chem. 2013, 405, 1559–1568. [Google Scholar] [CrossRef]
- Musin, E.V.; Dubrovskii, A.V.; Kim, A.L.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell. Int. J. Mol. Sci. 2022, 23, 9917. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature. Int. J. Mol. Sci. 2022, 23, 6608. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Ramazanov, B.R.; Tikhonenko, S.A. The Discovery of the Buffer Capacity of Various Types of Polyelectrolyte Microcapsules. Polymers 2021, 13, 4026. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Musin, E.V.; Chebykin, Y.S.; Tikhonenko, S.A. Characterization of Polyallylamine/Polystyrene Sulfonate Polyelectrolyte Microcapsules Formed on Solid Cores: Morphology. Polymers 2024, 16, 1521. [Google Scholar] [CrossRef]
- Déjugnat, C.; Sukhorukov, G.B. pH-Responsive Properties of Hollow Polyelectrolyte Microcapsules Templated on Various Cores. Langmuir 2004, 20, 7265–7269. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix Polyelectrolyte Microcapsules: New System for Macromolecule Encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Dong, W.; Gao, C.; Möhwald, H. Charge-Controlled Permeability of Polyelectrolyte Microcapsules. J. Phys. Chem. B 2005, 109, 13159–13165. [Google Scholar] [CrossRef]
- Haložan, D.; Riebentanz, U.; Brumen, M.; Donath, E. Polyelectrolyte microcapsules and coated CaCO3 particles as fluorescence activated sensors in flowmetry. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 115–121. [Google Scholar] [CrossRef]
- Heuvingh, J.; Zappa, M.; Fery, A. Salt Softening of Polyelectrolyte Multilayer Capsules. Langmuir 2005, 21, 3165–3171. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Möhwald, H.; Volodkin, D.V. pH- and salt-mediated response of layer-by-layer assembled PSS/PAH microcapsules: Fusion and polymer exchange. Soft Matter 2012, 8, 8659. [Google Scholar] [CrossRef]
- Prevot, M.; Déjugnat, C.; Möhwald, H.; Sukhorukov, G.B. Behavior of Temperature-Sensitive PNIPAM Confined in Polyelectrolyte Capsules. ChemPhysChem 2006, 7, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Haložan, D.; Déjugnat, C.; Brumen, M.; Sukhorukov, G.B. Entrapment of a Weak Polyanion and H+/Na+ Exchange in Confined Polyelectrolyte Microcapsules. J. Chem. Inf. Model. 2005, 45, 1589–1592. [Google Scholar] [CrossRef]
- Radtchenko, I.L.; Sukhorukov, G.B.; Leporatti, S.; Khomutov, G.B.; Donath, E.; Möhwald, H. Assembly of Alternated Multivalent Ion/Polyelectrolyte Layers on Colloidal Particles. Stability of the Multilayers and Encapsulation of Macromolecules into Polyelectrolyte Capsules. J. Colloid Interface Sci. 2000, 230, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan–DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2013, 14, 1732–1740. [Google Scholar] [CrossRef]
- Kim, A.L.; Musin, E.V.; Dubrovskii, A.V.; Tikhonenko, S.A. Determination of urea concentration using urease-containing polyelectrolyte microcapsules. Anal. Methods 2019, 11, 1585–1590. [Google Scholar] [CrossRef]
- Pavlov, A.M.; Saez, V.; Cobley, A.; Graves, J.; Sukhorukov, G.B.; Mason, T.J. Controlled protein release from microcapsules with composite shells using high frequency ultrasound—Potential for in vivo medical use. Soft Matter 2011, 7, 4341–4347. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Scheepers, D.; Chatillon, B.; Borneman, Z.; Nijmeijer, K. Influence of charge density and ionic strength on diallyldimethylammonium chloride (DADMAC)-based polyelectrolyte multilayer membrane formation. J. Memb. Sci. 2021, 617, 118619. [Google Scholar] [CrossRef]
- Ferrand-Drake del Castillo, G.; Hailes, R.L.N.; Dahlin, A. Large Changes in Protonation of Weak Polyelectrolyte Brushes with Salt Concentration—Implications for Protein Immobilization. J. Phys. Chem. Lett. 2020, 11, 5212–5218. [Google Scholar] [CrossRef]
- Sagou, J.-P.S.; Ahualli, S.; Thomas, F. Influence of ionic strength and polyelectrolyte concentration on the electrical conductivity of suspensions of soft colloidal polysaccharides. J. Colloid Interface Sci. 2015, 459, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Deng, S.; Advincula, R.C. Sustained Release Control via Photo-Cross-Linking of Polyelectrolyte Layer-by-Layer Hollow Capsules. Langmuir 2005, 21, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, A.V.; Shabarchina, L.I.; Kim, Y.A.; Sukhorukov, B.I. Influence of the temperature on polyelectrolyte microcapsules: Light scattering and confocal microscopy data. Russ. J. Phys. Chem. A 2006, 80, 1703–1707. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubrovskii, A.V.; Kim, A.L.; Tikhonenko, S.A. The Buffer Capacity of Polyelectrolyte Microcapsules Depends on the Type of Template. Polymers 2024, 16, 2261. https://doi.org/10.3390/polym16162261
Dubrovskii AV, Kim AL, Tikhonenko SA. The Buffer Capacity of Polyelectrolyte Microcapsules Depends on the Type of Template. Polymers. 2024; 16(16):2261. https://doi.org/10.3390/polym16162261
Chicago/Turabian StyleDubrovskii, Alexey V., Aleksandr L. Kim, and Sergey A. Tikhonenko. 2024. "The Buffer Capacity of Polyelectrolyte Microcapsules Depends on the Type of Template" Polymers 16, no. 16: 2261. https://doi.org/10.3390/polym16162261
APA StyleDubrovskii, A. V., Kim, A. L., & Tikhonenko, S. A. (2024). The Buffer Capacity of Polyelectrolyte Microcapsules Depends on the Type of Template. Polymers, 16(16), 2261. https://doi.org/10.3390/polym16162261





