A Study of Hydroxyl-Terminated Block Copolyether-Based Binder Curing Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Non-Isothermal Differential Scanning Calorimetry Tests
3. Results
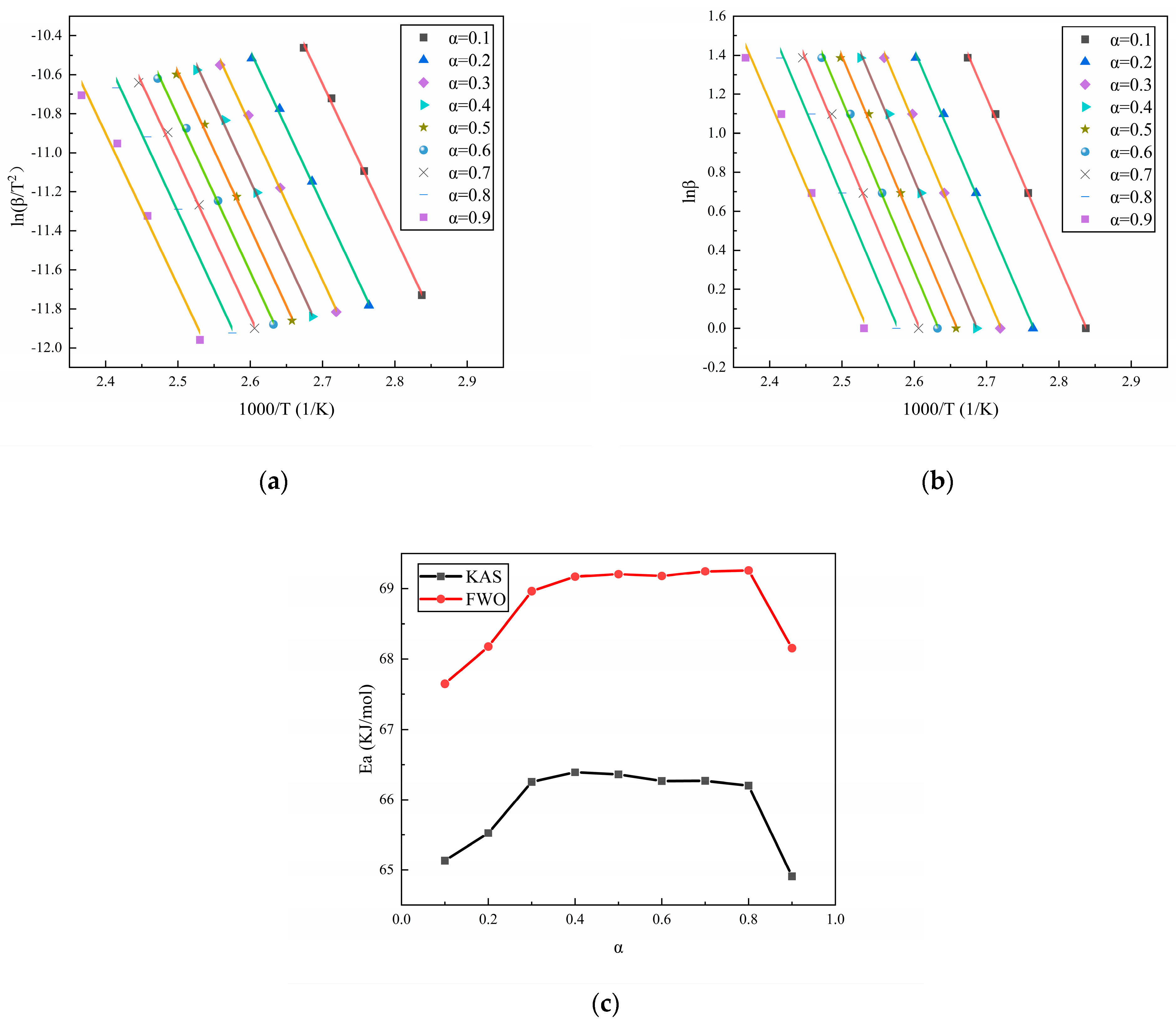
3.1. Curing Activation Energy
3.2. Curing Kinetics Model and Parameters
3.3. Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Jin, P.; Zhao, S.; Luo, Y. Mechanism of AP effect on slow cook-off response of HTPE propellant. Thermochim. Acta 2022, 715, 179291. [Google Scholar] [CrossRef]
- Shi, L.; Fu, X.; Li, Y.; Wu, S.; Meng, S.; Wang, J. Molecular Dynamic Simulations and Experiments Study on the Mechanical Properties of HTPE Binders. Polymers 2022, 14, 5491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-N.; Chang, H.; Li, J.-Q.; Li, X.-J.; Wang, H. High-strain-rate mechanical response of HTPE propellant under SHPB impact loading. AIP Adv. 2021, 11, 035145. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, B.; Wen, X.; Chen, K.; Jiang, S.; Luo, Y. Investigation on mechanical and thermal properties of HTPE/PCL propellant for wide temperature range use. J. Therm. Anal. Calorim. 2021, 147, 4971–4982. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Z.; Chen, L.; Liu, P.; Jia, L.; Dong, B.; Li, H.; Xu, S. A study on the decomposition pathways of HTPB and HTPE pyrolysis by mass spectrometric analysis. J. Anal. Appl. Pyrolysis 2023, 170, 105929. [Google Scholar] [CrossRef]
- Shen, C.; Yan, S.; Ou, Y.; Jiao, Q. Influence of Fluorinated Polyurethane Binder on the Agglomeration Behaviors of Aluminized Propellants. Polymers 2022, 14, 1124. [Google Scholar] [CrossRef]
- Shen, C.; Yan, S.; Tan, Y.; Ou, Y.; Jiao, Q.; Luo, Y. Enhancing energy release of aluminized propellants and explosives through fluorinated binder. Propellants Explos. Pyrotech. 2024, 49, e202300199. [Google Scholar] [CrossRef]
- Yao, Q.; Xia, M.; Wang, C.; Yang, F.; Yang, W.; Luo, Y. A new fluorocarbon adhesive: Inhibiting agglomeration during com-bustion of propellant via efficient F–Al2O3 preignition reaction. Carbon Energy 2024, 6, e467. [Google Scholar] [CrossRef]
- Xu, S.; Pang, A.; Kong, J. Decomposition mechanism and kinetic investigation of novel urea burning rate suppressants in AP/HTPE propellants. J. Therm. Anal. Calorim. 2023, 148, 12811–12820. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Jin, P.; Zhao, S.; Luo, Y. Mechanism of PSAN effect on slow cook-off response of HTPE propellant. J. Energetic Mater. 2022, 42, 313–330. [Google Scholar] [CrossRef]
- Nie, J.; Liang, J.; Zhang, H.; Zou, Y.; Jiao, Q.; Li, Y.; Guo, X.; Yan, S.; Zhu, Y. Evolution of structural damage of solid composite propellants under slow heating and effect on combustion characteristics. J. Mater. Res. Technol. 2023, 25, 5021–5037. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Non-isothermal DSC and rheological curing of ferrocene-functionalized, hydroxyl-terminated polybutadiene polyurethane. React. Funct. Polym. 2016, 107, 60–68. [Google Scholar]
- Lucio, B.; de la Fuente, J.L. Catalytic effects over formation of functional thermoplastic elastomers for rocket propellants. Polym. Adv. Technol. 2021, 33, 807–817. [Google Scholar] [CrossRef]
- Ducruet, N.; Delmotte, L.; Schrodj, G.; Stankiewicz, F.; Desgardin, N.; Vallat, M.-F.; Haidar, B. Evaluation of hydroxyl ter-minated polybutadiene-isophorone diisocyanate gel formation during crosslinking process. J. Appl. Polym. Sci. 2013, 128, 436–443. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Rheological kinetics of ferrocenylsilane functionalized polyurethanes based on 4,4′-methylenediphenyl diisocyanate for advanced energetic materials. J. Appl. Polym. Sci. 2021, 139, 51765. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Kinetic and chemorheological modelling of the polymerization of 2,4- Toluenediisocyanate and ferrocene-functionalized hydroxyl-terminated polybutadiene. Polymer 2018, 140, 290–303. [Google Scholar] [CrossRef]
- Duemichen, E.; Javdanitehran, M.; Erdmann, M.; Trappe, V.; Sturm, H.; Braun, U.; Ziegmann, G. Analyzing the network formation and curing kinetics of epoxy resins by in situ near-infrared measurements with variable heating rates. Thermochim. Acta 2015, 616, 49–60. [Google Scholar] [CrossRef]
- Russo, C.; Serra, À.; Fernández-Francos, X.; De la Flor, S. Characterization of sequential dual-curing of thiol-acrylate-epoxy systems with controlled thermal properties. Eur. Polym. J. 2019, 112, 376–388. [Google Scholar] [CrossRef]
- Magina, S.; Gama, N.; Carvalho, L.; Barros-Timmons, A.; Evtuguin, D.V. Lignosulfonate-Based Polyurethane Adhesives. Materials 2021, 14, 7072. [Google Scholar] [CrossRef]
- Ding, S.; Fang, Z.; Yu, Z.; Wang, Q. Curing Kinetics and Mechanical Properties of Epoxy–Cyanate Ester Composite Films for Microelectronic Applications. ACS Omega 2023, 8, 32907–32916. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Yarahmadi, E.; Didehban, K.; Ghiyasi, S.; Paran, S.M.R.; Puglia, D.; Ali, J.A.; Jannesari, A.; Saeb, M.R.; Ranjbar, Z.; et al. Cure kinetics of epoxy/graphene oxide (GO) nanocomposites: Effect of starch functionalization of GO nanosheets. Prog. Org. Coat. 2019, 136, 105217. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommenda-tions for analysis of thermal polymerization kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Ou, Y.; Sun, Y.; Guo, X.; Jiao, Q. Investigation on the thermal decomposition of hydroxyl terminated polyether based polyu-rethanes with inert and energetic plasticizers by DSC-TG-MS-FTIR. J. Anal. Appl. Pyrol. 2018, 132, 94–101. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, G.; Chen, K.; Yuan, S.; Luo, Y. Enhancing the Performance of an HTPE Binder by Adding a Novel Hyper-branched Multi-Arm Azide Copolyether. Propellants Explos. Pyrotech. 2020, 45, 1065–1076. [Google Scholar] [CrossRef]
- Chen, K.; Wen, X.; Li, G.; Pang, S.; Luo, Y. Improvement of mechanical properties of in situ-prepared HTPE binder in pro-pellants. RSC Adv. 2020, 10, 30150–30161. [Google Scholar] [CrossRef]
- Attaei, M.; Loureiro, M.V.; Vale, M.D.; Condeço, J.A.D.; Pinho, I.; Bordado, J.C.; Marques, A.C. Isophorone Diisocyanate (IPDI) Microencapsulation for Mono-Component Adhesives: Effect of the Active H and NCO Sources. Polymers 2018, 10, 825. [Google Scholar] [CrossRef]
- Wang, G.; Li, K.; Zou, W.; Hu, A.; Hu, C.; Zhu, Y.; Chen, C.; Guo, G.; Yang, A.; Drumright, R.; et al. Synthesis of HDI/IPDI hybrid isocyanurate and its application in polyurethane coating. Prog. Org. Coat. 2015, 78, 225–233. [Google Scholar] [CrossRef]
- Kim, C.K.; Bae, S.B.; Ahn, J.R.; Chung, I.J. Structure-property relationships of hydroxy-terminated polyether based polyu-rethane network. Polym. Bull. 2008, 61, 225–233. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, S.; Luo, Y. Cross-linking network structures and mechanical properties of novel HTPE/PCL binder for solid propellant. Polym. Bull. 2020, 78, 313–334. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Nallaperumal, A.M.; Manu, S.K.P.; Varghese, L.A.; Sekkar, V. DSC assisted kinetic analysis on the urethane network formation between castor oil based ester polyol and poly(methylene di phenyl isocyanate) (pMDI). Int. J. Polym. Anal. Charact. 2022, 27, 132–146. [Google Scholar] [CrossRef]
- Zvetkov, V.; Simeonova-Ivanova, E.; Calado, V. Comparative DSC kinetics of the reaction of DGEBA with aromatic diamines IV. Iso-conversional kinetic analysis. Thermochim. Acta 2014, 596, 42–48. [Google Scholar] [CrossRef]
- Olejnik, A.; Gosz, K.; Piszczyk, Ł. Kinetics of cross-linking processes of fast-curing polyurethane system. Thermochim. Acta 2020, 683, 178435. [Google Scholar] [CrossRef]
- Park, S.; Yang, L.; Park, B.-D.; Du, G. Thermal cure kinetics of highly branched polyurea resins at different molar ratios. J. Therm. Anal. Calorim. 2023, 148, 6389–6405. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Ginic-Markovic, M.; Clarke, S. A thermal and rheological investigation during the complex cure of a two-component thermoset polyurethane. J. Appl. Polym. Sci. 2009, 114, 3802–3810. [Google Scholar] [CrossRef]
- Zvetkov, V.; Calado, V. Comparative DSC kinetics of the reaction of DGEBA with aromatic diamines. III. Formal kinetic study of the reaction of DGEBA with diamino diphenyl methane. Thermochim. Acta 2013, 560, 95–103. [Google Scholar] [CrossRef]
- Tezel, G.B.; Sarmah, A.; Desai, S.; Vashisth, A.; Green, M.J. Kinetics of carbon nanotube-loaded epoxy curing: Rheometry, differential scanning calorimetry, and radio frequency heating. Carbon 2021, 175, 1–10. [Google Scholar] [CrossRef]
- Lee, S.; Choi, C.H.; Hong, I.-K.; Lee, J.W. Polyurethane curing kinetics for polymer bonded explosives: HTPB/IPDI binder. Korean J. Chem. Eng. 2015, 32, 1701–1706. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Chemorheology and Kinetics of High-Performance Polyurethane Binders Based on HMDI. Macromol. Mater. Eng. 2021, 306, 2000617. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Structural and thermal degradation properties of novel metallocene-polyurethanes. Polym. Degrad. Stab. 2017, 136, 39–47. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rastin, H.; Nonahal, M.; Ghaffari, M.; Jannesari, A.; Formela, K. Cure kinetics of epoxy/MWCNTs nanocomposites: Nonisothermal calorimetric and rheokinetic techniques. J. Appl. Polym. Sci. 2017, 134, 45221. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Kinetic and thermodynamic analysis of the polymerization of polyurethanes by a rheological method. Thermochim. Acta 2016, 625, 28–35. [Google Scholar] [CrossRef]











| Curing System | Peak Temperature Tmax (K) | Ea (kJ/mol) | ||||
|---|---|---|---|---|---|---|
| 1 K/min | 2 K/min | 3 K/min | 4 K/min | Kissinger | Ozawa | |
| N-100/HTPE | 376.753 | 388.952 | 395.251 | 400.745 | 66.53 | 69.37 |
| IPDI/HTPE | 381.872 | 392.055 | 399.711 | 403.886 | 73.01 | 75.60 |
| NI/HTPE | 378.736 | 390.987 | 396.699 | 403.084 | 66.94 | 69.79 |
| Designation | N-100/HTPE | IPDI/HTPE | NI/HTPE | ||
|---|---|---|---|---|---|
| m | Kissinger | calculated | 0.314 | 0.421 | 0.347 |
| fitting | 0.274 | 0.456 | 0.311 | ||
| Ozawa | calculated | 0.282 | 0.391 | 0.315 | |
| fitting | 0.242 | 0.428 | 0.279 | ||
| KAS | calculated | 0.325 | 0.506 | 0.358 | |
| fitting | 0.284 | 0.535 | 0.322 | ||
| FWO | calculated | 0.293 | 0.472 | 0.326 | |
| fitting | 0.252 | 0.504 | 0.290 | ||
| n | Kissinger | calculated | 1.200 | 1.407 | 1.220 |
| fitting | 1.156 | 1.409 | 1.176 | ||
| Ozawa | calculated | 1.214 | 1.419 | 1.234 | |
| fitting | 1.170 | 1.422 | 1.190 | ||
| KAS | calculated | 1.195 | 1.373 | 1.215 | |
| fitting | 1.151 | 1.371 | 1.172 | ||
| FWO | calculated | 1.209 | 1.386 | 1.229 | |
| fitting | 1.165 | 1.386 | 1.185 | ||
| lnA | Kissinger | calculated | 14.64 | 16.76 | 14.82 |
| fitting | 14.57 | 16.79 | 14.75 | ||
| Ozawa | calculated | 15.49 | 17.54 | 15.67 | |
| fitting | 15.42 | 17.57 | 15.61 | ||
| KAS | calculated | 14.35 | 14.58 | 14.51 | |
| fitting | 14.29 | 14.60 | 14.44 | ||
| FWO | calculated | 15.20 | 15.44 | 15.37 | |
| fitting | 15.14 | 15.46 | 15.30 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Ding, Z.; Zhu, C.; Li, T.; Liu, W.; Luo, Y. A Study of Hydroxyl-Terminated Block Copolyether-Based Binder Curing Kinetics. Polymers 2024, 16, 2246. https://doi.org/10.3390/polym16162246
Yang W, Ding Z, Zhu C, Li T, Liu W, Luo Y. A Study of Hydroxyl-Terminated Block Copolyether-Based Binder Curing Kinetics. Polymers. 2024; 16(16):2246. https://doi.org/10.3390/polym16162246
Chicago/Turabian StyleYang, Wu, Zhengmao Ding, Cong Zhu, Tianqi Li, Wenhao Liu, and Yunjun Luo. 2024. "A Study of Hydroxyl-Terminated Block Copolyether-Based Binder Curing Kinetics" Polymers 16, no. 16: 2246. https://doi.org/10.3390/polym16162246
APA StyleYang, W., Ding, Z., Zhu, C., Li, T., Liu, W., & Luo, Y. (2024). A Study of Hydroxyl-Terminated Block Copolyether-Based Binder Curing Kinetics. Polymers, 16(16), 2246. https://doi.org/10.3390/polym16162246







